Adipocyte Fatty Acid Binding Protein Promotes the Onset and Progression of Liver Fibrosis via Mediating the Crosstalk between Liver Sinusoidal Endothelial Cells and Hepatic Stellate Cells - PubMed (original) (raw)
. 2021 Jun;8(11):e2003721.
doi: 10.1002/advs.202003721. Epub 2021 Mar 27.
Lingling Shu 1 3 4, Zixuan Zhang 1 2, Jingjing Li 2, Jiuyu Zong 1 2, Lai Yee Cheong 1 3, Dewei Ye 5, Karen S L Lam 1 3, Erfei Song 6, Cunchuan Wang 6, Aimin Xu 1 2 3, Ruby L C Hoo 1 2 7
Affiliations
- PMID: 34105268
- PMCID: PMC8188197
- DOI: 10.1002/advs.202003721
Adipocyte Fatty Acid Binding Protein Promotes the Onset and Progression of Liver Fibrosis via Mediating the Crosstalk between Liver Sinusoidal Endothelial Cells and Hepatic Stellate Cells
Xiaoping Wu et al. Adv Sci (Weinh). 2021 Jun.
Abstract
Development of liver fibrosis results in drastic changes in the liver microenvironment, which in turn accelerates disease progression. Although the pathological function of various hepatic cells in fibrogenesis is identified, the crosstalk between them remains obscure. The present study demonstrates that hepatic expression of adipocyte fatty acid binding protein (A-FABP) is induced especially in the liver sinusoidal endothelial cells (LSECs) in mice after bile duct ligation (BDL). Genetic ablation and pharmacological inhibition of A-FABP attenuate BDL- or carbon tetrachloride-induced liver fibrosis in mice associating with reduced collagen accumulation, LSEC capillarization, and hepatic stellate cell (HSC) activation. Mechanistically, elevated A-FABP promotes LSEC capillarization by activating Hedgehog signaling, thus impairs the gatekeeper function of LSEC on HSC activation. LSEC-derived A-FABP also acts on HSCs in paracrine manner to potentiate the transactivation of transforming growth factor β1 (TGFβ1) by activating c-Jun N-terminal kinase (JNK)/c-Jun signaling. Elevated TGFβ1 subsequently exaggerates liver fibrosis. These findings uncover a novel pathological mechanism of liver fibrosis in which LSEC-derived A-FABP is a key regulator modulating the onset and progression of the disease. Targeting A-FABP may represent a potential approach against liver fibrosis.
Keywords: A-FABP; TGFβ1; hepatic stellate cells; liver fibrosis; liver sinusoidal endothelial cells.
© 2021 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Figure 1
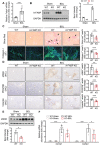
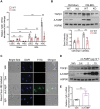
A‐FABP deficiency ameliorates BDL‐induced liver fibrosis in mice. A‐FABP KO mice and their WT littermates were subjected to BDL or sham operation for two weeks. A) Relative mRNA abundance of hepatic Fabp4 in WT mice (n = 8). B) Representative immunoblots of the hepatic expression of A‐FABP and GAPDH in mice and the band intensity of A‐FABP relative to GAPDH (n = 5). Representative images of C) Sirius red staining and immunofluorescence (IF) staining of collagen‐I, and D) immunohistochemistry (IHC) staining of α_SMA, PDGFR_β, and vimentin of mouse liver sections (100 ×, scale bar 250 µm) (n = 8). Black arrow in panel C indicates the mature collagen stained by Sirius red. Right panels are the densitometry analysis of the positive area of Sirius red, collagen‐I, α_SMA, PDGFR_β, and vimentin of mice in BDL group, respectively (n = 8). E) Representative immunoblots of the hepatic expression of _α_SMA and GAPDH in mice. Lower panel is the band intensity of _α_SMA of mice in BDL group relative to GAPDH (n = 4). F) Relative mRNA abundance of hepatic Col1a1 and Col3a1 (n = 8). Data are presented as mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001. Mann‐Whitney U test was used in (A) and (E). Unpaired Student's t test was used in (B), (C), and (D). Two‐way ANOVA followed by Tukey's test was used in (F).
Figure 2
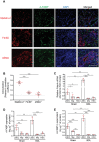
LSEC is the major hepatocellular source of A‐FABP in BDL‐induced liver fibrosis. WT mice were subjected to sham operation or BDL for 2 weeks. A) Representative images of immunofluorescence co‐staining of A‐FABP with stabilin‐2, F4/80, or _α_SMA of liver sections from BDL‐induced mice (scale bar 100 µm) (n = 10). B) Colocalization analysis of A‐FABP+ cells with stabilin‐2+, F4/80+, or α_SMA+ cells in co‐staining of panel A, data presented as Pearson's correlation coefficient (PCC) (n = 10). LSECs, macrophages (M_φ), and HSCs were isolated from WT mice after BDL or sham operation. C) Relative mRNA abundance of Fabp4 in various cell types (n = 5). D) The concentration of A‐FABP in various cell types normalized with the total protein content of cells (n = 5). E) LSECs, macrophages, and HSCs were isolated from WT mice with BDL or sham operation and cultured for 12 hours. The concentration of A‐FABP in conditioned media (CM) normalized with total protein content in cell lysate (n = 5). Data are presented as mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001. One‐way ANOVA followed by Tukey's test was used in (B). Unpaired Student's t test or Mann‐Whitney U test was used in (C), (D), and (E).
Figure 3
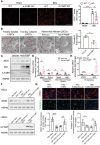
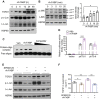
A‐FABP potentiates LSEC capillarization leading to initiation of HSC activation. A‐FABP KO mice and their WT littermates were subjected to BDL or sham operation for two weeks. A) Representative images of immunofluorescent staining of CD31 of mouse liver sections (scale bar 200 µm) (n = 8). Right panel is the densitometry analysis of CD31 positive area (n = 8). B–E) Primary A‐FABP KO LSECs were infected with adenovirus‐overexpressing luciferase (Ad‐Luci) or adenovirus‐overexpressing A‐FABP (Ad‐A‐FABP) (50 MOI) for 2 d after isolation. B) Phenotypic change of freshly isolated LSECs (3 hours after isolation), two‐day cultured LSECs, and adenovirus‐infected LSECs were examined by scanning electron microscopy (scale bar 10 µm). Red arrows indicate the fenestrae and sieve plates. Right panel is the quantification of the percentage of fenestrae to total surface area and represented as percentage of porosity (n = 3). C) Representative immunoblots of A‐FABP, iNOS, Gli2, and HSP90 in LSECs. D,E) mRNA abundance of D) LSEC capillarization‐related markers (Nos2, Edn‐1) and differentiated phenotype‐related markers (Nos3 and Klf2) and E) Hh signaling target genes (Ptch1 and Gli2). F,G) A‐FABP KO HSCs were cultured alone (HSC alone), cocultured with A‐FABP KO LSECs (HSC+LSEC), cocultured with A‐FABP KO LSECs infected with Ad‐Luci (HSC+LSEC/Ad‐Luci), or cocultured with A‐FABP KO LSECs infected with Ad‐A‐FABP (HSC+LSEC/Ad‐A‐FABP), respectively. F) Representative immunoblots of _α_SMA and HSP90 in HSCs (upper), and A‐FABP and HSP90 in LSECs (lower). G) Representative images of immunofluorescent staining of _α_SMA and collagen‐I in HSCs (scale bar 50 µm). Lower panels are the densitometry analysis of _α_SMA and collagen‐I positive area normalized to nucleus number and presented as fold change (n = 6). Data are presented as mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001. Two‐way ANOVA followed by Tukey's test was used in (A). Unpaired Student's t test or Mann‐Whitney U test was used in (B), (D), (E) and (G).
Figure 4
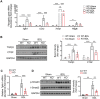
A‐FABP deficiency attenuates BDL‐induced activation of TGF_β_1/Smad signaling in the liver of mice. A‐FABP KO mice and their WT littermates were subjected to BDL or sham operation for two weeks (n = 8). A) Relative mRNA abundance of hepatic Tgfb1, Ccn2, Timp1, and Pdgfb (n = 8). B) Representative immunoblots of hepatic TGF_β_1, CTGF, and GAPDH and the band intensities of various proteins relative to GAPDH (n = 3–6). C) Relative mRNA abundance of Tgfbr1 in mouse liver (n = 8). D) Representative immunoblots of hepatic p‐Smad3 (Ser 423/425), t‐Smad3, and GAPDH and the band intensities of p‐Smad3 relative to t‐Smad‐3 (n = 5). Data are presented as mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001. Two‐way ANOVA followed by Tukey's test was used in (A), (B), (C), and (D).
Figure 5
LSEC‐derived A‐FABP exaggerates TGF_β_1 expression in activated HSCs. A) LSECs, macrophages (M_φ_), and HSCs were isolated from WT or A‐FABP KO mice after BDL or sham operation for two weeks. Relative mRNA abundance of Tgfb1 in various cell types (n = 5). B) Representative immunoblots of TGF_β_1, A‐FABP, and HSP90 of culture‐activated A‐FABP KO HSCs incubated with conditioned media (CM) of LSECs isolated from BDL‐ or sham‐operated mice for 24 hours. Lower panel is the band intensities of TGF_β_1 relative to HSP90 (n = 6). C) The representative images of culture‐activated A‐FABP KO HSCs treated with vehicle (veh, PBS), Alexa Fluor 488 dye, Alexa Fluor 488 labeled rA‐FABP (2 µg mL−1), or Alexa Fluor 488 labeled IgG (2 µg mL−1) for 30 min (200 ×, scale bar 25 µm) (n = 5). D) Representative immunoblots of TGF_β_1, A‐FABP, and HSP90 in culture‐activated A‐FABP KO HSCs treated with different doses of rA‐FABP or vehicle (veh, PBS) for 24 hours. E) The mRNA abundance of Tgfb1 in the culture‐activated A‐FABP KO HSCs treated with rA‐FABP (2 µg mL−1) or vehicle (veh, PBS) for 24 h (n = 5). Data are presented as mean ± SD. *P < 0.05, **P < 0.01. Unpaired Mann‐Whitney U test was used in (A). Two‐way ANOVA followed by Tukey's test was used in (B). Unpaired Student's t test was used in (E).
Figure 6
A‐FABP induces TGF_β_1 expression in HSCs by activating JNK/c‐Jun pathway. A,B) Culture‐activated A‐FABP KO HSCs were treated with rA‐FABP (2 µg mL−1) for different time durations. Representative immunoblots of A) TGF_β_1, p‐c‐Jun (Ser 63), t‐c‐Jun, and HSP90, and B) p‐JNK (Thr 183/ Tyr 185), t‐JNK, and HSP90 in HSCs. Right panel of (B) is the band intensities of p‐JNK relative to t‐JNK (n = 3). C) Culture‐activated A‐FABP KO HSCs were treated with rA‐FABP (2 µg mL−1) or vehicle (veh, PBS) for 24 h. Nuclear lysates were subjected to EMSA with the biotin‐labelled probe containing AP‐1 motif of mouse TGF_β_1 promoter with or without 200‐, 150‐, 100‐, and 50‐fold molar excess specific competitors. D) The relative luciferase activities of luciferase‐reporter constructs control vector (pGL3 basic), phTG‐5, or phTG‐6 in HEK293 cells treated with rA‐FABP (2 µg mL−1) or vehicle (veh, PBS) for 24 h normalized to renilla values (n = 6). E,F) Culture‐activated A‐FABP KO HSCs were pre‐incubated with or without SP600125 (5 × 10‐6
m
) for 1 h and followed by treatment with rA‐FABP (2 µg mL−1) for 24 h. E) Representative immunoblots of TGF_β_1, p‐c‐Jun (Ser 63), t‐c‐Jun, and HSP90 in HSCs. F) Concentration of TGF_β_1 in the conditioned media (CM) of HSCs (n = 5). Data are presented as mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001. One‐way ANOVA followed by Tukey's test was used in (B). Unpaired Student's t test was used in (D) and (F).
Figure 7
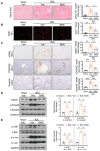
Pharmacological inhibition of A‐FABP alleviates BDL‐induced liver fibrosis. C57BL6/N mice were subjected to BDL or sham operation. From day four after surgery, sham‐operated or BDL‐subjected mice were treated with BMS309403 (BMS, 15 mg kg−1 day−1) or vehicle (veh, 4% Tween 80) daily by oral gavage for 10 d and sacrificed after 24 h of last gavage (n = 6). Representative images of A) Sirius red staining, B) IF staining of CD31, and C) IHC staining of α_SMA, PDGFR_β, and vimentin of mouse liver sections (scale bar, 250 µm). Right panels are densitometry analysis of positive area of Sirius red, CD31, α_SMA, PDGFR_β, and vimentin, respectively (n = 6). Representative immunoblots of D) TGF_β_1, p‐Smad3 (Ser 423/425), t‐Smad3, and GAPDH, and E) p‐JNK (Thr 183/ Tyr 185), t‐JNK, p‐c‐Jun (Ser 63), t‐c‐Jun, and GAPDH in mouse liver. Right panels are the band intensities of various proteins relative to GAPDH or their total protein (n = 4). Data are presented as mean ± SD. *P < 0.05, **P < 0.01. Unpaired Student's t test was used in (A), (B), and (C). Unpaired Mann‐Whitney U test was used in (D) and (E).
Figure 8
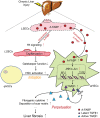
Schematic diagram illustrating the mechanism whereby A‐FABP potentiates liver fibrosis. In response to chronic liver injury, the expression and the subsequent secretion of A‐FABP from LSECs are induced. Elevated A‐FABP potentiates LSEC capillarization through activating Hh signaling pathway thus initiates HSC activation. On the other hand, LSEC‐derived A‐FABP acts in a paracrine manner which diffuses into HSCs and stimulates the transactivation of TGF_β_1 gene through activating the JNK/c‐Jun signaling. Enhanced TGF_β_1 perpetuates HSC activation and facilitates the fibrogenic events such as fibrogenic cytokine expression and deposition of scar matrix, therefore exaggerating liver fibrosis.
Similar articles
- Endothelial POFUT1 controls injury-induced liver fibrosis by repressing fibrinogen synthesis.
He S, Luo Y, Ma W, Wang X, Yan C, Hao W, Fang Y, Su H, Lai B, Liu J, Xiong Y, Bai T, Ren X, Liu E, Han H, Wu Y, Yuan Z, Wang Y. He S, et al. J Hepatol. 2024 Jul;81(1):135-148. doi: 10.1016/j.jhep.2024.02.032. Epub 2024 Mar 7. J Hepatol. 2024. PMID: 38460791 - Role of differentiation of liver sinusoidal endothelial cells in progression and regression of hepatic fibrosis in rats.
Xie G, Wang X, Wang L, Wang L, Atkinson RD, Kanel GC, Gaarde WA, Deleve LD. Xie G, et al. Gastroenterology. 2012 Apr;142(4):918-927.e6. doi: 10.1053/j.gastro.2011.12.017. Epub 2011 Dec 16. Gastroenterology. 2012. PMID: 22178212 Free PMC article. - Endothelial GATA4 controls liver fibrosis and regeneration by preventing a pathogenic switch in angiocrine signaling.
Winkler M, Staniczek T, Kürschner SW, Schmid CD, Schönhaber H, Cordero J, Kessler L, Mathes A, Sticht C, Neßling M, Uvarovskii A, Anders S, Zhang XJ, von Figura G, Hartmann D, Mogler C, Dobreva G, Schledzewski K, Géraud C, Koch PS, Goerdt S. Winkler M, et al. J Hepatol. 2021 Feb;74(2):380-393. doi: 10.1016/j.jhep.2020.08.033. Epub 2020 Sep 9. J Hepatol. 2021. PMID: 32916216 - Liver Sinusoidal Endothelial Cells in the Regulation of Immune Responses and Fibrosis in Metabolic Dysfunction-Associated Fatty Liver Disease.
Puri M, Sonawane S. Puri M, et al. Int J Mol Sci. 2025 Apr 23;26(9):3988. doi: 10.3390/ijms26093988. Int J Mol Sci. 2025. PMID: 40362227 Free PMC article. Review. - Interaction of non‑parenchymal hepatocytes in the process of hepatic fibrosis (Review).
Cheng QN, Yang X, Wu JF, Ai WB, Ni YR. Cheng QN, et al. Mol Med Rep. 2021 May;23(5):364. doi: 10.3892/mmr.2021.12003. Epub 2021 Mar 24. Mol Med Rep. 2021. PMID: 33760176 Free PMC article. Review.
Cited by
- Gene Signatures Detect Damaged Liver Sinusoidal Endothelial Cells in Chronic Liver Diseases.
Verhulst S, van Os EA, De Smet V, Eysackers N, Mannaerts I, van Grunsven LA. Verhulst S, et al. Front Med (Lausanne). 2021 Oct 20;8:750044. doi: 10.3389/fmed.2021.750044. eCollection 2021. Front Med (Lausanne). 2021. PMID: 34746184 Free PMC article. - Spotlights on extracellular vesicles in hepatocellular carcinoma diagnosis and treatment: an update review.
Wang C, Zhang X, Yu J, Bu J, Gu X, Wang Y, Zhu X, Lin J. Wang C, et al. Front Bioeng Biotechnol. 2023 Jun 29;11:1215518. doi: 10.3389/fbioe.2023.1215518. eCollection 2023. Front Bioeng Biotechnol. 2023. PMID: 37456728 Free PMC article. Review. - Adipokines in Non-Alcoholic Fatty Liver Disease: Are We on the Road toward New Biomarkers and Therapeutic Targets?
Francisco V, Sanz MJ, Real JT, Marques P, Capuozzo M, Ait Eldjoudi D, Gualillo O. Francisco V, et al. Biology (Basel). 2022 Aug 19;11(8):1237. doi: 10.3390/biology11081237. Biology (Basel). 2022. PMID: 36009862 Free PMC article. Review. - Endothelial Cell Dysfunction and Nonalcoholic Fatty Liver Disease (NAFLD): A Concise Review.
Nasiri-Ansari N, Androutsakos T, Flessa CM, Kyrou I, Siasos G, Randeva HS, Kassi E, Papavassiliou AG. Nasiri-Ansari N, et al. Cells. 2022 Aug 12;11(16):2511. doi: 10.3390/cells11162511. Cells. 2022. PMID: 36010588 Free PMC article. Review. - Three-Dimensional Cell Co-Culture Liver Models and Their Applications in Pharmaceutical Research.
Ma Y, Hu L, Tang J, Guo W, Feng Y, Liu Y, Tang F. Ma Y, et al. Int J Mol Sci. 2023 Mar 26;24(7):6248. doi: 10.3390/ijms24076248. Int J Mol Sci. 2023. PMID: 37047220 Free PMC article. Review.
References
- Friedman S. L., J. Hepatol. 2003, 38, S38. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 02131906/Health and Medical Research Fund
- 201605303000678/Shenzhen Basic Research Grant
- C7037-17W/Collaborative research fund from Research Grants Council of the Hong Kong Special Administrative Region
- AOE/M-707/18/Area of Excellence Scheme from Research Grants Council of the Hong Kong Special Administrative Region
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous