PCSK9 Modulates Macrophage Polarization-Mediated Ventricular Remodeling after Myocardial Infarction - PubMed (original) (raw)
PCSK9 Modulates Macrophage Polarization-Mediated Ventricular Remodeling after Myocardial Infarction
Feifei Wang et al. J Immunol Res. 2022.
Abstract
Background and aims: An increasing number of high-risk patients with coronary heart disease (similar to acute myocardial infarction (AMI)) are using PCSK9 inhibitors. However, whether PCSK9 affects myocardial repair and the molecular mechanism of PCSK9 modulation of immune inflammation after AMI are not known. The present research investigated the role of PCSK9 in the immunomodulation of macrophages after AMI and provided evidence for the clinical application of PCSK9 inhibitors after AMI to improve cardiac repair.
Methods and results: Wild-type C57BL6/J (WT) and PCSK9-/- mouse hearts were subjected to left anterior descending (LAD) coronary artery occlusion to establish an AMI model. Correlation analysis showed that higher PCSK9 expression indicated worse cardiac function after AMI, and PCSK9 knockout reduced infarct size, improved cardiac function, and attenuated inflammatory cell infiltration compared to WT mice. Notably, the curative effects of PCSK9 inhibition were abolished after the systemic depletion of macrophages using clodronate liposomes. PCSK9 showed a regulatory effect on macrophage polarization in vivo and in vitro. Our studies also revealed that activation of the TLR4/MyD88/NF-_κ_B axis was a possible mechanism of PCSK9 regulation of macrophage polarization.
Conclusion: Our data suggested that PCSK9 modulated macrophage polarization-mediated ventricular remodeling after myocardial infarction.
Copyright © 2022 Feifei Wang et al.
Conflict of interest statement
The authors report no conflicts of interest.
Figures
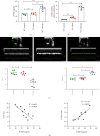
Figure 1
High expression of PCSK9 after acute myocardial infarction and the relationship between cardiac function. (a, b) Compared with WT control and WT sham group, the mice after AMI have a high level of PCSK9 protein and mRNA. (c) LVEF% and LVIDd measured by echocardiography 7 days after AMI, n = 6. (d) The correlation between the level of PCSK9 protein and cardiac function EF%, LVIDs in the mice after AMI, n = 10. ∗P < 0.05; ∗∗P < 0.01; ∗∗∗P < 0.001; ns: not significant.
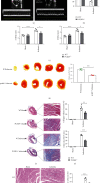
Figure 2
Inhibition of highly expressed PCSK9 reduced infarct size, inflammation, and myocardial fibrosis and improved cardiac function after 7 days of AMI. (a–c) Cardiac function measured by echocardiography, n = 5. (d) TTC staining showed the infarct size and quantitative analysis by ImageJ in each group, n = 5. (e) Masson staining for infarct size and myocardial fibrosis. Scale bar = 1 μ_m, n = 5. (f) Quantitative analysis by ImageJ for collagen volume fraction and percentage infarct size of hearts in (e). (g) HE staining for the infract regions in hearts. Scale bar = 50 μ_m, n = 5, and quantification of inflammatory cell infiltration. ∗_P < 0.05; ∗∗_P < 0.01; ∗∗∗P < 0.001; ns: not significant.
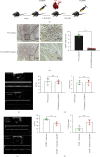
Figure 3
Systemic depletion of macrophages reduced the benefits of PCSK9 knockout in cardiac repair after myocardial infarction. (a) After being adaptively fed for 6 days, Cl2MDP or PBS were injected into the tail vein to systemically deplete macrophages. (b) Immunohistochemical staining for F4/80 expression in mouse hearts from Cl2MDP- and PBS-treated mice after myocardial infarction. Scale bar = 50 μ_m, n = 5. Quantitative analysis by ImageJ for F4/80+ cells of myocardium in (b). (c, d) Cardiac function measured by echocardiography after Cl2MDP and PBS treatment in the WT ischemia group, n = 4. (e, f) Cardiac function measured by echocardiography after Cl2MDP and PBS treatment in the PCSK9−/− ischemia group, n = 4. ∗_P < 0.05; ∗∗P < 0.01; ∗∗∗P < 0.001; ns: not significant.
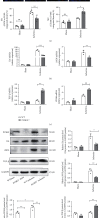
Figure 4
PCSK9 knockout inhibited M1 polarization and promoted M2 polarization in myocardial macrophages after infarction. (a) Representative immunofluorescence staining showing the percentages of M1 (F4/80+iNOS+CD206−) and M2 (F4/80+iNOS−CD206+) in WT/PCSK9−/− mouse myocardium after ischemia or sham. Nuclei were counterstained with DAPI. Scale bar = 50 _μ_m, n = 5. Quantitative analysis of the percentage of M1 and M2 macrophages of (a). (b, c) q-PCR analysis of IL-6, iNOS, TGF-β, and CD206 mRNA expression in WT/PCSK9−/− mouse myocardium after ischemia or sham, n = 3. (d) Representative images of Western blots for PCSK9, IL6, iNOS, and TGF-β in WT/PCSK9−/− mouse myocardium after ischemia or sham, n = 3.
Figure 5
In vitro, the exogenous PCSK9 protein induced inflammatory macrophages to acquire the M1 phenotype. The morphologic changes in macrophages stimulated by LPS/IL4. Cell shape changed from round to fusiform in LPS-stimulated RAW264.7 cells to ellipse in IL4-stimulated RAW264.7 cells. Scale bar = 50 μ_m. (b) 0.5 μ_g/mL PCSK9 protein significantly induced IL6 expression in RAW264.7 and have no effect on cell viability. (c) Representative flow cytometry plots showing the percentages of M1 (F4/80+/iNOS+/CD206−) and M2 (F4/80+/iNOS−/CD206+) phenotype in LPS/IL4-stimulated RAW264.7 cells after cocultivation with PCSK9 protein for 24 h, n = 3. Pooled flow cytometry data from (c). (e, f) q-PCR analysis of IL-6, iNOS, TGF-β, and CD206 mRNA expression in LPS/IL4-stimulated RAW264.7 cells after cocultivation with PCSK9 protein for 24 h, n = 3. (g) Representative images of Western blots for IL6, iNOS, and TGF-β in LPS/IL4-stimulated RAW264.7 cells after cocultivation with PCSK9 protein for 24 h, n = 3. Protein levels of IL6, iNOS, and TGF-β of (g). ∗_P < 0.05; ∗∗_P < 0.01; ∗∗∗P < 0.001; ns: not significant.
Figure 6
PCSK9 regulated M1 macrophage polarization by targeting TLR4. Representative images of Western blots for TLR4 and downstream MyD88/NF-_κ_B in WT/PCSK9−/− mouse myocardium after ischemia or sham, n = 3. (b) Protein levels of TLR4 and downstream MyD88/NF-_κ_B of (a). (c) Representative images of Western blots for TLR4 and downstream MyD88/NF-κ_B in LPS/IL4-stimulated RAW264.7 cells after cocultivation with PCSK9 protein for 24 h, n = 3. (d) Protein levels of TLR4 and downstream MyD88/NF-κ_B of (c). (e)TLR4 inhibitor (TAK242) was used to analyze whether TLR4 is involved in PCSK9-regulated macrophage polarization; (f) protein levels of IL6, iNOS, TLR4, and downstream MyD88/NF-κ_B of (e). ∗_P < 0.05; ∗∗_P < 0.01; ∗∗∗_P < 0.001; ns: not significant.
Similar articles
- PCSK9 expression in the ischaemic heart and its relationship to infarct size, cardiac function, and development of autophagy.
Ding Z, Wang X, Liu S, Shahanawaz J, Theus S, Fan Y, Deng X, Zhou S, Mehta JL. Ding Z, et al. Cardiovasc Res. 2018 Nov 1;114(13):1738-1751. doi: 10.1093/cvr/cvy128. Cardiovasc Res. 2018. PMID: 29800228 - Tissue factor cytoplasmic domain exacerbates post-infarct left ventricular remodeling via orchestrating cardiac inflammation and angiogenesis.
Chong SY, Zharkova O, Yatim SMJM, Wang X, Lim XC, Huang C, Tan CY, Jiang J, Ye L, Tan MS, Angeli V, Versteeg HH, Dewerchin M, Carmeliet P, Lam CSP, Chan MY, de Kleijn DPV, Wang JW. Chong SY, et al. Theranostics. 2021 Sep 3;11(19):9243-9261. doi: 10.7150/thno.63354. eCollection 2021. Theranostics. 2021. PMID: 34646369 Free PMC article. - Mesenchymal stromal cell-derived exosomes attenuate myocardial ischaemia-reperfusion injury through miR-182-regulated macrophage polarization.
Zhao J, Li X, Hu J, Chen F, Qiao S, Sun X, Gao L, Xie J, Xu B. Zhao J, et al. Cardiovasc Res. 2019 Jun 1;115(7):1205-1216. doi: 10.1093/cvr/cvz040. Cardiovasc Res. 2019. PMID: 30753344 Free PMC article. - Cardiac monocytes and macrophages after myocardial infarction.
Peet C, Ivetic A, Bromage DI, Shah AM. Peet C, et al. Cardiovasc Res. 2020 May 1;116(6):1101-1112. doi: 10.1093/cvr/cvz336. Cardiovasc Res. 2020. PMID: 31841135 Free PMC article. Review. - PCSK9: Associated with cardiac diseases and their risk factors?
Guo Y, Yan B, Tai S, Zhou S, Zheng XL. Guo Y, et al. Arch Biochem Biophys. 2021 Jun 15;704:108717. doi: 10.1016/j.abb.2020.108717. Epub 2020 Dec 9. Arch Biochem Biophys. 2021. PMID: 33307067 Review.
Cited by
- Proteomics and Lipidomics to unveil the contribution of PCSK9 beyond cholesterol lowering: a narrative review.
Gianazza E, Macchi C, Banfi C, Ruscica M. Gianazza E, et al. Front Cardiovasc Med. 2023 Jun 12;10:1191303. doi: 10.3389/fcvm.2023.1191303. eCollection 2023. Front Cardiovasc Med. 2023. PMID: 37378405 Free PMC article. Review. - The evolving landscape of PCSK9 inhibition in cancer.
Oza PP, Kashfi K. Oza PP, et al. Eur J Pharmacol. 2023 Jun 15;949:175721. doi: 10.1016/j.ejphar.2023.175721. Epub 2023 Apr 12. Eur J Pharmacol. 2023. PMID: 37059376 Free PMC article. Review. - Tregs delivered post-myocardial infarction adopt an injury-specific phenotype promoting cardiac repair via macrophages in mice.
Alshoubaki YK, Nayer B, Lu YZ, Salimova E, Lau SN, Tan JL, Amann-Zalcenstein D, Hickey PF, Del Monte-Nieto G, Vasanthakumar A, Martino MM. Alshoubaki YK, et al. Nat Commun. 2024 Aug 1;15(1):6480. doi: 10.1038/s41467-024-50806-y. Nat Commun. 2024. PMID: 39090108 Free PMC article. - Interorgan communication with the liver: novel mechanisms and therapeutic targets.
Zhao J, Zhang X, Li Y, Yu J, Chen Z, Niu Y, Ran S, Wang S, Ye W, Luo Z, Li X, Hao Y, Zong J, Xia C, Xia J, Wu J. Zhao J, et al. Front Immunol. 2023 Dec 12;14:1314123. doi: 10.3389/fimmu.2023.1314123. eCollection 2023. Front Immunol. 2023. PMID: 38155961 Free PMC article. Review. - The effect of macrophages and their exosomes in ischemic heart disease.
Wang M, Li C, Liu Y, Jin Y, Yu Y, Tan X, Zhang C. Wang M, et al. Front Immunol. 2024 May 10;15:1402468. doi: 10.3389/fimmu.2024.1402468. eCollection 2024. Front Immunol. 2024. PMID: 38799471 Free PMC article. Review.
References
- Zaid A., Roubtsova A., Essalmani R., et al. Proprotein convertase subtilisin/kexin type 9 (PCSK9): hepatocyte-specific low-density lipoprotein receptor degradation and critical role in mouse liver regeneration. Hepatology: official journal of the American Association for the Study of Liver Diseases . 2008;48(2):646–654. doi: 10.1002/hep.22354. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous