Asymptomatic and Presymptomatic SARS-CoV-2 Infections in Residents of a Long-Term Care Skilled Nursing Facility - King County, Washington, March 2020 - PubMed (original) (raw)
. 2020 Apr 3;69(13):377-381.
doi: 10.15585/mmwr.mm6913e1.
Kelly M Hatfield, Melissa Arons, Allison James, Joanne Taylor, Kevin Spicer, Ana C Bardossy, Lisa P Oakley, Sukarma Tanwar, Zeshan Chisty, Jeneita M Bell, Mark Methner, Josh Harney, Jesica R Jacobs, Christina M Carlson, Heather P McLaughlin, Nimalie Stone, Shauna Clark, Claire Brostrom-Smith, Libby C Page, Meagan Kay, James Lewis, Denny Russell, Brian Hiatt, Jessica Gant, Jeffrey S Duchin, Thomas A Clark, Margaret A Honein, Sujan C Reddy, John A Jernigan; Public Health – Seattle & King County; CDC COVID-19 Investigation Team
Collaborators, Affiliations
- PMID: 32240128
- PMCID: PMC7119514
- DOI: 10.15585/mmwr.mm6913e1
Asymptomatic and Presymptomatic SARS-CoV-2 Infections in Residents of a Long-Term Care Skilled Nursing Facility - King County, Washington, March 2020
Anne Kimball et al. MMWR Morb Mortal Wkly Rep. 2020.
Abstract
Older adults are susceptible to severe coronavirus disease 2019 (COVID-19) outcomes as a consequence of their age and, in some cases, underlying health conditions (1). A COVID-19 outbreak in a long-term care skilled nursing facility (SNF) in King County, Washington that was first identified on February 28, 2020, highlighted the potential for rapid spread among residents of these types of facilities (2). On March 1, a health care provider at a second long-term care skilled nursing facility (facility A) in King County, Washington, had a positive test result for SARS-CoV-2, the novel coronavirus that causes COVID-19, after working while symptomatic on February 26 and 28. By March 6, seven residents of this second facility were symptomatic and had positive test results for SARS-CoV-2. On March 13, CDC performed symptom assessments and SARS-CoV-2 testing for 76 (93%) of the 82 facility A residents to evaluate the utility of symptom screening for identification of COVID-19 in SNF residents. Residents were categorized as asymptomatic or symptomatic at the time of testing, based on the absence or presence of fever, cough, shortness of breath, or other symptoms on the day of testing or during the preceding 14 days. Among 23 (30%) residents with positive test results, 10 (43%) had symptoms on the date of testing, and 13 (57%) were asymptomatic. Seven days after testing, 10 of these 13 previously asymptomatic residents had developed symptoms and were recategorized as presymptomatic at the time of testing. The reverse transcription-polymerase chain reaction (RT-PCR) testing cycle threshold (Ct) values indicated large quantities of viral RNA in asymptomatic, presymptomatic, and symptomatic residents, suggesting the potential for transmission regardless of symptoms. Symptom-based screening in SNFs could fail to identify approximately half of residents with COVID-19. Long-term care facilities should take proactive steps to prevent introduction of SARS-CoV-2 (3). Once a confirmed case is identified in an SNF, all residents should be placed on isolation precautions if possible (3), with considerations for extended use or reuse of personal protective equipment (PPE) as needed (4).
Conflict of interest statement
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. No potential conflicts of interest were disclosed.
Figures
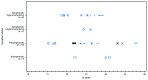
FIGURE
Cycle threshold (Ct) values* for residents of a long-term care skilled nursing facility with positive test results for SARS-CoV-2 by real-time reverse transcription–polymerase chain reaction on March 13, 2020 (n = 23), by symptom status†,§ at time of test — facility A, King County, Washington * Ct values are the number of cycles needed for detection of each genetic marker identified by real-time reverse transcription–polymerase chain reaction testing. A lower Ct value indicates a higher amount of viral RNA. Paired values for each resident are depicted using a different shape. Each resident has two Ct values for the two genetic markers (N1 and N2 nucleocapsid protein gene regions). † Typical symptoms include fever, cough, and shortness of breath. § Atypical symptoms include chills, malaise, sore throat, increased confusion, rhinorrhea or nasal congestion, myalgia, dizziness, headache, nausea, and diarrhea.
Similar articles
- Presymptomatic SARS-CoV-2 Infections and Transmission in a Skilled Nursing Facility.
Arons MM, Hatfield KM, Reddy SC, Kimball A, James A, Jacobs JR, Taylor J, Spicer K, Bardossy AC, Oakley LP, Tanwar S, Dyal JW, Harney J, Chisty Z, Bell JM, Methner M, Paul P, Carlson CM, McLaughlin HP, Thornburg N, Tong S, Tamin A, Tao Y, Uehara A, Harcourt J, Clark S, Brostrom-Smith C, Page LC, Kay M, Lewis J, Montgomery P, Stone ND, Clark TA, Honein MA, Duchin JS, Jernigan JA; Public Health–Seattle and King County and CDC COVID-19 Investigation Team. Arons MM, et al. N Engl J Med. 2020 May 28;382(22):2081-2090. doi: 10.1056/NEJMoa2008457. Epub 2020 Apr 24. N Engl J Med. 2020. PMID: 32329971 Free PMC article. - Universal and Serial Laboratory Testing for SARS-CoV-2 at a Long-Term Care Skilled Nursing Facility for Veterans - Los Angeles, California, 2020.
Dora AV, Winnett A, Jatt LP, Davar K, Watanabe M, Sohn L, Kern HS, Graber CJ, Goetz MB. Dora AV, et al. MMWR Morb Mortal Wkly Rep. 2020 May 29;69(21):651-655. doi: 10.15585/mmwr.mm6921e1. MMWR Morb Mortal Wkly Rep. 2020. PMID: 32463809 Free PMC article. - Serial Testing for SARS-CoV-2 and Virus Whole Genome Sequencing Inform Infection Risk at Two Skilled Nursing Facilities with COVID-19 Outbreaks - Minnesota, April-June 2020.
Taylor J, Carter RJ, Lehnertz N, Kazazian L, Sullivan M, Wang X, Garfin J, Diekman S, Plumb M, Bennet ME, Hale T, Vallabhaneni S, Namugenyi S, Carpenter D, Turner-Harper D, Booth M, Coursey EJ, Martin K, McMahon M, Beaudoin A, Lifson A, Holzbauer S, Reddy SC, Jernigan JA, Lynfield R; Minnesota Long-Term Care COVID-19 Response Group. Taylor J, et al. MMWR Morb Mortal Wkly Rep. 2020 Sep 18;69(37):1288-1295. doi: 10.15585/mmwr.mm6937a3. MMWR Morb Mortal Wkly Rep. 2020. PMID: 32966272 Free PMC article. - Thoracic imaging tests for the diagnosis of COVID-19.
Salameh JP, Leeflang MM, Hooft L, Islam N, McGrath TA, van der Pol CB, Frank RA, Prager R, Hare SS, Dennie C, Spijker R, Deeks JJ, Dinnes J, Jenniskens K, Korevaar DA, Cohen JF, Van den Bruel A, Takwoingi Y, van de Wijgert J, Damen JA, Wang J; Cochrane COVID-19 Diagnostic Test Accuracy Group; McInnes MD. Salameh JP, et al. Cochrane Database Syst Rev. 2020 Sep 30;9:CD013639. doi: 10.1002/14651858.CD013639.pub2. Cochrane Database Syst Rev. 2020. PMID: 32997361 Updated. - Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19): A Review.
Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Wiersinga WJ, et al. JAMA. 2020 Aug 25;324(8):782-793. doi: 10.1001/jama.2020.12839. JAMA. 2020. PMID: 32648899 Review.
Cited by
- Incidence of Nosocomial COVID-19 in Patients Hospitalized at a Large US Academic Medical Center.
Rhee C, Baker M, Vaidya V, Tucker R, Resnick A, Morris CA, Klompas M; CDC Prevention Epicenters Program. Rhee C, et al. JAMA Netw Open. 2020 Sep 1;3(9):e2020498. doi: 10.1001/jamanetworkopen.2020.20498. JAMA Netw Open. 2020. PMID: 32902653 Free PMC article. - Are presymptomatic SARS-CoV-2 infections in nursing home residents unrecognised symptomatic infections? Sequence and metadata from weekly testing in an extensive nursing home outbreak.
van den Besselaar JH, Sikkema RS, Koene FMHPA, van Buul LW, Oude Munnink BB, Frénay I, Witt RT, Koopmans MPG, Hertogh CMPM, Buurman BM. van den Besselaar JH, et al. Age Ageing. 2021 Sep 11;50(5):1454-1463. doi: 10.1093/ageing/afab081. Age Ageing. 2021. PMID: 33963830 Free PMC article. - Screening for SARS-CoV-2 Infection Within a Psychiatric Hospital and Considerations for Limiting Transmission Within Residential Psychiatric Facilities - Wyoming, 2020.
Callaghan AW, Chard AN, Arnold P, Loveland C, Hull N, Saraiya M, Saydah S, Dumont W, Frakes LG, Johnson D, Peltier R, Van Houten C, Trujillo AA, Moore J, Rose DA, Honein MA, Carrington D, Harrist A, Hills SL. Callaghan AW, et al. MMWR Morb Mortal Wkly Rep. 2020 Jul 3;69(26):825-829. doi: 10.15585/mmwr.mm6926a4. MMWR Morb Mortal Wkly Rep. 2020. PMID: 32614815 Free PMC article. - Fatal COVID-19 in a Patient with End-Stage Liver Disease Wait-Listed for Liver Transplantation: An Evidence-Based Review of COVID-19 Screening Modalities Prior to Transplant.
Rhee Y, Chan EL, Eswaran SL, Aloman C, Hertl M, Santos CAQ. Rhee Y, et al. Clin Liver Dis (Hoboken). 2020 Jun 30;15(6):246-250. doi: 10.1002/cld.990. eCollection 2020 Jun. Clin Liver Dis (Hoboken). 2020. PMID: 32617159 Free PMC article. Review. No abstract available. - COVID-19: data-driven dynamics, statistical and distributed delay models, and observations.
Liu X, Zheng X, Balachandran B. Liu X, et al. Nonlinear Dyn. 2020;101(3):1527-1543. doi: 10.1007/s11071-020-05863-5. Epub 2020 Aug 6. Nonlinear Dyn. 2020. PMID: 32836818 Free PMC article.
References
- CDC. Preparing for COVID-19: long-term care facilities, nursing homes. Atlanta, GA: US Department of Health and Human Services, CDC; 2020. https://www.cdc.gov/coronavirus/2019-ncov/healthcare-facilities/prevent-...
- CDC. Strategies for optimizing the supply of PPE. Atlanta, GA: US Department of Health and Human Services, CDC; 2020. https://www.cdc.gov/coronavirus/2019-ncov/hcp/ppe-strategy/index.html
- CDC. Interim guidelines for collecting, handling, and testing clinical specimens from persons for coronavirus disease 2019. (COVID-19). Atlanta, GA: US Department of Health and Human Services, CDC; 2020. https://www.cdc.gov/coronavirus/2019-nCoV/lab/guidelines-clinical-specim...
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous