Garcinol Attenuates Lipoprotein(a)-Induced Oxidative Stress and Inflammatory Cytokine Production in Ventricular Cardiomyocyte through α7-Nicotinic Acetylcholine Receptor-Mediated Inhibition of the p38 MAPK and NF-κB Signaling Pathways - PubMed (original) (raw)
Garcinol Attenuates Lipoprotein(a)-Induced Oxidative Stress and Inflammatory Cytokine Production in Ventricular Cardiomyocyte through α7-Nicotinic Acetylcholine Receptor-Mediated Inhibition of the p38 MAPK and NF-κB Signaling Pathways
Nen-Chung Chang et al. Antioxidants (Basel). 2021.
Abstract
Garcinol, a nicotinic acetylcholine receptor (nAChR) antagonist, has recently been established as an anti-inflammation agent. However, the molecular mechanism by which garcinol suppresses inflammation in the context of acute myocardial infarction (AMI) remains unclear. Hypothesis: We hypothesized that the administration of physiological doses of garcinol in mice with isoproterenol-induced AMI decreased the effect of lipoprotein(a) (Lp(a))-induced inflammation both in vivo and in vitro via the α7-nAChRs mediated p38 mitogen-activated protein kinase (MAPK)/nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB) signaling pathway. We analyzed altered reactive oxygen species (ROS) generation, the production of superoxide by mitochondria, cytokine expression patterns, and the role of the p38 MAPK/NF-κB signaling pathway after Lp(a)-stimulated human ventricular cardiomyocyte AC16 cells were treated with increasing doses of garcinol. C-reactive protein (CRP), interleukin (IL)-1β, IL-6, or tumor necrosis factor (TNF)-α production were detected by enzyme-linked immunosorbent assay. The Cell Counting Kit-8 assay was used to evaluate drug cytotoxicity. Western blots and confocal fluorescence microscopy were used to determine altered expression patterns of inflammatory biomarkers. We also examined whether the therapeutic effect of garcinol in AMI was mediated in part by α7-nAChR. Lp(a)-induced inflammatory cardiomyocytes had increased expression of membrane-bound α7-nAChRs in vitro and in vivo. Low-dose garcinol did not affect cardiomyocyte viability but significantly reduced mitochondrial ROS, CRP, IL-1β, IL-6, and TNF-α production in Lp(a)-stimulated cardiomyocytes (p < 0.05). The Lp(a)-induced phosphorylation of p38 MAPKs, CamKII, and NFκB, as well as NFκB-p65 nuclear translocation, was also suppressed (p < 0.05) by garcinol, while the inhibition of p38 MAPK by the inhibitor SB203580 decreased the phosphorylation of extracellular signal-regulated kinase (ERK) and p38 MAPK. Garcinol protected cardiomyocytes by inhibiting apoptosis and inflammation in mice with AMI. Furthermore, garcinol also enhanced the expression of microRNA-205 that suppressed the α7-nAChR-induced p38 MAPK/NF-κB signaling pathway. Garcinol suppresses Lp(a)-induced oxidative stress and inflammatory cytokines by α7-nAChR-mediated inhibition of p38 MAPK/NF-κB signaling in cardiomyocyte AC16 cells and isoproterenol-induced AMI mice.
Keywords: NF-κB signaling; garcinol; nicotinic receptor; α7-nAChR.
Conflict of interest statement
The authors declare that they have no potential financial competing interests that may in any way, gain or lose financially from the publication of this manuscript at present or in the future. Additionally, no non-financial competing interests are involved in the manuscript.
Figures
Figure 1
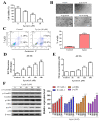
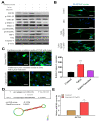
Effect of Lp(a) on reactive oxygen species (ROS) production and α7-nAChR (nicotinic acetylcholine receptor)-mediated phosphorylation in Ventricular cardiomyocyte AC16 cells. (A) Cell viability was significantly reduced in 5 and 10 μM Lp(a)-treated groups compared to control cells detected for 5 and 10 μM Lp(a)-treated groups compared to control cells. (B) Lp(a)-induced alterations in morphology after exposure to different concentration of Lp(a), (C) Lp(a)-induced apoptosis was detected using Annexin V-PE/7-AAD dual staining. (D) The cellular redox status was determined using 6-carboxy-2′,7′-dichlorodihydrofluorescein diacetate (carboxy-H2DCFDA). Serum-starved A16 cells was incubated with carboxy-H2DCFDA and treated with Lp(a) (1 μM) for the indicated time periods, and the fluorescence intensity was quantitated. (E) Serum-starved cells were treated with Lp(a) (1 μM) for the indicated time periods. The production of NO was determined by measuring nitrate concentrations. (F) Western blot analysis showed the concurrent changes in the expression of α7-nAChR and phosphorylation status of calmodulin-dependent kinase II (CamKII), p38 mitogen-activated protein kinase (MAPK) and extracellular signal-regulated kinase (ERK). * p < 0.05, ** p < 0.01, *** p < 0.001.
Figure 2
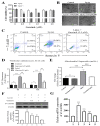
Garcinol reduced Lp(a)-induced reactive oxygen species (ROS) production and cytotoxicity in Ventricular cardiomyocyte AC16 cells. (A) Different concentrations of garcinol did not affect the viability of cardiomyocytes, but garcinol inhibited the cell cytotoxicity induced by Lp(a) in concentration-dependent manners. (B) Reduced alterations in morphology of Lp(a)-treated cardiomyocytes with simultaneous garcinol treatment. scale bars = 20 μm. (C) Garcinol inhibited Lp(a)-induced apoptosis in AC16 cell. Annexin V-PE/7-AAD dual staining was to detect apoptosis. (D) Garcinol suppressed Lp(a)-induced ROS production in human ventricular cardiomyocyte AC16 cells. AC16 were exposed to Lp(a) (1 μM), garcinol (2.5 μM) or Lp(a) (1 μM) with 6 h pretreatment with garcinol (2.5 μM). Six and 24 h after stimulation, AC16 cells were then incubated with DCFH-DA (20, 70-dichlorodihydrofluorescein diacetate), and the level of ROS production was detected by FACStar flow cytometer. (E) Ventricular cardiomyocyte AC16 cells were pretreated with Lp(a) (1 μM), garcinol (2.5 μM) or Lp(a) (1 μM) and mitochondrial superoxide mtO2•− production-specific dye, MitoSOXTM Red, before flow exposure. (F) The protein and (G) mRNA expression levels of α7-nAChR were significantly decreased by garcinol in a dose-dependent manner. * p < 0.05, ** p < 0.01, *** p < 0.001.
Figure 3
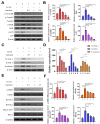
Garcinol inhibited the α7-nAChR-mediated phosphorylation and expression of adhesion molecules as well as RhoA-GTP activity in cardiomyocyte AC16 cells. (A) Western blot analysis showed that garcinol dose-dependently decreased the Lp(a)-induced activation of α7-nAChR and phosphorylation of CamKII, p38 MAPK and ERK. (B) Densitometry analysis of the immunoblots, shown as bar graphs normalized to β-actin. (C) Representative image of Western blot and (D) quantitative real-time polymerase chain reaction (qRT-PCR) mRNA analysis showing the dose-dependent reduction of Lp(a)-induced vascular cell adhesion molecule 1 (VCAM-1), intercellular adhesion molecule 1 (ICAM-1), and E-selectin expression by garcinol. (E) and (F) The Lp(a)-induced activation of the RhoA-GTP/Rho-kinases (ROCK1, ROCK2) were dose-dependently decreased by garcinol. * p < 0.05, ** p < 0.01, *** p < 0.001.
Figure 4
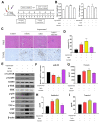
Garcinol inhibited Lp(a)-induced activation of α7-nAChR/p38 MAPK/NFkB in cardiomyocyte AC16 cells. (A) Enzyme-linked immunosorbent assay (ELISA) result showed that garcinol significantly decreased the Lp(a)-induced expression of α7-nAChR and proinflammatory cytokines (interleukin-6 (IL-6), tumor necrosis factor (TNF)-α, C-reactive protein (CRP), and NFkB). (B) Protein and (C) mRNA expression of α7-nAChR, IL-6, TNF-α, CRP, and NFkB were significantly reduced by garcinol. * p < 0.05, ** p < 0.01, *** p < 0.001.
Figure 5
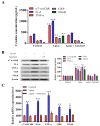
Garcinol prevented apoptosis and inhibited insulin-like growth factor-II receptor (IGF2R) through inactivation of phosphorylation of GSK-3β/ERK/p38 MAPK in ventricular cardiomyocyte AC16 cells. (A) Western blot analysis revealed that Lp(a) upregulated the expression of MAPK signaling-related proteins including p-GSK-3β, p-ERK1/2 and p-p38 MAPK. Garcinol significantly inhibited the Lp(a)-induced phosphorylation of GSK-3β, ERK1/2 and p38 MAPK, but had no obvious effect on total ERK1/2 and p38 MAPK expression. (B) In the immunofluorescence analysis of F-actin polymerization, nuclei and F-actin were stained, respectively, with 4′,6-diamidino-2-phenylindole (DAPI, blue) and rhodamine-phalloidin (red orange). An overlay of the two fluorescent signals was shown (scale bars = 20 μm). (C) Left panel: representative images of caspase-3 activation in AC16 cells treated with cardiomyocytes condition media in the presence of Lp(a) for 24 h with and without garcinol. Right panel: quantification of relative caspase-3 activation. A substantial reduction in caspase activation, suggesting less apoptosis, was observed in garcinol treatment in comparison to Lp(a) only treatment. (D) The mir-Target prediction showed the 3′UTR sites of CHRNA7 targeted by miR-205. (E) qPCR analysis showed miR-205 expression was significantly upregulated by garcinol in comparison to control. * p < 0.05, ** p < 0.01, *** p < 0.001.
Figure 6
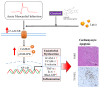
Garcinol suppressed Lp(a)-induced myocardial apoptosis and inflammation in a mouse model of myocardial infarction. (A) The protocol for the induction of myocardial infarction in C57/B6 mice. (B) The heart and liver weight of the control group, and Lp(a) treated mice as compared to the garcinol treatment in mice. (C) H&E and transferase dUTP nick end labeling (TUNEL) staining results of myocardium in the sham, Lp(a) and garcinol groups. (D) Quantitative analysis of percentage apoptosis. (E) Western blot showed a decrease in the expression of pro-inflammatory cytokines TNF-α, IL-6, CRP, NFκB, and phosphorylated CamKII/ERK/p38 MAPK by garcinol. β-actin was used as the loading control. (F) RT-qPCR analysis indicated that the relative miR-205 expression was significantly higher in mice treated with garcinol in comparison to Lp(a) group. (G–I) Enzyme-linked immunosorbent assay (ELISA) analysis results as compared to the treatment group, the significant reduction in the level of hemodynamic and cardiac function markers clusterin, endothelin-1 and troponin I. * p < 0.05, ** p < 0.01, *** p < 0.001.
Figure 7
Garcinol significantly decreased the Lp(a)/α7-nAChR-mediated protein expression levels of inflammatory cytokines and phosphorylation activation of CamKII/p38 MAPK signaling, resulting in reduced post-infarct cardiomyocyte apoptosis.
Similar articles
- Apolipoprotein (a)/Lipoprotein(a)-Induced Oxidative-Inflammatory _α_7-nAChR/p38 MAPK/IL-6/RhoA-GTP Signaling Axis and M1 Macrophage Polarization Modulate Inflammation-Associated Development of Coronary Artery Spasm.
Lin YK, Yeh CT, Kuo KT, Fong IH, Yadav VK, Kounis NG, Hu P, Hung MY. Lin YK, et al. Oxid Med Cell Longev. 2022 Jan 19;2022:9964689. doi: 10.1155/2022/9964689. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 35096275 Free PMC article. - GTS-21 attenuates lipopolysaccharide-induced inflammatory cytokine production in vitro by modulating the Akt and NF-κB signaling pathway through the α7 nicotinic acetylcholine receptor.
Yue Y, Liu R, Cheng W, Hu Y, Li J, Pan X, Peng J, Zhang P. Yue Y, et al. Int Immunopharmacol. 2015 Dec;29(2):504-512. doi: 10.1016/j.intimp.2015.10.005. Epub 2015 Oct 18. Int Immunopharmacol. 2015. PMID: 26490221 - Exogenous hydrogen sulfide protects against doxorubicin-induced inflammation and cytotoxicity by inhibiting p38MAPK/NFκB pathway in H9c2 cardiac cells.
Guo R, Wu K, Chen J, Mo L, Hua X, Zheng D, Chen P, Chen G, Xu W, Feng J. Guo R, et al. Cell Physiol Biochem. 2013;32(6):1668-80. doi: 10.1159/000356602. Epub 2013 Dec 13. Cell Physiol Biochem. 2013. PMID: 24356372 - Exploring the Role of Licorice and Its Derivatives in Cell Signaling Pathway NF-_κ_B and MAPK.
Fatima I, Sahar A, Tariq A, Naz T, Usman M. Fatima I, et al. J Nutr Metab. 2024 Oct 23;2024:9988167. doi: 10.1155/2024/9988167. eCollection 2024. J Nutr Metab. 2024. PMID: 39479405 Free PMC article. Review. - Inflammation-related signaling pathways in tendinopathy.
Jiang L, Liu T, Lyu K, Chen Y, Lu J, Wang X, Long L, Li S. Jiang L, et al. Open Life Sci. 2023 Sep 20;18(1):20220729. doi: 10.1515/biol-2022-0729. eCollection 2023. Open Life Sci. 2023. PMID: 37744452 Free PMC article. Review.
Cited by
- 14-Deoxygarcinol improves insulin sensitivity in high-fat diet-induced obese mice via mitigating NF-κB/Sirtuin 2-NLRP3-mediated adipose tissue remodeling.
Chen JL, Feng ZL, Zhou F, Lou RH, Peng C, Ye Y, Lin LG. Chen JL, et al. Acta Pharmacol Sin. 2023 Feb;44(2):434-445. doi: 10.1038/s41401-022-00958-8. Epub 2022 Aug 9. Acta Pharmacol Sin. 2023. PMID: 35945312 Free PMC article. - Plant-derived polyphenols in sow nutrition: An update.
Chen J, Huang Z, Cao X, Zou T, You J, Guan W. Chen J, et al. Anim Nutr. 2022 Oct 5;12:96-107. doi: 10.1016/j.aninu.2022.08.015. eCollection 2023 Mar. Anim Nutr. 2022. PMID: 36632620 Free PMC article. Review. - Causal effects for genetic variants of osteoprotegerin on the risk of acute myocardial infarction and coronary heart disease: A two-sample Mendelian randomization study.
Chao P, Zhang X, Zhang L, Cui X, Wang S, Yang Y. Chao P, et al. Front Cardiovasc Med. 2023 Mar 7;10:1041231. doi: 10.3389/fcvm.2023.1041231. eCollection 2023. Front Cardiovasc Med. 2023. PMID: 36960470 Free PMC article. - Apolipoprotein (a)/Lipoprotein(a)-Induced Oxidative-Inflammatory _α_7-nAChR/p38 MAPK/IL-6/RhoA-GTP Signaling Axis and M1 Macrophage Polarization Modulate Inflammation-Associated Development of Coronary Artery Spasm.
Lin YK, Yeh CT, Kuo KT, Fong IH, Yadav VK, Kounis NG, Hu P, Hung MY. Lin YK, et al. Oxid Med Cell Longev. 2022 Jan 19;2022:9964689. doi: 10.1155/2022/9964689. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 35096275 Free PMC article. - Coronary Artery Spasm-Related Heart Failure Syndrome: Literature Review.
Hung MJ, Yeh CT, Kounis NG, Koniari I, Hu P, Hung MY. Hung MJ, et al. Int J Mol Sci. 2023 Apr 19;24(8):7530. doi: 10.3390/ijms24087530. Int J Mol Sci. 2023. PMID: 37108691 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous