Interleukin 6 regulates the expression of programmed cell death ligand 1 in thyroid cancer - PubMed (original) (raw)
. 2021 Mar;112(3):997-1010.
doi: 10.1111/cas.14752. Epub 2021 Feb 24.
Affiliations
- PMID: 33247999
- PMCID: PMC7935800
- DOI: 10.1111/cas.14752
Interleukin 6 regulates the expression of programmed cell death ligand 1 in thyroid cancer
Guo-Qiang Zhang et al. Cancer Sci. 2021 Mar.
Erratum in
- Correction.
[No authors listed] [No authors listed] Cancer Sci. 2023 Apr;114(4):1773-1775. doi: 10.1111/cas.15675. Epub 2022 Dec 9. Cancer Sci. 2023. PMID: 36484180 Free PMC article. No abstract available.
Abstract
Programmed cell death ligand 1 (PD-L1), inducing T cell exhaustion to facilitate immune escape of tumor cells, is upregulated by interleukin 6 (IL-6) in T cell lymphoma and ovarian cancer. The purpose of this study is to investigate the expression of IL-6 and PD-L1 in thyroid cancer, and whether IL-6 regulates PD-L1 expression. As a result, IL-6 and PD-L1 were highly expressed in thyroid cancer tissues. Multivariate logistic analysis showed that tumor size, distant metastasis, and risk stratification were significantly associated with IL-6 expression (P < .05), and multifocality, lymph node metastasis, distant metastasis, risk stratification, and IL-6 expression were identified as the independent predictors of PD-L1 expression (P < .05). The invasiveness of thyroid cancer was significantly enhanced after IL-6 treatment or PD-L1 overexpression. PD-L1 positive rate correlated with IL-6 expression in cancer tissues (P < .001), and after IL-6 treatment, the PD-L1 expression in TPC-1 and BCPAP significantly increased. The mitogen-activated protein kinase pathway (MAPK) and the Janus-activated kinase (JAK)-signal transducers and activators of transcription 3 (STAT3) signaling pathways were activated by IL-6, and the IL-6-induced PD-L1 expression decreased after treatment with these two signaling pathway inhibitors. Knockdown of transcription factors c-Jun and stat3 suppressed the expression of PD-L1 induced by IL-6, and these two factors could bind to PD-L1 gene promoter directly and promote its transcription. It is concluded that IL-6 and PD-L1 are overexpressed in thyroid cancer and are related to tumor invasiveness. IL-6 upregulates PD-L1 expression through the MAPK and JAK-STAT3 signaling pathways, which function via transcription factors c-Jun and stat3.
Keywords: IL-6; PD-L1; mechanism; regulation; thyroid cancer.
© 2020 The Authors. Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Conflict of interest statement
The authors declare no competing interests.
Figures
FIGURE 1
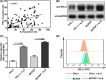
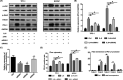
Overexpression of interleukin 6 (IL‐6) and programmed cell death ligand 1 (PD‐L1) in thyroid cancer. A, Immunohistochemistry (IHC) staining results of IL‐6 and PD‐L1 in thyroid cancer and normal control. B, IHC score results showed that IL‐6 and PD‐L1 were overexpressed in thyroid cancer. PA, para‐cancer tissues; CA, cancer tissues
FIGURE 2
Transwell invasion assay showed that interleukin 6 (IL‐6) and programmed cell death ligand 1 (PD‐L1) overexpression enhance the invasiveness of thyroid cancer
FIGURE 3
Induction of interleukin 6 (IL‐6) on programmed cell death ligand 1 (PD‐L1) expression in thyroid cancer. A, The PD‐L1 positive rate positively correlated with IL‐6 expression in thyroid cancer. B and C, Western blotting results showed that the total PD‐L1 protein in TPC‐1 and BCPAP increased significantly after IL‐6 treatment. D, Flow cytometry results showed that IL‐6 upregulated the PD‐L1 membrane expression significantly
FIGURE 4
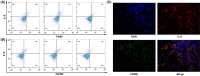
M2‐phenotype macrophages produce interleukin 6 (IL‐6) in thyroid cancer. A, Flow cytometry showed that IL‐6 was distributed in F4/80 staining–positive tumor‐associated macrophages (TAMs). B, Flow cytometry showed that IL‐6 was distributed in CD206‐positive M2‐phenotype macrophages. C, Double immunofluorescence analysis illustrated the colocalization of IL‐6 and M2 phenotype within thyroid cancer
FIGURE 5
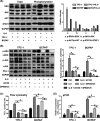
Interleukin 6 (IL‐6) upregulated programmed cell death ligand 1 (PD‐L1) expression through the mitogen‐activated protein kinase (MAPK) pathway. A, In TPC‐1 and BAPCP, after IL‐6 treatment, the phosphorylation levels of extracellular signal–regulated kinase (ERK), C‐Jun NH(2)–terminal kinase (JNK), and signal transducers and activators of transcription 3 (STAT3) significantly increased, while those of protein serine‐threonine kinase (AKT) did not. B, After treatment with U0126 (ERK inhibitor) or SP600125 (JNK inhibitor), the IL‐6–induced total PD‐L1 protein reduced, and combination of them had a synergistic effect on PD‐L1 expression. C, Flow cytometry result showed that the membrane PD‐L1 expression increased after IL‐6 treatment but decreased after subsequent treatment with U0126 or SP600125. D, The level of PD‐L1 mRNA in the IL‐6 group was higher than that in the control group. It was inhibited after treatment with U0126 or SP600125 which worked synergistically. #Indicates P < .05. *Indicates P ≥ .05
FIGURE 6
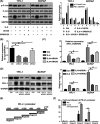
Interleukin 6 (IL‐6) promotes programmed cell death ligand 1 (PD‐L1) gene transcription through the mitogen‐activated protein kinase (MAPK) pathway, which functions via transcription factor c‐Jun. A, The level of p‐c‐Jun increased with the activation of signaling pathways induced by IL‐6 but decreased with the subsequent inhibitors U0126 or SP600125. The expression trend of p‐c‐Jun after different treatments was similar to that of PD‐L1. B, Three small interfering RNAs (siRNAs) were selected to knock down c‐Jun expression. qPCR results showed that the expression of c‐Jun was significantly reduced by siRNA1 and siRNA2 but not by siRNA3. C, qPCR results showed that, compared with IL‐6 treatment alone, the PD‐L1 mRNA level significantly decreased with the combination treatment of IL‐6 and siRNA. D, Western blot results showed that the IL‐6–induced total PD‐L1 protein decreased after treatment with c‐Jun siRNA1 or siRNA2. E, The PD‐L1 gene promoter. F, C‐Jun binding sites were mainly concentrated in region 3, and fold enrichment increased significantly after c‐Jun overexpression. #indicates P < .05. *indicates P ≥ .05
FIGURE 7
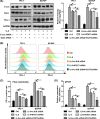
Interleukin 6 (IL‐6) promotes programmed cell death ligand 1 (PD‐L1) gene transcription through the Janus‐activated kinase (JAK)–signal transducers and activators of transcription 3 (STAT3) pathway, which functions via transcription factor stat3. A, The level of p‐stat3 increased after IL‐6 treatment but decreased after subsequent treatment with JAK inhibitor Ruxolitinib or STAT3 small interfering RNA (siRNA). The expression trend of p‐stat3 after different treatments was similar to that of PD‐L1. B, The relative expression of PD‐L1 in different groups. C, Three siRNAs were selected to knock down stat3 expression. Stat3 siRNA1 and siRNA2 significantly reduced the expression of stat3, while siRNA3 did not. D, Flow cytometry result showed that the membrane PD‐L1 expression increased after IL‐6 treatment, but decreased after subsequent treatment with Ruxolitinib or stat3 siRNA. E, Stat3 binding sites were mainly concentrated in promoter regions 2‐4, and fold enrichment increased significantly after stat3 overexpression. #indicates P < .05. *indicates P ≥ .05
FIGURE 8
Synergistic effect of the mitogen‐activated protein kinase (MAPK) and Janus‐activated kinase (JAK)–signal transducers and activators of transcription 3 (STAT3) signaling pathways on interleukin 6 (IL‐6)‐induced programmed cell death ligand 1 (PD‐L1) expression. A, IL‐6–induced PD‐L1 expression decreased after treatment with c‐Jun small interfering RNA (siRNA) or stat3 siRNA, and its expression further decreased after treatment with the combination of c‐Jun siRNA and stat3 siRNA. B and C, The constitutive membrane PD‐L1 expression was 24% and 43% on TPC‐1 and BCPAP, and increased to 58% and 95% after IL‐6 treatment. After treatment with c‐Jun siRNA or stat3 siRNA, the induced PD‐L1 expression decreased to 40% or 37% on TPC‐1 and to 63% or 61% on BCPAP. After combining siRNAs target c‐Jun and stat3, the membrane positive rate further decreased to 31% and 42% on TPC‐1 and BCPAP. D, The level of PD‐L1 mRNA increases after IL‐6 treatment, but decreases after subsequent treatment with c‐Jun siRNA or stat3 siRNA. Combination of these two siRNAs has a synergistic effect on the inhibition of IL‐6–induced PD‐L1 mRNA. #indicates P < .05
FIGURE 9
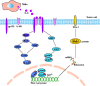
The mechanism by which interleukin 6 (IL‐6) , secreted by tumor‐associated macrophages (TAMs), regulates the expression of programmed cell death ligand 1 (PD‐L1) through the mitogen‐activated protein kinase (MAPK) and Janus‐activated kinase (JAK)–signal transducers and activators of transcription 3 (STAT3) signaling pathway. ERK, extracellular signal–regulated kinase; JNK, C‐Jun NH(2)–terminal kinase
Similar articles
- CKS1B promotes cell proliferation and invasion by activating STAT3/PD-L1 and phosphorylation of Akt signaling in papillary thyroid carcinoma.
Wang H, Zhang Z, Yan Z, Ma S. Wang H, et al. J Clin Lab Anal. 2021 Jan;35(1):e23565. doi: 10.1002/jcla.23565. Epub 2020 Sep 22. J Clin Lab Anal. 2021. PMID: 32960462 Free PMC article. - Cell-intrinsic PD-L1 signaling drives immunosuppression by myeloid-derived suppressor cells through IL-6/Jak/Stat3 in PD-L1-high lung cancer.
Jeong H, Koh J, Kim S, Yim J, Song SG, Kim H, Li Y, Lee SH, Chung YK, Kim H, Lee CH, Kim HY, Keam B, Lee SH, Chung DH, Jeon YK. Jeong H, et al. J Immunother Cancer. 2025 Mar 6;13(3):e010612. doi: 10.1136/jitc-2024-010612. J Immunother Cancer. 2025. PMID: 40050048 Free PMC article. - PD-L1 and thyroid cytology: A possible diagnostic and prognostic marker.
Dell'Aquila M, Granitto A, Martini M, Capodimonti S, Cocomazzi A, Musarra T, Fiorentino V, Pontecorvi A, Lombardi CP, Fadda G, Pantanowitz L, Larocca LM, Rossi ED. Dell'Aquila M, et al. Cancer Cytopathol. 2020 Mar;128(3):177-189. doi: 10.1002/cncy.22224. Epub 2019 Dec 10. Cancer Cytopathol. 2020. PMID: 31821747 - Regulation of PD-L1: a novel role of pro-survival signalling in cancer.
Chen J, Jiang CC, Jin L, Zhang XD. Chen J, et al. Ann Oncol. 2016 Mar;27(3):409-16. doi: 10.1093/annonc/mdv615. Epub 2015 Dec 17. Ann Oncol. 2016. PMID: 26681673 Review. - Programmed Death-Ligand 1 (PD-L1) Is a Potential Biomarker of Disease-Free Survival in Papillary Thyroid Carcinoma: a Systematic Review and Meta-Analysis of PD-L1 Immunoexpression in Follicular Epithelial Derived Thyroid Carcinoma.
Girolami I, Pantanowitz L, Mete O, Brunelli M, Marletta S, Colato C, Trimboli P, Crescenzi A, Bongiovanni M, Barbareschi M, Eccher A. Girolami I, et al. Endocr Pathol. 2020 Sep;31(3):291-300. doi: 10.1007/s12022-020-09630-5. Endocr Pathol. 2020. PMID: 32468210
Cited by
- Dual Inhibition of BRAF-MAPK and STAT3 Signaling Pathways in Resveratrol-Suppressed Anaplastic Thyroid Cancer Cells with BRAF Mutations.
Lu MD, Li H, Nie JH, Li S, Ye HS, Li TT, Wu ML, Liu J. Lu MD, et al. Int J Mol Sci. 2022 Nov 19;23(22):14385. doi: 10.3390/ijms232214385. Int J Mol Sci. 2022. PMID: 36430869 Free PMC article. - High aggressiveness of papillary thyroid cancer: from clinical evidence to regulatory cellular networks.
Zhang J, Xu S. Zhang J, et al. Cell Death Discov. 2024 Aug 26;10(1):378. doi: 10.1038/s41420-024-02157-2. Cell Death Discov. 2024. PMID: 39187514 Free PMC article. Review. - Harnessing Immunity to Treat Advanced Thyroid Cancer.
Komatsuda H, Kono M, Wakisaka R, Sato R, Inoue T, Kumai T, Takahara M. Komatsuda H, et al. Vaccines (Basel). 2023 Dec 30;12(1):45. doi: 10.3390/vaccines12010045. Vaccines (Basel). 2023. PMID: 38250858 Free PMC article. Review. - Association Between Gestational Diabetes Mellitus and Risk of Overall and Site-Specific Cancers (Pancreatic, Liver, Thyroid, Lung): A Systematic Review and Meta-Analysis.
Tian L, Wen Y, Liu C, Li T, Fan J. Tian L, et al. Life (Basel). 2025 May 19;15(5):808. doi: 10.3390/life15050808. Life (Basel). 2025. PMID: 40430234 Free PMC article. Review. - Metformin Downregulates PD-L1 Expression in Esophageal Squamous Cell Catrcinoma by Inhibiting IL-6 Signaling Pathway.
Lu Y, Xin D, Guan L, Xu M, Yang Y, Chen Y, Yang Y, Wang-Gillam A, Wang L, Zong S, Wang F. Lu Y, et al. Front Oncol. 2021 Nov 22;11:762523. doi: 10.3389/fonc.2021.762523. eCollection 2021. Front Oncol. 2021. PMID: 34881181 Free PMC article.
References
- Wiltshire JJ, Drake TM, Uttley L, Balasubramanian SP. Systematic review of trends in the incidence rates of thyroid cancer. Thyroid. 2016;26(11):1541‐1552. - PubMed
- de Visser KE, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development. Nat Rev Cancer. 2006;6(1):24‐37. - PubMed
- Yao M, Brummer G, Acevedo D, Cheng N. Cytokine regulation of metastasis and tumorigenicity. Adv Cancer Res. 2016;132:265‐367. - PubMed
- Akdis M, Burgler S, Crameri R, et al. Interleukins, from 1 to 37, and interferon‐γ: receptors, functions, and roles in diseases. J Allergy Clin Immunol. 2011;127(3):701‐721.e1‐70. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous