Interferon-γ primes macrophages for pathogen ligand-induced killing via a caspase-8 and mitochondrial cell death pathway - PubMed (original) (raw)
. 2022 Mar 8;55(3):423-441.e9.
doi: 10.1016/j.immuni.2022.01.003. Epub 2022 Feb 8.
Jiyi Pang 2, Ashley Weir 1, Isabella Y Kong 1, Melanie Fritsch 3, Maryam Rashidi 1, James P Cooney 1, Kathryn C Davidson 1, Mary Speir 4, Tirta M Djajawi 4, Sebastian Hughes 1, Liana Mackiewicz 5, Merle Dayton 5, Holly Anderton 1, Marcel Doerflinger 1, Yexuan Deng 1, Allan Shuai Huang 1, Stephanie A Conos 4, Hazel Tye 4, Seong H Chow 6, Arfatur Rahman 7, Raymond S Norton 8, Thomas Naderer 6, Sandra E Nicholson 1, Gaetan Burgio 9, Si Ming Man 9, Joanna R Groom 1, Marco J Herold 1, Edwin D Hawkins 1, Kate E Lawlor 4, Andreas Strasser 1, John Silke 1, Marc Pellegrini 1, Hamid Kashkar 3, Rebecca Feltham 10, James E Vince 11
Affiliations
- PMID: 35139355
- PMCID: PMC8822620
- DOI: 10.1016/j.immuni.2022.01.003
Interferon-γ primes macrophages for pathogen ligand-induced killing via a caspase-8 and mitochondrial cell death pathway
Daniel S Simpson et al. Immunity. 2022.
Abstract
Cell death plays an important role during pathogen infections. Here, we report that interferon-γ (IFNγ) sensitizes macrophages to Toll-like receptor (TLR)-induced death that requires macrophage-intrinsic death ligands and caspase-8 enzymatic activity, which trigger the mitochondrial apoptotic effectors, BAX and BAK. The pro-apoptotic caspase-8 substrate BID was dispensable for BAX and BAK activation. Instead, caspase-8 reduced pro-survival BCL-2 transcription and increased inducible nitric oxide synthase (iNOS), thus facilitating BAX and BAK signaling. IFNγ-primed, TLR-induced macrophage killing required iNOS, which licensed apoptotic caspase-8 activity and reduced the BAX and BAK inhibitors, A1 and MCL-1. The deletion of iNOS or caspase-8 limited SARS-CoV-2-induced disease in mice, while caspase-8 caused lethality independent of iNOS in a model of hemophagocytic lymphohistiocytosis. These findings reveal that iNOS selectively licenses programmed cell death, which may explain how nitric oxide impacts disease severity in SARS-CoV-2 infection and other iNOS-associated inflammatory conditions.
Keywords: BAX and BAK; COVID-19; SARS-CoV-2; TNF; Toll-like receptor; apoptosis; caspase-8; hemophagocytic lymphohistiocytosis; iNOS; interferon.
Copyright © 2022 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare that D.S.S., J.P., A.W., I.Y.K., M.R., J.P.C., K.C.D., S.H., H.A., M.D., Y.D., L.M., M.D., A.S.H., S.E.N., J.R.G., M.J.H., E.D.H., A.S., J.S., M.P., R.F., and J.E.V. are employees or former employees of the Walter and Eliza Hall Medical Institute, which receives milestone payments from Genentech and AbbVie for the development of ABT-199 for cancer therapy. J.E.V. sits on the advisory board of Avammune Therapeutics.
Figures
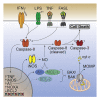
Graphical abstract
Figure 1
IFNγ primes macrophages for TLR-induced cell death (A and B) Wild-type (WT) bone-marrow-derived macrophages (BMDMs) were treated with (A) IFNγ (50 ng/mL) or (B) IFNβ (1,000 U/mL, n = 5) overnight, then with either LPS (50 ng/mL, n = 5), Pam3CSK4 (P3C, 500 ng/mL, n = 6), or PolyI:C (10 μg/mL, n = 6) for 24 h. Cell death was assessed by propidium iodide (PI) exclusion as measured by flow cytometry. (C) WT or Tnf −/− Fasl gld/gld Trail −/− BMDMs were treated with IFNγ (50 ng/mL) overnight then with LPS (50 ng/mL) (left), or P3C (500 ng/mL) (right) for 24 or 48 h ± TNF (100 ng/mL). Cell death was assessed by PI exclusion as measured by flow cytometry (n = 5). (D) Immunoblot of WT BMDMs treated with IFNγ (50 ng/mL) overnight then with LPS (50 ng/mL) for 8, 12, or 24 h. Treatment with the BCL-2, BCL-XL, and BCL-W inhibitor, ABT-737 (737, 1 μM) and cycloheximide (CHX, 10 μg/mL) ± Q-VD-OPh (QVD, 20 μM) for 4 h was used as a control (n = 3). (E) Immunoblot of Tnf −/− Fasl gld/gld Trail −/− BMDMs that were treated with IFNγ (50 ng/mL) overnight then with LPS (50 ng/mL) for 12, 24, or 48 h (n = 2). Data represent the mean value ± SD, or a representative immunoblot, from n independent experiments. p > 0.05 (n.s.), p ≤ 0.001 (∗∗∗), p ≤ 0.0001 (∗∗∗∗). See also Figures S1 and S2 and Videos S1, S2, S3, and S4.
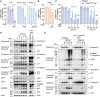
Figure 2
Both extrinsic caspase-8 and the mitochondrial apoptosis effector proteins BAX/BAK contribute to IFNγ/LPS-induced macrophage killing (A) WT, Mlkl −/−, or Casp8 −/− Mlkl −/− BMDMs were treated with IFNγ (50 ng/mL) overnight then with LPS (50 ng/mL) for 24 or 48 h. Treatment with LPS and Compound A (Cp. A, 1 μM) (extrinsic apoptosis) or LPS and Z-VAD-fmk (Z-VAD, 20 μM) (necroptosis) for 24 h were used as controls. Cell death was assessed by PI exclusion as measured by flow cytometry (n = 4). (B) Immunoblot analysis of WT, Mlkl −/−, or Casp8 −/− Mlkl −/− BMDMs that were treated with IFNγ (50 ng/mL) overnight then with LPS (50 ng/mL) for 8, 12 or 24 h. WT BMDMs were treated with LPS and Cp. A for 12 h as a control (n = 3). (C) WT and Bax −/− Bak −/− BMDMs were treated with IFNγ (50 ng/mL) overnight then with LPS (50 ng/mL) for 24 or 48 h. ABT-737 (1 μM) plus cycloheximide (CHX, 10 μg/mL) treatment for 6 h was used as a control. Cell death was assessed by PI exclusion as measured by flow cytometry (n = 4). (D) Immunoblot of WT and Bax −/− Bak −/− BMDMs that were treated with IFNγ (50 ng/mL) overnight then with LPS (50 ng/mL) for 12, 24, or 48 h (n = 3). Data represent the mean value ± SD, or a representative immunoblot, from n independent experiments. p > 0.05 (n.s.), p ≤ 0.05 (∗), p ≤ 0.01 (∗∗), p ≤ 0.001 (∗∗∗), p ≤ 0.0001 (∗∗∗∗). See also Figure S3.
Figure 3
Caspase-8 triggers mitochondrial cytochrome c loss and cell death through a BID-independent mechanism following IFNγ/LPS treatment (A) WT, Mlkl −/−, or Casp8 −/− Mlkl −/− BMDMs were treated with IFNγ (50 ng/mL) overnight then with LPS (50 ng/mL) for 12, 24, 36, or 48 h. Treatment with ABT-737 (1 μM) and cycloheximide (CHX, 10 μg/mL) for 5 h was used as a positive control for BAX/BAK activation (see Figure 2C). Cytochrome c retention was measured by intracellular cytochrome c staining and flow cytometric analysis (n = 3). (B) WT and Bid −/− BMDMs were treated with IFNγ (50 ng/mL) overnight then with LPS (50 ng/mL) for 24 or 48 h. Cell death was assessed by PI exclusion as measured by flow cytometry (n = 6). (C) Immunoblot of WT and Bid −/− BMDMs that were treated with IFNγ (50 ng/mL) overnight then with LPS (50 ng/mL) for 8 or 16 h. WT BMDMs treated with ABT-737 (1 μM) plus cycloheximide (CHX, 10 μg/mL) for 4 h were used as a positive control (n = 3). (D) WT or Bid −/− BMDMs were treated with IFNγ (50 ng/mL) overnight then with LPS (50 ng/mL) for 16 or 24 h. Treatment with ABT-737 (1 μM) and cycloheximide (CHX, 10 μg/mL) for 6 h was used as a positive control for BAX/BAK activation. Cytochrome c retention was measured by intracellular cytochrome c staining and flow cytometric analysis (n = 3). (E) Immunoblot analysis of WT BMDMs that had been primed with IFNγ (50 ng/mL) overnight then stimulated with LPS (50 ng/mL) for 8, 12, or 24 h (n = 3). Data represent the mean value ± SD, or a representative immunoblot, from n independent experiments. p > 0.05 (n.s.), p ≤ 0.05 (∗), p ≤ 0.001 (∗∗∗), p ≤ 0.0001 (∗∗∗∗).
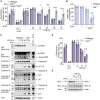
Figure 4
Caspase-8 is required for transcriptional programming of macrophages upon stimulation with IFNγ/LPS, resulting in elevated NOXA and reduced BCL-2 transcripts (A–D) Mlkl −/− and Casp8 −/− Mlkl −/− BMDMs were treated with IFNγ (50 ng/mL) overnight then with LPS (50 ng/mL) for 7 h followed by RNA isolation and 3′ mRNA-sequencing. (A) Multidimensional scaling (MDS) plot and (B) differentially expressed genes (DEGs) that are up- or down-regulated in Casp8 −/− Mlkl −/− BMDMs in comparison to Mlkl −/− cells are shown. The effect of Casp8 deletion on genes associated with cell death was assessed by (C) a volcano plot and (D) a heatmap plot of DEGs involved in distinct cell death signaling pathways (see Table S1) that are enriched in Mlkl −/− BMDMs versus Casp8 −/− Mlkl −/− BMDMs . Adjusted p ≤ 0.05 and cut-off values logFC ≥ 1 or logFC ≤ −1 (n = 3). (E) Immunoblot of WT, Mlkl −/−, or Casp8 −/− Mlkl −/− BMDMs that were treated with IFNγ (50 ng/mL) overnight then with LPS (50 ng/mL) for 2, 4, 6, or 8 h (n = 2). (F) WT or Casp8 −/− Mlkl −/− BMDMs were treated with IFNγ (50 ng/mL) overnight then with LPS (50 ng/mL) for 4, 8, or 16 h. Bcl2 expression was assessed by quantitative PCR (qPCR) using Hprt as a housekeeping gene. Baseline Bcl2 expression (dCT) is shown (right) as a control (n = 3). (G) Casp8 +/+ (WT [circles] or Ripk3 −/− [triangles]) or Casp8 −/− (Casp8 −/− Mlkl −/− [circles] or Casp8 −/− Ripk3 −/− [triangles]) BMDMs were treated with IFNγ (50 ng/mL) overnight then with LPS (50 ng/mL) ± the BCL-2 inhibitor, ABT-199 (1 μM), or DMSO for 16 or 24 h. Treatments with LPS and Compound A (Cp. A 1 μM) (extrinsic apoptosis) or ABT-737 (1 μM) plus cycloheximide (CHX, 10 μg/mL) (intrinsic apoptosis) were used as controls. Cell death was assessed by PI exclusion as measured by flow cytometry (n = 4). (H) WT BMDMs were treated with the MCL-1 inhibitor S63845 (10 μM) ± ABT-199 (1 μM) for 12 h. Cell death was assessed by PI exclusion as measured by flow cytometry (n = 3). Data represent the mean value ± SD, or a representative immunoblot, from n independent experiments. p > 0.05 (n.s.), p ≤ 0.05 (∗), p ≤ 0.01 (∗∗), p ≤ 0.001 (∗∗∗). See also Figures S4 and S5 and Tables S1–S3.
Figure 5
Caspase-8 enzymatic activity is required for IFNγ/LPS-induced killing of macrophages (A and B) WT, Ripk3 −/−, Casp8 −/− Ripk3 −/−, or Casp8 C362S/C362S Ripk3 −/− (Casp8 CS/CS Ripk3 −/−) BMDMs were primed with IFNγ (50 ng/mL) overnight then stimulated with LPS (50 ng/mL) for 24 or 48 h. Treatment with LPS and Compound A (Cp. A, 1 μM) for 24 h (extrinsic apoptosis), LPS and IDN-6556 (IDN, 5 μM) for 24 h (necroptosis), and ABT-737 (1 μM) and cycloheximide (CHX, 10 μg/mL) for 6 h (intrinsic apoptosis) were used as controls. (A) Cell death and (B) cytochrome c retention was measured by PI exclusion as measured by flow cytometry or intracellular cytochrome c staining and flow cytometric analysis (n = 3). (C and D) Immunoblot of WT, Ripk3 −/−, Casp8 −/− Ripk3 −/−, or Casp8 CS/CS Ripk3 −/− BMDMs primed with IFNγ (50 ng/mL) overnight and stimulated with LPS (50 ng/mL) for (C) 16 and 24 h, or (D) 2–8 h. Ponceau stain is provided as a loading control (n = 2). (E) WT or Mlkl −/− BMDMs were treated with IFNγ (50 ng/mL) overnight then with LPS (50 ng/mL) ± IDN (5 μM) or DMSO for 16, 24, or 48 h. LPS and IDN or IFNγ and IDN treatment for 24 h (necroptosis) was used as a control. Cell death was assessed by PI exclusion as measured by flow cytometry (n = 3). (F) Immunoblot of WT or Mlkl −/− BMDMs that were treated with IFNγ (50 ng/mL) overnight then with LPS (50 ng/mL) ± IDN (5 μM) or DMSO for 8 or 16 h (n = 2). Data represent the mean value ± SD, or a representative immunoblot, from n independent experiments. p > 0.05 (n.s.), p ≤ 0.01 (∗∗), p ≤ 0.0001 (∗∗∗∗). See also Figure S6.
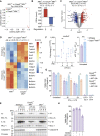
Figure 6
IFNγ/LPS-induced iNOS sensitizes macrophages to caspase-8 and BAX/BAK-mediated death (A) WT BMDMs were primed with IFNγ (50 ng/mL) overnight then stimulated with LPS (50 ng/mL) for 2–24 h. Nitrite (NO2−) production and iNOS expression were measured by the Griess assay and immunoblot (n = 3). (B and C) WT BMDMs were primed with IFNγ (50 ng/mL) overnight then stimulated with LPS (50 ng/mL) ± the iNOS inhibitor 1400W (10 μM) or DMSO for 24 h. (B) Nitrite (NO2−) production and cell death were measured by the Griess assay and (C) PI exclusion as measured by flow cytometry (n = 4). (D) WT BMDMs were primed with IFNγ (50 ng/mL) overnight then stimulated with LPS (50 ng/mL) ± 1400W (10 μM) for 24 h. The nitric oxide donor SNAP (200 μM) or DMSO were provided 8 h post-treatment with LPS. Cell death was assessed by PI exclusion as measured by flow cytometry (n = 5). (E) WT BMDMs were treated with 1400W (10 μM) ± IFNγ (50 ng/mL) overnight then stimulated with LPS (50 ng/mL) or TNF (100 ng/mL) for 24 h. SNAP (200 μM) or DMSO were added to cells 8 h post-treatment with LPS or TNF. Cell death was assessed by PI exclusion as measured by flow cytometry (n = 4). (F, G, and H) WT or Nos2 −/− BMDMs were primed with IFNγ (50 ng/mL) overnight then stimulated with LPS (50 ng/mL) or Pam-3-CSK4 (P3C, 500 ng/mL) for 16, 24, or 48 h. ABT-737 (1 μM) and cycloheximide (CHX, 10 μg/mL) treatment for (G) 6 h or (H) 2 h was used as a positive control. (F) Cell death and (G) cytochrome c retention was assessed by PI exclusion as measured by flow cytometry or intracellular cytochrome c staining and flow cytometric analysis (n = 6). (H) Cell death pathway activation was assessed by immunoblot of cell supernatants (S/N) and cell lysates (n = 2). Data represent the mean value ± SD, or a representative immunoblot, from n independent experiments. p ≤ 0.05 (∗), p ≤ 0.001 (∗∗∗), p ≤ 0.0001 (∗∗∗∗). See also Figure S6 and S7.
Figure 7
iNOS and caspase-8 influence the host response to SARS-CoV-2, but only caspase-8 impacts hemophagocytic lymphohistiocytosis (HLH) disease severity (A and B) Rectal temperatures of (A) wild-type (WT, n = 12), Ripk3 −/− (n = 6), Casp8 −/− Ripk3 −/− (n = 10), and Tnf −/− Fasl gld/gld Trail −/− (n = 6) mice, or (B) WT (n = 14) and Nos2 −/− (n = 12) mice injected with PolyI:C (10 mg/kg) for 24 h followed by LPS (5 mg/kg) to induce HLH-like disease. (C) Cleaved caspase-3 immunohistochemistry of endpoint small intestine sections taken from mice treated as described (A and B). Each image represents a separate mouse. Positive cells are indicated with red arrows. Scale bar, 100 μm. (D and E) Endpoint plasma TNF, IL-1β and IL-6 concentrations of mice treated as described in (D) A and (E) B. (F and G) (F) TCID50 infectious units per lung and (G) percentage weight loss of WT (n = 22), Nos2 +/− (n = 18) or Nos2 −/− (n = 20) mice infected with 1.5 × 107 TCID50 infectious units of SARS-CoV-2 for three days. (H and I) (H) TCID50 infectious units per lung and (I) percentage weight loss of WT (n = 21), Mlkl −/− (n = 23), Casp8 +/− Mlkl −/− (n = 17), or Casp8 −/− Mlkl −/− (n = 15) mice infected with 1.5 × 107 TCID50 infectious units of SARS-CoV-2 for three days. Data represent the mean value ± SEM pooled from at least 2 independent experimental cohorts of mice. p > 0.05 (n.s.), p ≤ 0.05 (∗), p ≤ 0.01 (∗∗), p ≤ 0.001 (∗∗∗), p ≤ 0.0001 (∗∗∗∗).
Comment in
- The domiNO effect turns macrophage activation deadly.
Robertson SJ, Best SM. Robertson SJ, et al. Immunity. 2022 Mar 8;55(3):382-384. doi: 10.1016/j.immuni.2022.02.010. Immunity. 2022. PMID: 35263563 Free PMC article.
Similar articles
- The Mitochondrial Apoptotic Effectors BAX/BAK Activate Caspase-3 and -7 to Trigger NLRP3 Inflammasome and Caspase-8 Driven IL-1β Activation.
Vince JE, De Nardo D, Gao W, Vince AJ, Hall C, McArthur K, Simpson D, Vijayaraj S, Lindqvist LM, Bouillet P, Rizzacasa MA, Man SM, Silke J, Masters SL, Lessene G, Huang DCS, Gray DHD, Kile BT, Shao F, Lawlor KE. Vince JE, et al. Cell Rep. 2018 Nov 27;25(9):2339-2353.e4. doi: 10.1016/j.celrep.2018.10.103. Cell Rep. 2018. PMID: 30485804 - YopJ-induced caspase-1 activation in Yersinia-infected macrophages: independent of apoptosis, linked to necrosis, dispensable for innate host defense.
Zheng Y, Lilo S, Mena P, Bliska JB. Zheng Y, et al. PLoS One. 2012;7(4):e36019. doi: 10.1371/journal.pone.0036019. Epub 2012 Apr 26. PLoS One. 2012. PMID: 22563435 Free PMC article. - The HIV-1-specific protein Casp8p41 induces death of infected cells through Bax/Bak.
Sainski AM, Natesampillai S, Cummins NW, Bren GD, Taylor J, Saenz DT, Poeschla EM, Badley AD. Sainski AM, et al. J Virol. 2011 Aug;85(16):7965-75. doi: 10.1128/JVI.02515-10. Epub 2011 Jun 8. J Virol. 2011. PMID: 21653671 Free PMC article. - Pro-apoptotic complexes of BAX and BAK on the outer mitochondrial membrane.
Wolf P, Schoeniger A, Edlich F. Wolf P, et al. Biochim Biophys Acta Mol Cell Res. 2022 Oct;1869(10):119317. doi: 10.1016/j.bbamcr.2022.119317. Epub 2022 Jun 22. Biochim Biophys Acta Mol Cell Res. 2022. PMID: 35752202 Review. - BCL-2 proteins and apoptosis: Recent insights and unknowns.
Edlich F. Edlich F. Biochem Biophys Res Commun. 2018 May 27;500(1):26-34. doi: 10.1016/j.bbrc.2017.06.190. Epub 2017 Jul 1. Biochem Biophys Res Commun. 2018. PMID: 28676391 Review.
Cited by
- Endocrine dysregulation in COVID-19: molecular mechanisms and insights.
Iosef C, Matusa AM, Han VKM, Fraser DD. Iosef C, et al. Front Endocrinol (Lausanne). 2024 Oct 22;15:1459724. doi: 10.3389/fendo.2024.1459724. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 39502570 Free PMC article. Review. - Dynamic interplay of autophagy and membrane repair during Mycobacterium tuberculosis Infection.
Augenstreich J, Phan AT, Allen CNS, Poddar A, Chen H, Srinivasan L, Briken V. Augenstreich J, et al. PLoS Pathog. 2025 Jan 2;21(1):e1012830. doi: 10.1371/journal.ppat.1012830. eCollection 2025 Jan. PLoS Pathog. 2025. PMID: 39746091 Free PMC article. - A spatiotemporal comparative analysis on tumor immune microenvironment characteristics between neoadjuvant chemotherapy and preoperative immunotherapy for ESCC.
Zhou Z, Zhang H, Du J, Yang J, Pan W, Zhang Q, Wang H, Tang P, Ba Y, Zhang H. Zhou Z, et al. Cell Death Dis. 2024 Sep 10;15(9):663. doi: 10.1038/s41419-024-06986-y. Cell Death Dis. 2024. PMID: 39256364 Free PMC article. - Oral nitrate-reducing bacteria as potential probiotics for blood pressure homeostasis.
Chai X, Liu L, Chen F. Chai X, et al. Front Cardiovasc Med. 2024 Apr 4;11:1337281. doi: 10.3389/fcvm.2024.1337281. eCollection 2024. Front Cardiovasc Med. 2024. PMID: 38638884 Free PMC article. Review. - RIPK1 and RIPK3 inhibitors: potential weapons against inflammation to treat diabetic complications.
Ke D, Zhang Z, Liu J, Chen P, Dai Y, Sun X, Chu Y, Li L. Ke D, et al. Front Immunol. 2023 Oct 26;14:1274654. doi: 10.3389/fimmu.2023.1274654. eCollection 2023. Front Immunol. 2023. PMID: 37954576 Free PMC article. Review.
References
- Allam R., Lawlor K.E., Yu E.C., Mildenhall A.L., Moujalled D.M., Lewis R.S., Ke F., Mason K.D., White M.J., Stacey K.J., et al. Mitochondrial apoptosis is dispensable for NLRP3 inflammasome activation but non-apoptotic caspase-8 is required for inflammasome priming. EMBO Rep. 2014;15:982–990. doi: 10.15252/embr.201438463. - DOI - PMC - PubMed
- Alvarez-Diaz S., Dillon C.P., Lalaoui N., Tanzer M.C., Rodriguez D.A., Lin A., Lebois M., Hakem R., Josefsson E.C., O’Reilly L.A., et al. The Pseudokinase MLKL and the Kinase RIPK3 Have Distinct Roles in Autoimmune Disease Caused by Loss of Death-Receptor-Induced Apoptosis. Immunity. 2016;45:513–526. doi: 10.1016/j.immuni.2016.07.016. - DOI - PMC - PubMed
- Bossaller L., Chiang P.I., Schmidt-Lauber C., Ganesan S., Kaiser W.J., Rathinam V.A., Mocarski E.S., Subramanian D., Green D.R., Silverman N., et al. Cutting edge: FAS (CD95) mediates noncanonical IL-1β and IL-18 maturation via caspase-8 in an RIP3-independent manner. J. Immunol. 2012;189:5508–5512. doi: 10.4049/jimmunol.1202121. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous