The Proper Administration Sequence of Radiotherapy and Anti-Vascular Agent-DMXAA Is Essential to Inhibit the Growth of Melanoma Tumors - PubMed (original) (raw)
The Proper Administration Sequence of Radiotherapy and Anti-Vascular Agent-DMXAA Is Essential to Inhibit the Growth of Melanoma Tumors
Alina Drzyzga et al. Cancers (Basel). 2021.
Abstract
Vascular disrupting agents (VDAs), such as DMXAA, effectively destroy tumor blood vessels and cause the formation of large areas of necrosis in the central parts of the tumors. However, the use of VDAs is associated with hypoxia activation and residues of rim cells on the edge of the tumor that are responsible for tumor regrowth. The aim of the study was to combine DMXAA with radiotherapy (brachytherapy) and find the appropriate administration sequence to obtain the maximum synergistic therapeutic effect. We show that the combination in which tumors were irradiated prior to VDAs administration is more effective in murine melanoma growth inhibition than in either of the agents individually or in reverse combination. For the first time, the significance of immune cells' activation in such a combination is demonstrated. The inhibition of tumor growth is linked to the reduction of tumor blood vessels, the increased infiltration of CD8+ cytotoxic T lymphocytes and NK cells and the polarization of macrophages to the cytotoxic M1 phenotype. The reverse combination of therapeutic agents showed no therapeutic effect and even abolished the effect of DMXAA. The combination of brachytherapy and vascular disrupting agent effectively inhibits the growth of melanoma tumors but requires careful planning of the sequence of administration of the agents.
Keywords: brachytherapy; combined anti-cancer therapy; immunotherapy; radiotherapy; vascular disrupting agents.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
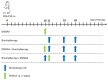
Scheme 1
Diagram showing sequence of brachytherapy and DMXAA administrations.
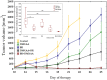
Figure 1
Inhibition of B16-F10 tumor growth using combination therapy of DMXAA and brachytherapy. Mice with tumors were treated with DMXAA (25 mg/kg) (day 10 or 11) and brachytherapy in a dose of 6 Gy in 3 fractional doses (days 10 or 11 and 15, 18). Tumor growth inhibition was measured (mean ± SEM). Statistical analysis was performed at day 22. * p < 0.05, ** p < 0.01 Tukey’s HSD test.
Figure 2
Hematoxylin eosin staining of B16-F10 tumor tissue. Twenty days after tumor inoculation tumors were removed and stained with hematoxylin and eosin. Tumor sections were imaged using light microscope. The scale bar is 1000 µm in the upper pictures and 50 µm in the lower pictures.
Figure 3
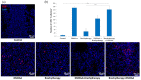
Effect of combination therapy on tumor vascularization. Twenty days after tumor inoculation tumors were collected. (a) Tumor sections were stained with anti-CD31 antibody. CD31 positive endothelial cells (Alexa Fluor 594, red) and nuclei (DAPI, blue) were visualized using confocal microscope. Photographs were taken in 5 randomly chosen fields (magn. 20×) per section in at least 4 tumors of each group. Representative photographs are shown. (b) Percentage of tumor area covered by blood vessels was calculated (mean ± SEM), * p < 0.05, ** p < 0.01 Kruskal–Wallis multiple comparisons.
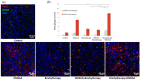
Figure 4
Effect of therapy on infiltration of tumors by macrophages. Twenty days after tumor inoculation tumors were collected. (a) Tumor sections were stained with anti-F4/80 and anti-CD206 antibodies. F4/80 positive total macrophages (Alexa Fluor 594, red), CD206 positive M2 macrophages (FITC, green) and nuclei (DAPI, blue) were visualized using confocal microscope. Photographs were taken in 5 randomly chosen fields (magn. 20×) per section in at least 4 tumors of each group. Representative photographs are shown. (b) Percentage of the area covered by selected populations of macrophages was calculated (mean ± SEM) *** p < 0.001 Kruskal–Wallis multiple comparisons. (c) M1 macrophages were stained using anti-iNOS antibody. F4/80 positive total macrophages (AlexaFluor488, green), iNOS positive M1 macrophages (TexasRed, red) and nuclei (DAPI, blue) were visualized using confocal microscope. Representative photographs are shown. (d) Percentage of the area covered by iNOS positive cells was calculated (mean ± SEM) *** p < 0.001 Kruskal–Wallis multiple comparisons.
Figure 4
Effect of therapy on infiltration of tumors by macrophages. Twenty days after tumor inoculation tumors were collected. (a) Tumor sections were stained with anti-F4/80 and anti-CD206 antibodies. F4/80 positive total macrophages (Alexa Fluor 594, red), CD206 positive M2 macrophages (FITC, green) and nuclei (DAPI, blue) were visualized using confocal microscope. Photographs were taken in 5 randomly chosen fields (magn. 20×) per section in at least 4 tumors of each group. Representative photographs are shown. (b) Percentage of the area covered by selected populations of macrophages was calculated (mean ± SEM) *** p < 0.001 Kruskal–Wallis multiple comparisons. (c) M1 macrophages were stained using anti-iNOS antibody. F4/80 positive total macrophages (AlexaFluor488, green), iNOS positive M1 macrophages (TexasRed, red) and nuclei (DAPI, blue) were visualized using confocal microscope. Representative photographs are shown. (d) Percentage of the area covered by iNOS positive cells was calculated (mean ± SEM) *** p < 0.001 Kruskal–Wallis multiple comparisons.
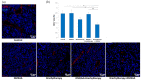
Figure 5
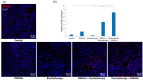
Effect of combination therapy on infiltration of tumors by CD8+ T lymphocytes. Twenty days after tumor inoculation tumors were collected. (a) Tumor sections were stained with anti-CD8 antibody. CD8 positive T lymphocytes (Alexa Fluor 594, red) and nuclei (DAPI, blue) were visualized using confocal microscope. Photographs were taken in 5 randomly chosen fields (magn. 20×) per section in at least 4 tumors of each group. Representative photographs are shown. (b) Total number of CD8 positive cells was calculated per mm2 of tumor section (mean ± SEM) *** p < 0.001 Kruskal–Wallis multiple comparisons.
Figure 6
Effect of combination therapy on infiltration of tumors by NK cells. Twenty days after tumor inoculation tumors were collected. (a) Tumor sections were stained with anti-NKp46 antibody. NKp46 positive NK cells (Alexa Fluor 594, red) and nuclei (DAPI, blue) were visualized using confocal microscope. Photograph were taken in 5 randomly chosen fields (magn. 20×) per section in at least 3 tumors of each group. Representative photographs are shown. (b) Total number of NKp46 positive cells was calculated per mm2 of tumor section (mean ± SEM) * p < 0.05 *** p < 0.001 Kruskal–Wallis multiple comparisons.
Figure 7
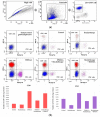
Determination of immune cells infiltration using cytometry analysis. Twenty days after tumor inoculation tumors were collected. (a) Gating strategy of flow cytometry. After gating the population of single cells, viable lymphocytes with negative 7-AAD and positive CD45 staining were selected. (b) Representative flow cytometry dot plot graphs of CD8+ and CD4+ T cells for each experimental group. The number of CD8+ and CD4+ T lymphocytes was determined in the total viable CD45+ cells using appropriate antibodies. (c) Representative flow cytometry histograms of NK cells for each experimental group. The number of NK cells was determined in the total viable CD45+ cells using appropriate antibodies. Analysis was performed in at least 7 tumors of each group. In all experimental groups selected cells were gated to appropriate isotype control for each tumor individually. The number of immune cells was determined in a Lympholyte gradient enriched cell population. Total number of CD8+, CD4+ and NK cells was calculated per mg of tumor tissue.
Figure 7
Determination of immune cells infiltration using cytometry analysis. Twenty days after tumor inoculation tumors were collected. (a) Gating strategy of flow cytometry. After gating the population of single cells, viable lymphocytes with negative 7-AAD and positive CD45 staining were selected. (b) Representative flow cytometry dot plot graphs of CD8+ and CD4+ T cells for each experimental group. The number of CD8+ and CD4+ T lymphocytes was determined in the total viable CD45+ cells using appropriate antibodies. (c) Representative flow cytometry histograms of NK cells for each experimental group. The number of NK cells was determined in the total viable CD45+ cells using appropriate antibodies. Analysis was performed in at least 7 tumors of each group. In all experimental groups selected cells were gated to appropriate isotype control for each tumor individually. The number of immune cells was determined in a Lympholyte gradient enriched cell population. Total number of CD8+, CD4+ and NK cells was calculated per mg of tumor tissue.
Similar articles
- Combination of anti-vascular agent - DMXAA and HIF-1α inhibitor - digoxin inhibits the growth of melanoma tumors.
Smolarczyk R, Cichoń T, Pilny E, Jarosz-Biej M, Poczkaj A, Kułach N, Szala S. Smolarczyk R, et al. Sci Rep. 2018 May 9;8(1):7355. doi: 10.1038/s41598-018-25688-y. Sci Rep. 2018. PMID: 29743548 Free PMC article. - Activation of tumor-associated macrophages by the vascular disrupting agent 5,6-dimethylxanthenone-4-acetic acid induces an effective CD8+ T-cell-mediated antitumor immune response in murine models of lung cancer and mesothelioma.
Jassar AS, Suzuki E, Kapoor V, Sun J, Silverberg MB, Cheung L, Burdick MD, Strieter RM, Ching LM, Kaiser LR, Albelda SM. Jassar AS, et al. Cancer Res. 2005 Dec 15;65(24):11752-61. doi: 10.1158/0008-5472.CAN-05-1658. Cancer Res. 2005. PMID: 16357188 - The vascular disrupting agent, DMXAA, directly activates dendritic cells through a MyD88-independent mechanism and generates antitumor cytotoxic T lymphocytes.
Wallace A, LaRosa DF, Kapoor V, Sun J, Cheng G, Jassar A, Blouin A, Ching LM, Albelda SM. Wallace A, et al. Cancer Res. 2007 Jul 15;67(14):7011-9. doi: 10.1158/0008-5472.CAN-06-3757. Cancer Res. 2007. PMID: 17638914 - An overview on Vadimezan (DMXAA): The vascular disrupting agent.
Daei Farshchi Adli A, Jahanban-Esfahlan R, Seidi K, Samandari-Rad S, Zarghami N. Daei Farshchi Adli A, et al. Chem Biol Drug Des. 2018 May;91(5):996-1006. doi: 10.1111/cbdd.13166. Epub 2018 Jan 24. Chem Biol Drug Des. 2018. PMID: 29288534 Review. - Small molecule vascular disrupting agents: potential new drugs for cancer treatment.
Cai SX. Cai SX. Recent Pat Anticancer Drug Discov. 2007 Jan;2(1):79-101. doi: 10.2174/157489207779561462. Recent Pat Anticancer Drug Discov. 2007. PMID: 18221055 Review.
Cited by
- Targeting the tumour vasculature: from vessel destruction to promotion.
Guelfi S, Hodivala-Dilke K, Bergers G. Guelfi S, et al. Nat Rev Cancer. 2024 Oct;24(10):655-675. doi: 10.1038/s41568-024-00736-0. Epub 2024 Aug 29. Nat Rev Cancer. 2024. PMID: 39210063 Review. - Combination of STING and TLR 7/8 Agonists as Vaccine Adjuvants for Cancer Immunotherapy.
Bhatnagar S, Revuri V, Shah M, Larson P, Shao Z, Yu D, Prabha S, Griffith TS, Ferguson D, Panyam J. Bhatnagar S, et al. Cancers (Basel). 2022 Dec 11;14(24):6091. doi: 10.3390/cancers14246091. Cancers (Basel). 2022. PMID: 36551577 Free PMC article. - Vascular Disruptive Hydrogel Platform for Enhanced Chemotherapy and Anti-Angiogenesis through Alleviation of Immune Surveillance.
Li F, Shao X, Liu D, Jiao X, Yang X, Yang W, Liu X. Li F, et al. Pharmaceutics. 2022 Aug 28;14(9):1809. doi: 10.3390/pharmaceutics14091809. Pharmaceutics. 2022. PMID: 36145556 Free PMC article. - Prognostic Significance of STING Immunoexpression in Relation to HPV16 Infection in Patients with Squamous Cell Carcinomas of Oral Cavity and Oropharynx.
Biesaga B, Smolarczyk R, Mucha-Małecka A, Czapla J, Ryś J, Małecki K. Biesaga B, et al. Biomedicines. 2022 Oct 12;10(10):2538. doi: 10.3390/biomedicines10102538. Biomedicines. 2022. PMID: 36289800 Free PMC article. - Demonstrating Tumor Vascular Disrupting Activity of the Small-Molecule Dihydronaphthalene Tubulin-Binding Agent OXi6196 as a Potential Therapeutic for Cancer Treatment.
Liu L, Schuetze R, Gerberich JL, Lopez R, Odutola SO, Tanpure RP, Charlton-Sevcik AK, Tidmore JK, Taylor EA, Kapur P, Hammers H, Trawick ML, Pinney KG, Mason RP. Liu L, et al. Cancers (Basel). 2022 Aug 30;14(17):4208. doi: 10.3390/cancers14174208. Cancers (Basel). 2022. PMID: 36077745 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials