Metabolic reprogramming of tumor-associated macrophages by collagen turnover promotes fibrosis in pancreatic cancer - PubMed (original) (raw)
Metabolic reprogramming of tumor-associated macrophages by collagen turnover promotes fibrosis in pancreatic cancer
Madeleine M LaRue et al. Proc Natl Acad Sci U S A. 2022.
Abstract
A hallmark of pancreatic tumors is their highly desmoplastic stroma composed of fibroblasts, immune cells, and a dense network of collagen fibers. Tumor-associated macrophages are one of the most abundant immune cell populations in the pancreatic tumor stroma. Their protumorigenic function has been attributed predominantly to their capacity to promote immune evasion and metastasis. Tumor-assoc iated macrophages are also well known for their role in the remodeling of the stroma via collagen production and degradation, with the latter being mediated by mannose receptor (MRC1)-dependent endocytosis of collagen. Here we show that MRC1-mediated collagen internalization and subsequent lysosomal degradation by macrophages harboring a tumor-associated phenotype are accompanied by the accumulation of collagen-derived intracellular free amino acids and increased arginine biosynthesis. The resulting increase in intracellular arginine levels leads to the up-regulation of inducible nitric oxide synthase and the production of reactive nitrogen species. Furthermore, reactive nitrogen species derived from internalized and degraded collagen promotes a profibrotic phenotype in pancreatic stellate cells resulting in enhanced intratumoral collagen deposition. Overall, our findings identify a role for extracellular matrix remodeling in the functional modulation of tumor-associated macrophages via metabolic rewiring.
Keywords: collagen; fibrosis; macrophage; pancreatic cancer; stellate cell.
Conflict of interest statement
Competing interest statement: A.C.K. has financial interests in Vescor Therapeutics, LLC; is an inventor on patents pertaining to KRAS-regulated metabolic pathways, redox control pathways in pancreatic cancer, targeting GOT1 as a therapeutic approach, and the autophagy control of iron metabolism; is on the Scientific Advisory Board of Rafael/Cornerstone Pharmaceuticals; and is a consultant for Deciphera and Abbvie. D.B.-S. is on the Scientific Advisory Board of Rafael/Cornerstone Pharmaceuticals and Samumed LLC.
Figures
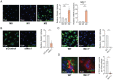
Fig. 1.
Macrophages internalize and degrade collagen via Mrc1-mediated endocytosis. (A) Representative fluorescence microscopy images (Left) and quantification (Center) of BMDMs from C57BL/6 mice either unstimulated (M0) or treated with LPS (M1) or IL-4 (M2) fed OG-gelatin. (Right) Analysis of mRNA expression by quantitative PCR of Mrc1 normalized to a GAPDH housekeeping gene and presented relative to M0. (B) Representative fluorescence microscopy images (Left) and quantification (Right) showing IL-4–treated BMDMs (M2) transfected with siRNA targeting Mrc1 or control siRNA and fed OG-gelatin. (C) Representative fluorescence microscopy images (Left) and quantification (Right) of IL-4–treated BMDMs (M2) from mannose receptor (Mrc1) knockout mice or wild-type littermate controls fed OG gelatin. Data represents average uptake index within BMDMs from two mice sampled, with at least n = 3 technical replicates per mouse. (D) KPC pancreatic cells were injected subcutaneously into flanks of Mrc1 knockout mice or wild-type littermate controls. At ∼300 mm3, tumors were injected with OG gelatin (green) and uptake by F4/80+ cells (red) was analyzed by indirect immunofluorescence of tumor cryosections. Nuclei are labeled with DAPI (blue). Graph (Right) represents the average number of OG gelatin puncta observed in randomly selected F4/80+ cells per tumor sampled (50 macrophages per tumor). Quantification of all fluorescent images are presented in arbitrary units and relative to values obtained for control conditions (untreated, DMSO, or wild-type). For all graphs, values are mean ± SE for at least n = 3 independent experiments, unless otherwise noted. Statistical significance was determined using a Student’s t test. *P < 0.05; ***P < 0.0001; ns, not significant.
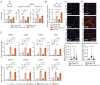
Fig. 2.
Collagen uptake and degradation augments intracellular arginine bioavailability and enhances expression of arginine metabolism enzymes in macrophages. (A) Analysis of mRNA expression by quantitative PCR of iNos (Left) or Arg1 (Right) in IL-4–treated BMDMs transfected with siRNA targeting control (siCtl) or Mrc1 (siMrc1) fed 1% gelatin. (B) Representative Western blot (Left) and quantification (Right) of iNOS protein expression in IL-4–treated BMDMs transfected with siRNA targeting Mrc1 (siMrc1), or control (siCtl), and fed 1% gelatin (n = 3). Tubulin serves as loading control. Data were normalized to siCtl + gelatin condition. (C and D) Flow cytometry analysis showing percent CD45+F4/80+Ly6C−iNOS+ARG1+ cells from (C) IL-4–treated BMDMs without (control) or with 1% gelatin or (D) 2-wk-implanted KPC orthotopic pancreatic tumors and 3-mo-old KPC GEMM mouse pancreata. Representative flow cytometry plots (Left) and quantification (Right, n = 3) are shown. (E) CD68+iNOS+ARG1+ macrophages in human PDAs by OPAL high-throughput multicolor IHC analysis. 20x magnification (Right). Indicated box (Left) is enlarged. Arrows identify several clusters of CD68+iNOS+ARG1+ macrophages. Image is 1 representative of ∼180 from three tumors analyzed. (F) Flow cytometry analysis showing percent CD45+F4/80+Ly6C−iNOS+ARG1+ cells in MRC1high versus MRC1low macrophage populations in 2-wk-implanted KPC orthotopic pancreatic tumors. (G) Analysis of mRNA expression of iNos (Left) and Arg1 (Right) by quantitative PCR in IL-4–treated BMDMs treated as shown and normalized to DMSO control. (H) GC-MS metabolomics analysis showing intracellular arginine levels (fmol per 1 × 106 cells) in IL-4–treated BMDMs transfected with siMrc1 or siCtl and treated with 1% dialyzed gelatin or PBS (control). Absolute values of arginine are shown due to interexperimental variability in baseline intracellular levels of arginine observed in M2 macrophages. (I, Left) Diagram of intracellular arginine biosynthesis and target of MDLA inhibition. (Right) Analysis of mRNA expression of iNos by quantitative PCR in IL-4–treated BMDMs treated as shown and normalized to control. For all graphs, values are mean ± SE for at least n = 3 independent experiments. All mRNA data were normalized to GAPDH housekeeping gene during analysis. Statistical significance was determined using a Student’s t test or one-way ANOVA test. Adjusted P values for two group comparisons are plotted. *P < 0.05; **P < 0.001; ***P < 0.0001; ns, not significant.
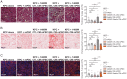
Fig. 3.
Macrophages produce reactive nitrogen species during collagen uptake and degradation that promote a profibrotic phenotype in pancreatic stellate cells. (A) Total nitrite (μmol/L), endogenous nitrite (μmol/L), and nitrate (μmol/L) measured using a colorimetric NO Detection Kit in cell culture supernatent collected from untreated (Control CM), gelatin-fed (Gelatin CM), 1400W-treated and gelatin-fed (1400W Gelatin CM), or LPS-treated (LPS CM) macrophages. (B) Peroxynitrite production by macrophages determined by quantifying the 574 nm/476 nm fluorescence intensity ratio of a peroxynitrite-specific ratiometric probe, normalized to control. Ten randomly selected cells were analyzed per frame. Each frame was averaged per condition and normalized to control. (C) Relative mRNA expression of Hsp47, αSma, Ctgf, Cdh10, Col1a1, Col2a1, Col3a1, and Col4a1 in murine PSCs measured by qPCR analysis and normalized to control. All mRNA data were normalized to a housekeeping gene, GAPDH, during analysis. (D) Intracellular and (E) extracellular immunofluorescence staining of COL1A1 (orange) and nuclei (blue) in conditioned medium from IL-4–treated BMDMs fed with PBS (Control CM), 1% gelatin (Gelatin CM), or 1400W + 1% gelatin (1400W Gelatin CM). All fluorescent images quantified in arbitrary units and presented relative to values obtained for control. For qPCR graphs, values are mean ± SE for at least n = 3 independent experiments. For immunofluoresence analysis, each dot represents quantification in one field of view, n = 2 independent experiments. Statistical significance was determined using a one-way ANOVA test. Adjusted P values for two group comparisons are plotted. *P < 0.05; **P < 0.001; ***P < 0.0001; ns, not significant.
Fig. 4.
PSCs exposed to macrophage-derived reactive nitrogen species enhance fibrosis and pancreatic tumor growth in vivo. (A) Masson’s trichrome stain, (B) Picrosirius red stain, and (C) Vimentin immunofluorescence (orange) and nuclei (blue) in sections from tumors implanted with KPC pancreatic cancer cells alone (−) or coimplanted with murine pancreatic stellate cells cultured in complete medium (mPSC) or conditioned medium from BMDMs treated with 1400W + PBS (1400W CTL CM mPSC), 1% gelatin (GEL CM mPSC), or 1400W + 1% gelatin (1400W GEL CM mPSC) for 24 h prior to injecting cells subcutaneously into the rear flanks of nude mice. For all graphs, values are mean ± SE n = 8 mice. All fluorescent images quantified in arbitrary units and presented relative to values obtained for control. Statistical significance was determined using a one-way ANOVA test. Adjusted P values for two group comparisons are plotted. *P < 0.05; **P < 0.001; ***P < 0.0001; ns, not significant.
Similar articles
- Immune Cell Modulation of the Extracellular Matrix Contributes to the Pathogenesis of Pancreatic Cancer.
Ahmad RS, Eubank TD, Lukomski S, Boone BA. Ahmad RS, et al. Biomolecules. 2021 Jun 17;11(6):901. doi: 10.3390/biom11060901. Biomolecules. 2021. PMID: 34204306 Free PMC article. Review. - Tumor Cell-Derived IL1β Promotes Desmoplasia and Immune Suppression in Pancreatic Cancer.
Das S, Shapiro B, Vucic EA, Vogt S, Bar-Sagi D. Das S, et al. Cancer Res. 2020 Mar 1;80(5):1088-1101. doi: 10.1158/0008-5472.CAN-19-2080. Epub 2020 Jan 8. Cancer Res. 2020. PMID: 31915130 Free PMC article. - Fibrogenesis in pancreatic cancer is a dynamic process regulated by macrophage-stellate cell interaction.
Shi C, Washington MK, Chaturvedi R, Drosos Y, Revetta FL, Weaver CJ, Buzhardt E, Yull FE, Blackwell TS, Sosa-Pineda B, Whitehead RH, Beauchamp RD, Wilson KT, Means AL. Shi C, et al. Lab Invest. 2014 Apr;94(4):409-21. doi: 10.1038/labinvest.2014.10. Epub 2014 Feb 17. Lab Invest. 2014. PMID: 24535260 Free PMC article. - Pancreatic Tumor Microenvironment.
Wang K, He H. Wang K, et al. Adv Exp Med Biol. 2020;1296:243-257. doi: 10.1007/978-3-030-59038-3_15. Adv Exp Med Biol. 2020. PMID: 34185297 - Pancreatic stellate cells and the interleukin family: Linking fibrosis and immunity to pancreatic ductal adenocarcinoma (Review).
Li H, Liu D, Li K, Wang Y, Zhang G, Qi L, Xie K. Li H, et al. Mol Med Rep. 2024 Sep;30(3):159. doi: 10.3892/mmr.2024.13283. Epub 2024 Jul 12. Mol Med Rep. 2024. PMID: 38994764 Free PMC article. Review.
Cited by
- Expression of Selected miRNAs in Normal and Cancer-Associated Fibroblasts and in BxPc3 and MIA PaCa-2 Cell Lines of Pancreatic Ductal Adenocarcinoma.
Mandys V, Popov A, Gürlich R, Havránek J, Pfeiferová L, Kolář M, Vránová J, Smetana K Jr, Lacina L, Szabo P. Mandys V, et al. Int J Mol Sci. 2023 Feb 10;24(4):3617. doi: 10.3390/ijms24043617. Int J Mol Sci. 2023. PMID: 36835029 Free PMC article. - MIF/NR3C2 axis regulates glucose metabolism reprogramming in pancreatic cancer through MAPK-ERK and AP-1 pathways.
Yang S, Tang W, Azizian A, Gaedcke J, Ohara Y, Cawley H, Hanna N, Ghadimi M, Lal T, Sen S, Creighton CJ, Gao J, Putluri N, Ambs S, Hussain P. Yang S, et al. Carcinogenesis. 2024 Aug 12;45(8):582-594. doi: 10.1093/carcin/bgae025. Carcinogenesis. 2024. PMID: 38629149 - Establishment of homotrimer collagen type I signature and its association with clinical manifestation and tertiary lymphoid structures formation in liver cancer.
Shen XT, Chen ZC, Wang XY, Wang XF, Xie SZ, Zheng X, Yang LY, Lu L. Shen XT, et al. Heliyon. 2024 May 17;10(11):e31320. doi: 10.1016/j.heliyon.2024.e31320. eCollection 2024 Jun 15. Heliyon. 2024. PMID: 38841477 Free PMC article. - Barriers and opportunities in pancreatic cancer immunotherapy.
Ju Y, Xu D, Liao MM, Sun Y, Bao WD, Yao F, Ma L. Ju Y, et al. NPJ Precis Oncol. 2024 Sep 12;8(1):199. doi: 10.1038/s41698-024-00681-z. NPJ Precis Oncol. 2024. PMID: 39266715 Free PMC article. Review. - The Macrophage Landscape Across the Lifespan of a Human Cardiac Allograft.
Li X, Turaga D, Li RG, Tsai CR, Quinn JN, Zhao Y, Wilson R, Carlson K, Wang J, Spinner JA, Hickey EJ, Adachi I, Martin JF. Li X, et al. Circulation. 2024 May 21;149(21):1650-1666. doi: 10.1161/CIRCULATIONAHA.123.065294. Epub 2024 Feb 12. Circulation. 2024. PMID: 38344825
References
- American Cancer Society, Cancer Facts & Figures 2021 (American Cancer Society, Atlanta, 2021).
- Neesse A., et al. , Stromal biology and therapy in pancreatic cancer. Gut 60, 861–868 (2011). - PubMed
- Bulle D. J., van der Merwe S., Van Cutsem E., Verslype C., van Pelt J. J., Relevance of the stroma in pancreatic ductal adenocarcinoma and its challenges for translational research. J. Cancer Treatment Diagn. 2, 1–15 (2017).
MeSH terms
Substances
Grants and funding
- P30 CA016087/CA/NCI NIH HHS/United States
- R35 CA210263/CA/NCI NIH HHS/United States
- CA210263/HHS | NIH | National Cancer Institute (NCI)
- R35 CA232124/CA/NCI NIH HHS/United States
- P01 CA117969/CA/NCI NIH HHS/United States
- CA232124/HHS | NIH | National Cancer Institute (NCI)
- T32 GM066704/GM/NIGMS NIH HHS/United States
- S10 OD021747/OD/NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous