Prolonged tau clearance and stress vulnerability rescue by pharmacological activation of autophagy in tauopathy neurons - PubMed (original) (raw)
Prolonged tau clearance and stress vulnerability rescue by pharmacological activation of autophagy in tauopathy neurons
M Catarina Silva et al. Nat Commun. 2020.
Abstract
Tauopathies are neurodegenerative diseases associated with accumulation of abnormal tau protein in the brain. Patient iPSC-derived neuronal cell models replicate disease-relevant phenotypes ex vivo that can be pharmacologically targeted for drug discovery. Here, we explored autophagy as a mechanism to reduce tau burden in human neurons and, from a small-molecule screen, identify the mTOR inhibitors OSI-027, AZD2014 and AZD8055. These compounds are more potent than rapamycin, and robustly downregulate phosphorylated and insoluble tau, consequently reducing tau-mediated neuronal stress vulnerability. MTORC1 inhibition and autophagy activity are directly linked to tau clearance. Notably, single-dose treatment followed by washout leads to a prolonged reduction of tau levels and toxicity for 12 days, which is mirrored by a sustained effect on mTORC1 inhibition and autophagy. This new insight into the pharmacodynamics of mTOR inhibitors in regulation of neuronal autophagy may contribute to development of therapies for tauopathies.
Conflict of interest statement
At the time of this study, S.T., I.K.G., T.J., D.G.B. and N.J.B. were fulltime employees by and shareholders in AstraZeneca. S.J.H. is a member of the SAB and equity holder in Rodin Therapeutics, Psy Therapeutics, Frequency Therapeutics, and Souvien Therapeutics, none of whom were involved in this study. S.J.H. has received consulting or speaking fees from Sunovion, Biogen, AstraZeneca, Amgen, and Merck. B.C.D. is a consultant with Arkuda, Axovant, Biogen, Lilly, Merck, Novartis and Wave Life Sciences; and has editorial duties with honoraria with Elsevier. B.C.D. also received royalties from Oxford University Press and Cambridge University Press. None of these entities were involved in this study.
Figures
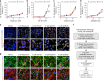
Fig. 1. Microscopy screen for compounds that activate autophagy in human Control-1 NPCs and neurons.
a–d Compound dose-effect on number of LysoTracker+ and CYTO-ID+ fluorescent vesicles in NPCs (8330-8-RC1), after 24 h treatment with rapamycin, OSI-027, AZD2014, and AZD8055 (+500 nM CQ). Data points correspond to mean number of fluorescent vesicles per nuclei ± SEM from n = 5 biological replicates. e NPCs (8330-8-RC1) treated with compounds for 24 h at a dose that promoted maximum vesicle formation without toxicity. Scale bars are 50 μm. Representative images from n = 5 biological replicates. Inserts correspond to zoomed-in images below (scale bars are 25μm). f Screening strategy followed. g Representative images of 5-week differentiated neurons (8330-8-RC1) treated with compounds for 24 h (+500 nM CQ). LysoTracker+ (top) and CYTO-ID+ (bottom) vesicles are shown in the background of MAP2 neuronal staining (n = 3 biological replicates). Scale bars are 25 μm. Source data are provided as a Source Data file.
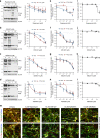
Fig. 2. Compound dose-dependent reduction of tau in a neuronal cell model of tauopathy.
Tau-A152T (FTD19-L5-RC6) 6-week differentiated neurons were treated for 24 h, and tau protein levels and neuronal viability were measured. a–d Western blot of total tau (TAU5) and P-tauS396, with representative blots shown on the left and mean densitometry shown on the right (b–d samples were run on the same gel, with the same vehicle sample, and image was cropped at the dotted line only for the purpose of this figure). Bands used for quantification are within brackets. Data points represent mean densitometry relative to vehicle-treated samples (a.u., arbitrary units) ± SEM of n = 4 biological replicates (n = 6 for vehicle and highest dose). e–h Tau ELISA dose-response curves. Data points represent mean tau per μg of total protein, relative to vehicle-treated samples ± SEM. Results represent n = 4 with technical replicates per ELISA plate. Statistical significance (a–h) was calculated using two-way ANOVA with post hoc Dunnett’s multiple comparisons test relative to vehicle, with ns_P_ > 0.05, *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, ****P ≤ 0.0001. i–l Dose-response curves for neuronal viability. Data points represent mean % viability relative to vehicle ± SEM, for n = 3 biological replicates. m IF of A152T neurons with total tau (K9JA) and P-tauS396/S404 (PHF-1) antibodies in red, and neuronal marker MAP2 in green. Scale bars are 50μm. Representative images of n = 3. Source data are provided as a Source Data file.
Fig. 3. Rescue of tau phenotypes in 8-week differentiated A152T (FTD19-L5-RC6) neurons upon 24 h treatment.
a Summary of the assay for protein fractionation based on Triton-X (T) and SDS (S) detergents differential solubility. b–d Western blot analysis of tau after mTORi compound treatment and detergent fractionation. b Samples were run on the same blot, image cropped (dotted line) only for the purpose of this figure. Brackets indicate protein bands for densitometry analysis. Graph bars represent mean densitometry ± SEM, and black diamond-dots indicate individual data points for soluble (c) and insoluble (d) total tau (TAU5) and P-tauS396 levels relative to vehicle samples. n = 4 biological replicates. Statistical significance relative to vehicle was calculated using two-tailed unpaired _t_-test and P values are indicated in each graph. e–l Rescue of neuronal vulnerability to 30 μM Aβ(1-42) (red) and 400 μM NMDA (blue), by pre-treatment with rapamycin (e, i), OSI-027 (f, j), AZD2014 (g, k) or AZD8055 (h, l). Assay summary in Supplementary Fig. 4f. Bars represent mean % viability relative to vehicle-treated neurons ± SEM, and black diamond-dots represent individual data points for n = 3 biological replicates with 2 technical replicates per experiment. Statistical significance was calculated with a two-tailed unpaired t_-test and P values are indicated (ns_P > 0.05, *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, ****P ≤ 0.0001). Source data are provided as a Source Data file.
Fig. 4. Compound activation of autophagy as shown by upregulation of pathway-specific markers and pathway-dependence analysis.
a Simplified schematic of the proposed mechanism for mTORi-mediated tau clearance through autophagy. mTORi are shown in black, and autophagy inhibitors in red. b Western blot of autophagy-specific markers (in blue, a) in A152T neurons (6-week differentiated, FTD19-L5-RC6) upon 24 h treatment (samples were run on the same gel, image was cropped on the dotted line only for the purpose of this figure). Blot is representative of n = 4 biological replicates. c–h Graph bars represent mean densitometry relative to vehicle ± SEM, and black diamond-dots indicate individual data points for n = 4 biological replicates (n = 5 for LC3-II). i Assay to test mTORi effect pathway-dependence: A152T (FTD19-L5-RC6) neurons were treated for 6 h with autophagy inhibitors (SAR405, 3-MA, BAF.A1) followed by mTORi (10 μM OSI-027, 10 μM AZD2014) for a total of 24 h. j, k Western blot densitometry of total tau (TAU5) and P-tauS396 levels. Graph bars represent mean tau densitometry ± SEM and black diamond-dots indicate individual data points for n = 3 biological replicates. Corresponding representative blots are included in Supplementary Fig. 5b, c. Source data are provided as a Source Data file.
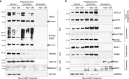
Fig. 5. mTORi promoted tau sequestration into phagolysosomes.
Tau-A152T neurons (6-week differentiated FTD19-L5-RC6) were treated with vehicle (–), 10 μM AZD2014 (AZD) or 10 μM OSI-027 (OSI) for 8 h. Cell lysates were fractionated by density gradient and western blot analysis shows a total tau (TAU5) and P-tauS396 levels in cytosol, lysosomes/phagolysosomes and exosomes fractions; and b fraction-specific markers. Key: Nuc, nuclear negative control; Cyt, cytosol; Mit, mitochondrial. Blots shown are representative of n = 2 biological replicates.
Fig. 6. Demonstration of mTORC1 as the primary target of mTORi in human ex vivo neurons.
a Simplified schematic of the targeted pathways by mTORi (in black), through mTORC1 and autophagy clearance of tau. Red-hexagons indicate phospho-substrates measured. Dotted lines indicate predicted or indirect interactions. b–d Compound dose-response curves (0.1 nM–50 μM) for mTORC1 activity in Control-1 neurons (6-week differentiated 8330-8-RC1, 24 h treatment), measured by western blot of substrates phosphorylation (representative blots in Supplementary Fig. 7a). Data points represent mean densitometry of each phospho-marker normalized to total levels, for n = 2 biological replicates. e–i Western blot of A152T neurons (6-week differentiated FTD19-L5-RC6) treated for 24 h and immunoprobed for mTORC1 substrates and BECN1 levels and phosphorylation. Representative blot (e) of n = 2 biological replicates and 2 technical replicates. Graph bars (f–i) represent mean densitometry of phospho-markers normalized to total levels of each protein, relative to vehicle ± SD. Black circles represent individual data points for n = 2 biological replicates and 2 technical replicates. Statistical significance was calculated with a two-tailed unpaired t_-test (**P_ ≤ 0.01, ****P ≤ 0.0001). j, k A152T neurons (FTD19-L5-RC6) were pre treated with mTOR activator MHY-1485 (a) for 6 h, followed by rapamycin, OSI-027 or AZD2014 for a total of 24 h. Effect on total tau (TAU5) and P-tauS396 was measured by western blot (representative images of n = 3), relative to the effect of each mTORi alone (bars outlined in blue, k). Graph bars represent mean tau densitometry ± SEM and diamond-dots represent individual data points for n = 3 biological replicates. Samples (e, j) were run on the same gel and images were cropped at the dotted lines only for the purpose of this figure. Source data are provided as a Source Data file.
Fig. 7. Single-dose mTORi has sustained effect on tau phenotypes.
a Assay to measure effect of mTORi 24h-treatment on tau levels and neuronal viability over a time-course of 20 days. b–i A152T neurons (6-week differentiated FTD19-L5-RC6) were treated with rapamycin, OSI-027 or AZD2014 for 24 h, followed by compound washout (red arrow). b–f Total tau (TAU5), P-tauS396 and the neuronal microtubule marker β-III-tubulin were measured by western blot over a period of 20 days post treatment. Representative blots are shown, and graph data points represent mean protein densitometry (bands within brackets) ± SEM for n = 3 biological replicates. Dotted lines correspond to vehicle-treated protein levels. g–i Time-course analysis of effect on neuronal viability. Data points represent average % viability relative to vehicle ± SEM for n = 3 biological replicates. Source data are provided as a Source Data file.
Fig. 8. Time-course analysis of mTORi effect on neuronal vulnerability to stress, autophagy and mTORC1 activity.
a Assay to measure mTORi time-course effect on neuronal vulnerability to stress. A152T neurons (FTD19-L5-RC6, 6 weeks differentiated) were treated with 10 μM AZD2014 or OSI-027 for 24 h (day 0–1), followed by media change. Then, on each day indicated, neurons were stressed with either 400 μM NMDA or 30 μM Aβ(1-42) for 18 h, and viability was measured. b Graph bars represent mean % viability, relative to vehicle ± SEM and diamond dots represent individual data points for n = 3 (and 4 technical replicates per experiment). Statistical significance was calculated with a two-tailed unpaired _t_-test (*P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, ****P ≤ 0.0001). c–q Neurons were treated with 3 μM rapamycin (c–g), 10 μM OSI027 (h–l) or 10 μM AZD2014 (m–q) and analyzed by western blot over a period of 20 days (Supplementary Fig. 9a, b). c–f, h–k, m–p Time-course densitometry of LC3-II, ATG12, LAMP1 and p62 autophagy protein levels. Data points represent mean densitometry, relative to vehicle-treated samples (dotted lines) ± SEM for n = 3 biological replicates. g, l, q Time-course measure of mTORC1 substrates’ phosphorylation (p70S6K, S6, 4E-BP1). Data points represent mean densitometry, relative to vehicle-treated samples (dotted lines) for n = 2 biological replicates. Source data are provided as a Source Data file.
Similar articles
- Dual mTORC1/mTORC2 blocker as a possible therapy for tauopathy in cellular model.
Salama M, Elhussiny M, Magdy A, Omran AG, Alsayed A, Ashry R, Mohamed W. Salama M, et al. Metab Brain Dis. 2018 Apr;33(2):583-587. doi: 10.1007/s11011-017-0137-7. Epub 2017 Oct 27. Metab Brain Dis. 2018. PMID: 29080085 - mTOR regulates tau phosphorylation and degradation: implications for Alzheimer's disease and other tauopathies.
Caccamo A, Magrì A, Medina DX, Wisely EV, López-Aranda MF, Silva AJ, Oddo S. Caccamo A, et al. Aging Cell. 2013 Jun;12(3):370-80. doi: 10.1111/acel.12057. Epub 2013 Mar 24. Aging Cell. 2013. PMID: 23425014 Free PMC article. - Methylthioninium chloride (methylene blue) induces autophagy and attenuates tauopathy in vitro and in vivo.
Congdon EE, Wu JW, Myeku N, Figueroa YH, Herman M, Marinec PS, Gestwicki JE, Dickey CA, Yu WH, Duff KE. Congdon EE, et al. Autophagy. 2012 Apr;8(4):609-22. doi: 10.4161/auto.19048. Epub 2012 Apr 1. Autophagy. 2012. PMID: 22361619 Free PMC article. - Infantile tauopathies: Hemimegalencephaly; tuberous sclerosis complex; focal cortical dysplasia 2; ganglioglioma.
Sarnat HB, Flores-Sarnat L. Sarnat HB, et al. Brain Dev. 2015 Jun;37(6):553-62. doi: 10.1016/j.braindev.2014.08.010. Epub 2014 Oct 19. Brain Dev. 2015. PMID: 25451314 Review. - Autophagy and Tau Protein.
Hamano T, Enomoto S, Shirafuji N, Ikawa M, Yamamura O, Yen SH, Nakamoto Y. Hamano T, et al. Int J Mol Sci. 2021 Jul 12;22(14):7475. doi: 10.3390/ijms22147475. Int J Mol Sci. 2021. PMID: 34299093 Free PMC article. Review.
Cited by
- Conserved gene signatures shared among MAPT mutations reveal defects in calcium signaling.
Minaya MA, Mahali S, Iyer AK, Eteleeb AM, Martinez R, Huang G, Budde J, Temple S, Nana AL, Seeley WW, Spina S, Grinberg LT, Harari O, Karch CM. Minaya MA, et al. Front Mol Biosci. 2023 Feb 9;10:1051494. doi: 10.3389/fmolb.2023.1051494. eCollection 2023. Front Mol Biosci. 2023. PMID: 36845551 Free PMC article. - BH3-only proteins Puma and Beclin1 regulate autophagic death in neurons in response to Amyloid-β.
Saha A, Saleem S, Paidi RK, Biswas SC. Saha A, et al. Cell Death Discov. 2021 Nov 15;7(1):356. doi: 10.1038/s41420-021-00748-x. Cell Death Discov. 2021. PMID: 34782612 Free PMC article. - Bi-Directional Relationship Between Autophagy and Inflammasomes in Neurodegenerative Disorders.
Panda C, Mahapatra RK. Panda C, et al. Cell Mol Neurobiol. 2023 Jan;43(1):115-137. doi: 10.1007/s10571-021-01184-2. Epub 2022 Jan 23. Cell Mol Neurobiol. 2023. PMID: 35066716 Free PMC article. Review. - Compromised autophagy and mitophagy in brain ageing and Alzheimer's diseases.
Caponio D, Veverová K, Zhang SQ, Shi L, Wong G, Vyhnalek M, Fang EF. Caponio D, et al. Aging Brain. 2022 Nov 24;2:100056. doi: 10.1016/j.nbas.2022.100056. eCollection 2022. Aging Brain. 2022. PMID: 36908880 Free PMC article. Review. - Autophagy in health and disease: From molecular mechanisms to therapeutic target.
Lu G, Wang Y, Shi Y, Zhang Z, Huang C, He W, Wang C, Shen HM. Lu G, et al. MedComm (2020). 2022 Jul 10;3(3):e150. doi: 10.1002/mco2.150. eCollection 2022 Sep. MedComm (2020). 2022. PMID: 35845350 Free PMC article. Review.
References
- Goedert M. Tau protein and neurodegeneration. Semin Cell Dev. Biol. 2004;15:45–49. - PubMed
- Gotz J, Halliday G, Nisbet RM. Molecular pathogenesis of the tauopathies. Annu. Rev. Pathol. 2019;14:239–261. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous