IL‑6 plays a crucial role in epithelial‑mesenchymal transition and pro‑metastasis induced by sorafenib in liver cancer - PubMed (original) (raw)
IL‑6 plays a crucial role in epithelial‑mesenchymal transition and pro‑metastasis induced by sorafenib in liver cancer
Ke-Wei Zhang et al. Oncol Rep. 2021 Mar.
Abstract
Interleukin‑6 (IL‑6) is involved in various biological responses, including tumor progression, metastasis and chemoresistance. However, the role and molecular mechanism of IL‑6 in the treatment of sorafenib in liver cancer remain unclear. In the present study, through western blot analysis, Transwell assay, flow cytometric assay, ELISA analysis and immunohistochemistry it was revealed that sorafenib promoted metastasis and induced epithelial‑mesenchymal transition (EMT) in liver cancer cells in vitro and in vivo, and significantly increased IL‑6 expression. Endogenous or exogenous IL‑6 affected metastasis and EMT progression in liver cancer cells through Janus kinase 2/signal transducer and activator of transcription 3 (STAT3) signaling. Knocked out IL‑6 markedly attenuated the pro‑metastasis effect of sorafenib and increased the susceptibility of liver cancer cells to it. In conclusion, the present results indicated that IL‑6/STAT3 signaling may be a novel therapeutic strategy for liver cancer.
Keywords: liver cancer; interleukin-6; sorafenib; drug resistance; epithelial-mesenchymal transition.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Figure 1.
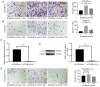
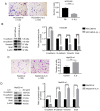
Sorafenib increases the metastatic potential of liver cancer cells and IL-6 knockout attenuates the pro-invasive effect induced by the treatment of sorafenib. (A and B) Transwell assays revealed that sorafenib increased the metastatic potential of HCCLM3-wt and HepG2-wt cells (both ***P<0.001). (C and D) IL-6 expression was disrupted by TALEN. Stably transfected clones were validated through RT-qPCR and western blot analysis (both ****P<0.0001). (E) Transwell assays revealed that IL-6 knockout attenuated the pro-invasive effect induced by sorafenib in HCCLM3-IL-6(−) (P>0.05). HCC, hepatocellular carcinoma; IL-6, interleukin-6; wt, wild-type; TALEN, transcription activator-like effector nucleases; RT-qPCR, reverse transcription quantitative polymerase chain reaction.
Figure 2.
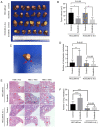
Sorafenib decreases the tumor volume and increases the intrahepatic metastatic potential and lung metastatic potential of liver cancer cells in vivo. IL-6 knockout attenuated the pro-invasive effect induced by the treatment of sorafenib in vivo. (A and B) Sorafenib decreased the tumor volume in the HCCLM3-wt and HCCLM3-IL-6(−) groups (both *P<0.05). (C and D) Sorafenib increased the number of IHMs in the HCCLM3-wt sorafenib group compared with the HCCLM3-wt control, while sorafenib did not increase the number of IHMs in the HCCLM3-IL-6(−) sorafenib group compared with the HCCLM3-IL-6(−) control. (**P<0.01 and P>0.05, respectively). The number of IHMs in the HCCLM3-wt control was not higher than that in the HCCLM3-IL-6(−) control (P>0.05). (E and F) Sorafenib increased the number of lung metastases in the HCCLM3-wt sorafenib group compared with the HCCLM3-wt control, while sorafenib did not increase the number of lung metastases in the HCCLM3-IL-6(−) sorafenib group compared with the HCCLM3-IL-6(−) control. (****P<0.0001 and P>0.05, respectively). The number of lung metastases in the HCCLM3-wt control was higher than that in the HCCLM3-IL-6(−) control (****P<0.0001). IL-6, interleukin-6; IHMs, intrahepatic metastases; HCC, hepatocellular carcinoma; wt, wild-type.
Figure 3.
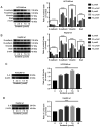
Sorafenib induces EMT and upregulates IL-6 in HCCLM3-wt and HepG2-wt cells, as revealed by western blot analysis. (A and B) E-cadherin was downregulated, and N-cadherin, vimentin and Snail were upregulated by sorafenib in HCCLM3-wt and HepG2-wt cells (all ****P<0.0001). (C and D) Sorafenib upregulated IL-6 in HCCLM3-wt and HepG2-wt cells (both ****P<0.0001). EMT, epithelial-mesenchymal transition; IL-6, interleukin-6; HCC, hepatocellular carcinoma; wt, wild-type.
Figure 4.
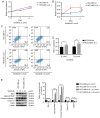
IL-6 knockout inhibits tumor cell growth, as revealed by CCK-8 assay, flow cytometric analysis and western blot analysis. (A) CCK-8 assay for cell proliferation of HCCLM3-wt and HCCLM3-IL-6(−) cells. IL-6 knockout inhibited liver cancer cell proliferation, as revealed by CCK-8 assay (***P<0.001). (B) Flow cytometric cycle assay of HCCLM3-wt and HCCLM3-IL-6(−) cells revealed that the knockout of IL-6 increased the proportion of cells at the G1 phase and decreased that of cells in the S phase (both *P<0.05). (C) Flow cytometric apoptosis assay of HCCLM3-wt and HCCLM3-IL-6(−) cells revealed that the knockout of IL-6 increased the cell apoptosis ratio as indicated by western blot analysis (*P<0.05). (D) Western blot analysis revealed that anti-apoptotic marker (Bcl-2) and cell cycle markers (cyclin D1 and CDK2) were downregulated in HCCLM3-IL-6(−) cells, as compared with HCCLM3-wt cells, whereas pro-apoptotic markers cleaved caspase-3 and cleaved PARP were upregulated in HCCLM3-IL-6(−) cells (all ****P<0.0001). CCK-8, Cell Counting Kit-8; wt, wild-type; IL-6, interleukin-6; HCC, hepatocellular carcinoma; Bcl-2, B-cell lymphoma-2.
Figure 5.
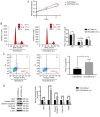
The knockout of IL-6 decreases the metastatic ability of HCCLM3 cells, and exogenous IL-6 increases the metastasis ability of HepG2 cells, as revealed by Transwell assay and western blot analysis. (A) The knockout of IL-6 decreased the metastatic potential of HCCLM3-wt cells, as revealed by Transwell assay (***P<0.001). (B) IL-6 knockout upregulated E-cadherin, and downregulated N-cadherin, vimentin and Snail in HCCLM3-IL-6(−) cells, as compared with HCCLM3-wt cells (all ****P<0.0001). (C) Exogenous IL-6 increased the metastatic ability of HepG2-wt cells as revealed by Transwell assay (**P<0.01). (D) Exogenous IL-6 downregulated E-cadherin, and upregulated N-cadherin, vimentin and Snail in HepG2-wt cells (all ****P<0.0001). IL-6, interleukin-6; HCC, hepatocellular carcinoma; wt, wild-type.
Figure 6.
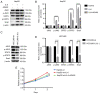
IL-6 knockout increases the susceptivity of HCCLM3 cells to sorafenib, as revealed by CCK-8 assay, flow cytometric analysis and western blot analysis. (A and B) CCK-8 assays for cell proliferation of HCCLM3-wt and HCCLM3-IL-6(−) cells revealed that the knockout of IL-6 increased the growth inhibition effect induced by 5 and 10 µmol/l sorafenib (***P<0.001 and *P<0.05, respectively). (C and D) Flow cytometric apoptosis assay of HCCLM3-wt and HCCLM3-IL-6(−) cells revealed that the knockout IL-6 increased the apoptosis induced by 5 and 10 µmol/l sorafenib (*P<0.05 and **P<0.01, respectively). (E) Western blot analysis revealed that the level of anti-apoptotic marker Bcl-2 and cell cycle markers cyclin D1 and CDK2 were lower in HCCLM3-IL-6(−) than HCCLM3-wt cells, whereas pro-apoptotic markers cleaved caspase-3 and cleaved PARP were higher in HCCLM3-IL-6(−) than in HCCLM3-wt cells following the administration of 5 and 10 µmol/l sorafenib (all ****P<0.0001). HCC, hepatocellular carcinoma; wt, wild-type; Bcl-2, B-cell lymphoma-2; IL-6, interleukin-6.
Figure 7.
IL-6 induces liver cancer EMT through JAK/STAT3/Snail pathway hyperactivation. (A and B) Exogenous IL-6 hyperactivation of p-JAK2, p-STAT3 and increased Snail expression in HepG2-wt cells, whereas AG490 blocked the effect induced by IL-6 (all ****P<0.0001). (C and D) IL-6 knockout decreased p-JAK2, p-STAT3 and Snail expression in HCCLM3-IL-6(−) cells, as compared with HCCLM3-wt cells (all ****P<0.0001). (E) CCK-8 assay revealed that exogenous IL-6 could promote HepG2-wt cell proliferation, while this effect was significantly blocked by AG490 (***P<0.001). IL-6, interleukin-6; EMT, epithelial-mesenchymal transition; HCC, hepatocellular carcinoma; wt, wild-type.
Similar articles
- Shikonin blocks human lung adenocarcinoma cell migration and invasion in the inflammatory microenvironment via the IL‑6/STAT3 signaling pathway.
Pan T, Zhang F, Li F, Gao X, Li Z, Li X, Ren X. Pan T, et al. Oncol Rep. 2020 Sep;44(3):1049-1063. doi: 10.3892/or.2020.7683. Epub 2020 Jul 9. Oncol Rep. 2020. PMID: 32705271 Free PMC article. - CAFs enhance paclitaxel resistance by inducing EMT through the IL‑6/JAK2/STAT3 pathway.
Wang L, Zhang F, Cui JY, Chen L, Chen YT, Liu BW. Wang L, et al. Oncol Rep. 2018 May;39(5):2081-2090. doi: 10.3892/or.2018.6311. Epub 2018 Mar 14. Oncol Rep. 2018. PMID: 29565447 Free PMC article. - Norcantharidin inhibits IL-6-induced epithelial‑mesenchymal transition via the JAK2/STAT3/TWIST signaling pathway in hepatocellular carcinoma cells.
Gao Y, Li W, Liu R, Guo Q, Li J, Bao Y, Zheng H, Jiang S, Hua B. Gao Y, et al. Oncol Rep. 2017 Aug;38(2):1224-1232. doi: 10.3892/or.2017.5775. Epub 2017 Jun 30. Oncol Rep. 2017. PMID: 28677802 - Role of STAT3 in cancer cell epithelial‑mesenchymal transition (Review).
Zhang G, Hou S, Li S, Wang Y, Cui W. Zhang G, et al. Int J Oncol. 2024 May;64(5):48. doi: 10.3892/ijo.2024.5636. Epub 2024 Mar 15. Int J Oncol. 2024. PMID: 38488027 Free PMC article. Review. - The transition from inflammation to cancer in the liver.
Villanueva A, Luedde T. Villanueva A, et al. Clin Liver Dis (Hoboken). 2016 Oct 27;8(4):89-93. doi: 10.1002/cld.578. eCollection 2016 Oct. Clin Liver Dis (Hoboken). 2016. PMID: 31041071 Free PMC article. Review. No abstract available.
Cited by
- Modulation of the tumour microenvironment in hepatocellular carcinoma by tyrosine kinase inhibitors: from modulation to combination therapy targeting the microenvironment.
Chen R, Li Q, Xu S, Ye C, Tian T, Jiang Q, Shan J, Ruan J. Chen R, et al. Cancer Cell Int. 2022 Feb 11;22(1):73. doi: 10.1186/s12935-021-02435-4. Cancer Cell Int. 2022. PMID: 35148789 Free PMC article. Review. - Interleukin-6 at the Host-Tumor Interface: STAT3 in Biomolecular Condensates in Cancer Cells.
Sehgal PB. Sehgal PB. Cells. 2022 Mar 30;11(7):1164. doi: 10.3390/cells11071164. Cells. 2022. PMID: 35406728 Free PMC article. Review. - Src-FAK Signaling Mediates Interleukin 6-Induced HCT116 Colorectal Cancer Epithelial-Mesenchymal Transition.
Huang YH, Chen HK, Hsu YF, Chen HC, Chuang CH, Huang SW, Hsu MJ. Huang YH, et al. Int J Mol Sci. 2023 Apr 2;24(7):6650. doi: 10.3390/ijms24076650. Int J Mol Sci. 2023. PMID: 37047623 Free PMC article. - Alpha-Lipoic Acid-Mediated Inhibition of LTB4 Synthesis Suppresses Epithelial-Mesenchymal Transition, Modulating Functional and Tumorigenic Capacities in Non-Small Cell Lung Cancer A549 Cells.
Torres MJ, Ríos JC, Valle A, Indo S, Gv KB, López-Moncada F, Faúndez M, Castellón EA, Contreras HR. Torres MJ, et al. Curr Ther Res Clin Exp. 2024 Nov 29;102:100765. doi: 10.1016/j.curtheres.2024.100765. eCollection 2025. Curr Ther Res Clin Exp. 2024. PMID: 39816494 Free PMC article. - Tumor necrosis factor-α coordinates with transforming growth factor-β1 to induce epithelial-mesenchymal transition and migration via the NF-κB/NOX4 pathway in bronchial epithelial cells.
Li K, Zhou R, Ma M, Jin C, Jiao L, Zhang S, Tian M, Zhou F. Li K, et al. Mol Biol Rep. 2022 Oct;49(10):9325-9333. doi: 10.1007/s11033-022-07777-4. Epub 2022 Aug 1. Mol Biol Rep. 2022. PMID: 35913579
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous