Human umbilical cord mesenchymal stem cell-derived exosomal miR-146a-5p reduces microglial-mediated neuroinflammation via suppression of the IRAK1/TRAF6 signaling pathway after ischemic stroke - PubMed (original) (raw)
. 2021 Jan 21;13(2):3060-3079.
doi: 10.18632/aging.202466. Epub 2021 Jan 21.
Xiaoxiong Zou 1, Run Zhang 1, Yu Xie 1, Zhiming Feng 1, Feng Li 1, Jianbang Han 1, Haitao Sun 1, Qian Ouyang 1, Shiting Hua 1, Bingke Lv 1, Tian Hua 1, Zhizheng Liu 1, Yingqian Cai 1, Yuxi Zou 1, Yanping Tang 1, Xiaodan Jiang 1 2
Affiliations
- PMID: 33479185
- PMCID: PMC7880318
- DOI: 10.18632/aging.202466
Human umbilical cord mesenchymal stem cell-derived exosomal miR-146a-5p reduces microglial-mediated neuroinflammation via suppression of the IRAK1/TRAF6 signaling pathway after ischemic stroke
Zhongfei Zhang et al. Aging (Albany NY). 2021.
Abstract
To investigate the therapeutic mechanism of action of transplanted stem cells and develop exosome-based nanotherapeutics for ischemic stroke, we assessed the effect of exosomes (Exos) produced by human umbilical cord mesenchymal stem cells (hUMSCs) on microglia-mediated neuroinflammation after ischemic stroke. Our results found that injected hUMSC-Exos were able to access the site of ischemic damage and could be internalized by cells both in vivo and in vitro. In vitro, treatment with hUMSC-Exos attenuated microglia-mediated inflammation after oxygen-glucose deprivation (OGD). In vivo results demonstrated that treatment with hUMSC-Exos significantly reduced infarct volume, attenuated behavioral deficits, and ameliorated microglia activation, as measured three days post-transient brain ischemia. Furthermore, miR-146a-5p knockdown (miR-146a-5p k/d Exos) partially reversed the neuroprotective effect of hUMSC-Exos. Our mechanistic study demonstrated that miR-146a-5p in hUMSC-Exos reduces microglial-mediated neuroinflammatory response through IRAK1/TRAF6 pathway. We conclude that miR-146a-5p derived from hUMSC-Exos can attenuate microglia-mediated neuroinflammation and consequent neural deficits following ischemic stroke. These results elucidate a potential therapeutic mechanism of action of mesenchymal stem cells and provide evidence that hUMSC-Exos represent a potential cell-free therapeutic option for ischemic stroke.
Keywords: exosomes; ischemic stroke; mesenchymal stem/stromal cell; microRNA; neuroinflammation.
Conflict of interest statement
CONFLICTS OF INTEREST: The authors declare that they have no conflicts of interest.
Figures
Figure 1
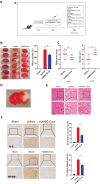
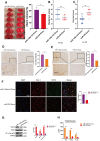
Analysis of human umbilical mesenchymal stem cells (hUMSCs) and hUMSC-derived exosomes (hUMSC-Exos). (A) Representative micrographs of cultured hUMSCs at passage 3 (P3). Scale bar: 200 μm. (B) Flow cytometry analysis of hUMSC CD73, CD105, CD90, CD11b, CD19, CD34, CD45, and HLA-DR expression. (C) Representative electron micrographs of hUMSC-Exos. Scale bar: 200 nm. (D) Exosome particle size and concentration. (E) Western blot analysis of Exos-specific markers CD9, ALIX, and TSG101. Each blot represents three independent experiments of two samples each.
Figure 2
Treatment with hUMSC-Exos attenuates microglia-mediated inflammation and neurological deficits after ischemic stroke. (A) Schematic of the protocol. (B) Representative photomicrographs of TTC-stained tissue from the control, vehicle-only, and experimental groups, with associated infarct size as calculated using ImageJ software. Data are expressed as mean ± SEM (n = 12 per group). Significant differences are indicated (_*p < 0.05). (C) Neurological deficit scores in the vehicle-only and experimental groups 72 hours post-reperfusion. Data are expressed as mean ± SEM (n = 12 per group). Significant differences are indicated (*_p < 0.05, _**p < 0.01). (D) The red box indicates the cerebral ischemic penumbra. (E) H&E staining. Scale bar: 50 μm. (F) Representative photomicrographs of IL-6 and NFκB in the ischemic penumbra 72 hours post-reperfusion, with associated relative intensities as calculated using ImageJ software. Scale bar: 50 μm. Data are expressed as mean ± SEM (n = 6 per group). Significant differences are indicated (*_p < 0.05).
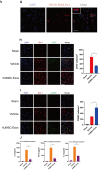
Figure 2
Treatment with hUMSC-Exos attenuates microglia-mediated inflammation and neurological deficits after ischemic stroke. (G) Red fluorescence indicates PKH26-labeled exosomes which have accessed the site of cerebral damage. Scale bar: 50 μm. (H) Microglial M1 markers IBA-1 and CD16 in the ischemic penumbra 3 days following ischemic stroke, in the control, vehicle-only, and experimental groups. Scale bar: 50 μm. Associated M1 counts are shown (A, B). (I) Microglial M2 markers IBA-1 and CD206 in the ischemic penumbra 3 days following ischemic stroke, in the control, vehicle-only, and experimental groups. Scale bar: 50 μm. Associated M2 counts - from the same animals in which M1 counts were determined - are shown (C, D). Significant differences are indicated (_*_p < 0.05). (J) Lower protein levels of pro-inflammatory cytokines IL-6, TNF-α, and IL-1β in the experimental group. Data are expressed as mean ± SEM (experiments were performed in triplicate). Significant differences are indicated (*p < 0.05, **p < 0.01).
Figure 3
Treatment with hUMSC-Exos reduces microglial pro-inflammatory activity in vitro. (A) Confocal imaging demonstrating uptake of PKH-26-labeled exosomes (red) by BV2 microglia. Scale bar: 50 μm. (B) Lower protein levels of pro-inflammatory cytokines IL-6, TNF-α, and IL-1β in the hUMSC-Exos treatment group. (C) Levels of IL-6, TNF-α, and IL-1β mRNA as detected using qRT-PCR. Data are expressed as mean ± SEM (experiments were performed at least in triplicate). Significant differences are indicated (*p < 0.05, **p < 0.01).
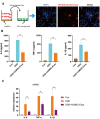
Figure 4
Exosomal miRNAs are implicated in hUMSC-Exos-mediated attenuation of microglial pro-inflammatory activity. (A, B) After 24 hours’ siRNA-Drosha transfection, hUMSC Drosha knockdown efficiency was evaluated by qPCR quantitation of Drosha mRNA and western blot-based quantitation of Drosha protein. Western blots are representative of three independent experimental replicates. (C, D) Exosomal miR-146a-5p and miR-21-5p content was significantly decreased after Drosha knockdown. (E) Protein levels of the pro-inflammatory cytokines IL-6, TNF-α, and IL-1β in hUMSC-Exos were decreased after Drosha knockdown. (F) Detection of IL-6, TNF-α, and IL-1β mRNA levels via qRT-PCR. Data are expressed as mean ± SEM. (A-F) Each experiment is representative of n = 3 per group. Significant differences are indicated (*p < 0.05, **p < 0.01).
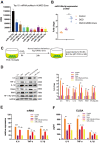
Figure 5
Exosomal miR-146a-5p decreases microglial pro-inflammatory activity by suppressing the IRAK1/TRAF6 signaling pathway in vitro. (A) Expression levels of the top ten hUMSC-Exosomal miRNAs, including MiR-146a-5p. (B) After post-OGD exposure to hUMSC-Exos, BV2 microglia exhibited significantly increased miR-146a-5p content. Data were normalized to levels of U6. (C) In vitro experimental scheme. (D) Expression of pro-inflammatory cytokines IL-6, TNF-α, and IL-1β, as well as signaling pathway IRAK1, TRAF6, and NFκB (p65) in microglia treated with wild-type versus miR-146a-5p knockdown hUMSC-Exos. (E) Determination of IL-6, TNF-α, and IL-1β mRNA levels via qRT-PCR. (F) Determination of supernatant IL-6, TNF-α, and IL-1β protein levels via ELISA. Data are expressed as mean ± SEM. (A–F) Each experiment is representative of n = 3 per group. Significant differences are indicated (*p < 0.05, **p < 0.01, ***p < 0,001).
Figure 6
Treatment with hUMSC-Exos decreases neuroinflammation and is neuroprotective by down-regulating IRAK1/TRAF6 signaling pathway activity in vivo. (A) Representative photomicrographs of TTC-stained tissue from wild-type versus miR-146a-5p knockdown hUMSC-Exos groups, with infarct size as calculated using ImageJ software. Data are expressed as mean ± SEM (n = 6 per group). Significant differences are indicated (*p < 0.05). (B, C) Neurological deficit scores in vehicle-only versus experimental groups at 72 hours post-reperfusion. Data are expressed as mean ± SEM (n = 12 per group). Significant differences are indicated (_*_p < 0.05, _**p < 0.01). (D, E) Representative photomicrographs of IL-6 and NFκB in the ischemic penumbra 72 hours post-reperfusion, with associated relative intensities as calculated using ImageJ software. Scale bar: 50 μm. Data are expressed as mean ± SEM (n = 6 per group). Significant differences are indicated (*_p < 0.05). (F) Microglial M1 markers IBA-1 and CD16 in the ischemic penumbra 3 days following ischemic stroke. (G) Expression of signaling pathway IRAK1, TRAF6, and NFκB (p65) in the wild-type versus miR-146a-5p knockdown groups. (H) Determination of IL-6, TNF- α, and IL-1β protein levels via ELISA. Data are expressed as mean ± SEM (experiments were performed in triplicate). Significant differences are indicated (*p < 0.05, **p < 0.01, ***p < 0,001).
Figure 7
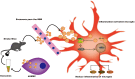
A potential mechanism contributing to the hUMSC-Exos-induced decrease in microglia-mediated neuroinflammation after ischemic stroke. After injection of hUMSC-Exos into the tail vein of the murine ischemic stroke model, they traversed the blood-brain barrier and were internalized by microglia at the site of cerebral injury. Exosomal miR-146a-5p may decrease microglia-mediated neuroinflammation by suppressing the IRAK1/TRAF6 signaling pathway.
Similar articles
- Exosomal miRNA-17-5p derived from human umbilical cord mesenchymal stem cells improves ovarian function in premature ovarian insufficiency by regulating SIRT7.
Ding C, Zhu L, Shen H, Lu J, Zou Q, Huang C, Li H, Huang B. Ding C, et al. Stem Cells. 2020 Sep;38(9):1137-1148. doi: 10.1002/stem.3204. Epub 2020 May 29. Stem Cells. 2020. PMID: 32442343 - Exosomes Derived from MicroRNA-146a-5p-Enriched Bone Marrow Mesenchymal Stem Cells Alleviate Intracerebral Hemorrhage by Inhibiting Neuronal Apoptosis and Microglial M1 Polarization.
Duan S, Wang F, Cao J, Wang C. Duan S, et al. Drug Des Devel Ther. 2020 Aug 5;14:3143-3158. doi: 10.2147/DDDT.S255828. eCollection 2020. Drug Des Devel Ther. 2020. PMID: 32821084 Free PMC article. - MicroRNA engineered umbilical cord stem cell-derived exosomes direct tendon regeneration by mTOR signaling.
Yao Z, Li J, Xiong H, Cui H, Ning J, Wang S, Ouyang X, Qian Y, Fan C. Yao Z, et al. J Nanobiotechnology. 2021 Jun 5;19(1):169. doi: 10.1186/s12951-021-00906-4. J Nanobiotechnology. 2021. PMID: 34090456 Free PMC article. - The potential therapeutic effect of human umbilical cord mesenchymal stem cell-derived exosomes in bronchopulmonary dysplasia.
Cheng T, Mao M, Liu Y, Xie L, Shi F, Liu H, Li X. Cheng T, et al. Life Sci. 2024 Nov 15;357:123047. doi: 10.1016/j.lfs.2024.123047. Epub 2024 Sep 12. Life Sci. 2024. PMID: 39260518 Review. - Potential Druggability of Mesenchymal Stem/Stromal Cell-derived Exosomes.
Zhang F, Zhang L, Yu H. Zhang F, et al. Curr Stem Cell Res Ther. 2024;19(9):1195-1209. doi: 10.2174/011574888X311270240319084835. Curr Stem Cell Res Ther. 2024. PMID: 38523514 Review.
Cited by
- Inhibition of Cerebral Ischemia/Reperfusion Injury by MSCs-Derived Small Extracellular Vesicles in Rodent Models: A Systematic Review and Meta-Analysis.
Zhang L, Pei C, Hou D, Yang G, Yu D. Zhang L, et al. Neural Plast. 2022 Oct 6;2022:3933252. doi: 10.1155/2022/3933252. eCollection 2022. Neural Plast. 2022. PMID: 36338577 Free PMC article. - Mesenchymal stem cell-derived exosomes: Shaping the next era of stroke treatment.
Waseem A, Saudamini, Haque R, Janowski M, Raza SS. Waseem A, et al. Neuroprotection. 2023 Dec;1(2):99-116. doi: 10.1002/nep3.30. Epub 2023 Dec 30. Neuroprotection. 2023. PMID: 38283953 Free PMC article. - Microglia Polarization: A Novel Target of Exosome for Stroke Treatment.
Wan T, Huang Y, Gao X, Wu W, Guo W. Wan T, et al. Front Cell Dev Biol. 2022 Mar 9;10:842320. doi: 10.3389/fcell.2022.842320. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35356292 Free PMC article. Review. - Huc-MSCs-derived exosomes attenuate inflammatory pain by regulating microglia pyroptosis and autophagy via the miR-146a-5p/TRAF6 axis.
Hua T, Yang M, Song H, Kong E, Deng M, Li Y, Li J, Liu Z, Fu H, Wang Y, Yuan H. Hua T, et al. J Nanobiotechnology. 2022 Jul 14;20(1):324. doi: 10.1186/s12951-022-01522-6. J Nanobiotechnology. 2022. PMID: 35836229 Free PMC article.
References
- Feigin VL, Nguyen G, Cercy K, Johnson CO, Alam T, Parmar PG, Abajobir AA, Abate KH, Abd-Allah F, Abejie AN, Abyu GY, Ademi Z, Agarwal G, et al., and GBD 2016 Lifetime Risk of Stroke Collaborators. Global, regional, and country-specific lifetime risks of stroke, 1990 and 2016. N Engl J Med. 2018; 379:2429–37. 10.1056/NEJMoa1804492 - DOI - PMC - PubMed
- Chen H, Guan B, Wang B, Pu H, Bai X, Chen X, Liu J, Li C, Qiu J, Yang D, Liu K, Wang Q, Qi S, Shen J. Glycyrrhizin prevents hemorrhagic transformation and improves neurological outcome in ischemic stroke with delayed thrombolysis through targeting peroxynitrite-mediated HMGB1 signaling. Transl Stroke Res. 2020; 11:967–82. 10.1007/s12975-019-00772-1 - DOI - PubMed
- Xue J, Yu Y, Zhang X, Zhang C, Zhao Y, Liu B, Zhang L, Wang L, Chen R, Gao X, Jiao P, Song G, Jiang XC, Qin S. Sphingomyelin synthase 2 inhibition ameliorates cerebral ischemic reperfusion injury through reducing the recruitment of toll-like receptor 4 to lipid rafts. J Am Heart Assoc. 2019; 8:e012885. 10.1161/JAHA.119.012885 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical