Succinic semialdehyde derived from the gut microbiota can promote the proliferation of adult T-cell leukemia/lymphoma cells - PubMed (original) (raw)
Succinic semialdehyde derived from the gut microbiota can promote the proliferation of adult T-cell leukemia/lymphoma cells
Nodoka Chiba et al. Heliyon. 2024.
Abstract
Adult T-cell leukemia/lymphoma (ATLL) is a refractory blood cancer with severe immunodeficiency resulting from retroviral infection. ATLL develops in only 5 % of HTLV-1-infected individuals, but the entire mechanism of ATLL progression remains unknown. Since recent studies have reported that the gut microbiome influences the progression of various diseases, we hypothesized that ATLL is also related to the gut microbiome and aimed to investigate this relationship. We analyzed the taxonomic and functional profiles of the gut microbiota of ATLL patients (n = 28) and HTLV-1-infected individuals (n = 37). We found that the succinic semialdehyde (SSA) synthesis pathway was significantly enriched in the gut microbiome of ATLL patients (P = 0.000682), and Klebsiella, whose abundance was significantly greater in ATLL patients and high-risk HTLV-1-infected individuals (P = 0.0326), was the main contributor to this pathway. Administration of SSAs to ATLL cell lines resulted in significant cell proliferation. Herein, we propose that the gut microbiome can regulate ATLL progression via metabolites.
© 2024 The Authors.
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:Takuji Yamada reports a relationship with Metagen Therapeutics Inc that includes: board membership. Takuji Yamada reports a relationship with Metagen Inc that includes: board membership. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
Fig. 1
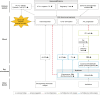
Overview of this study.
Fig. 2
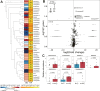
Comparison of the gut microbial community structure between patients with ATLL and asymptomatic HTLV-1 carriers. Blue, healthy controls; green, asymptomatic HTLV-1 carriers; red, patients with ATLL. (A) PCA plot of the subjects' gut microbiota taxonomic composition. (B) Variation in the relative abundance of the four dominant genera in the subjects' guts. P values were calculated by the two-tailed Brunner–Munzel test. (C) Relative abundance of the top 30 genera in patients with ATLL, asymptomatic HTLV-1 carriers, and healthy controls. A phylogenetic tree was constructed from 16S rRNA gene sequences. (D) Alpha diversity of the subjects' gut microbiota. P values were calculated by the two-tailed Mann‒Whitney U test. The Shannon index did not significantly differ among all the groups.
Fig. 3
Differential analysis of the abundance of each genus in the gut microbiota. A + HiC, a combined group of patients with ATLL and high-risk HTLV-1 carriers; H + C, a combined group of healthy controls and asymptomatic HTLV-1 carriers. (A) The distribution of P values for 46 bacterial genera with significant differences in abundance according to the two-tailed Brunner–Munzel test (left column, “BM”) and Kruskal‒Wallis test (right column, “KW”). Bacteria with significantly increased abundance compared to H + C are represented as red, and those with decreased abundance are represented as blue on the left side of the heatmap. The color intensity reflects the magnitude of the -log10 (P value). ∗, P < 0.05; ∗∗, P < 0.01. A phylogenetic tree was constructed from the V3-V4 region of the 16S rRNA gene sequences. (B) Comparison of the abundance of each bacterial genus between the A + HiC and H + C groups. The X-axis represents the ratio of the mean relative abundance of each group (fold change) transformed by log2; the Y-axis represents the P value transformed by -log10. The horizontal line represents P = 0.05. P values were calculated by the two-tailed Brunner–Munzel test. The plot size represents the prevalence of each bacterium (number of subjects) in the A + HiC group. (C) The relative abundance of seven genera was significantly greater in the A + HiC group (red) than in H + C group (blue). P values were calculated by the two-tailed Brunner–Munzel test.
Fig. 4
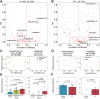
Correlations between tumor markers and the relative abundance of each bacteria. (A), (B) Distribution of Spearman correlation coefficients between sIL-2Ra levels and the relative abundance of each individual bacteria (X-axis) and the R2 score (Y-axis) for patients with ATLL and high-risk HTLV-1 carriers in cohort 1 (A) and cohort 2 (B). The bacterial names in the figure represent the bacterial genera whose abundance increased with the progression of ATLL identified in the previous section. Red plots represent bacteria that were found in five or more subjects; subjects had measurable bacterial abundance values and sIL-2Ra levels. A relative abundance of 0 was omitted before the correlation coefficient was calculation. (C), (D) The distribution of sIL-2Ra levels (X-axis) and relative abundance (Y-axis) of Eubacterium_G (left) and Klebsiella (right). The relative abundance values were converted by log10. Corr, Spearman's correlation coefficient: R2, coefficient of determination. The plot color represents ATLL subtypes: green, high-risk HTLV-1 carrier; yellow, smoldering type; pink, acute type; and cyan, lymphoma type. (C) Patients with ATLL and high-risk HTLV-1 carriers in cohort 1 and (D) patients with ATLL in cohort 2. (E), (F) The relative abundance of Eubacterium_G (left) and Klebsiella (right). The relative abundance values were converted by log10. P values were calculated by the two-tailed Brunner–Munzel test. (E) Cohort 1 and (F) cohort 2.
Fig. 5
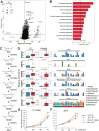
Gut microbial functional modules with altered abundance in the ATLL patient group. (A) Comparison of KO abundances between patients with ATLL and healthy controls. X-axis, fold change (log2-transformed) in the mean relative abundance of each bacterium in each group; Y-axis, -log10-transformed P values from pairwise comparisons. The horizontal line represents P = 0.05. P values were calculated by the two-tailed Brunner–Munzel test. The plot size shows the KO prevalence (number of subjects) in the ATLL patient group. (B) Enrichment analysis of KOs that were significantly more abundant in patients with ATLL. P values are transformed by -log10. The vertical line represents P = 0.05. ∗, q < 0.05. (C) SSA synthesis module. Abbreviations: HPC, homoprotocatechuate; CHMS, 5-carboxymethyl-2-hydroxymuconic-semialdehyde; CHM, 5-carboxymethyl-2-hydroxymuconate; OPET, 5-oxo-pent-3-ene-1,2,5-tricarboxylate; HHDD, 2-hydroxyhepta-2,4-diene-1,7-dioate; OHED, 2-oxo-hept-3-ene-1,7-dioate; HHED, 2,4-dihydroxy-hept-2-ene-1,7-dioate; SSA, succinic semialdehyde. (D) Association of KO abundance and genus Klebsiella with the SSA synthesis module. The box plot represents the relative abundance of the KOs (left) and ortholog genes assigned to the KO from Klebsiella (right). The bar plot represents the bacterial composition of the KOs of each subject (right). The top two genera with the highest average relative abundance in at least one KO are displayed. (E) (F) Cell growth rate. (E) IL-2-dependent ILT-Mat cells and (F) IL-2-independent MT-1 cells treated with SSA. The values represent the means ± s.d. ∗P < 0.05; unpaired two-tailed Student's _t_-test.
Fig. 6
Hypotheses and previous findings on the mechanism of development and progression of ATLL. We summarized our hypotheses and previous findings related to the development and progression of ATLL.
Similar articles
- Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.
Salisbury L, Baraitser L. Salisbury L, et al. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review. - Human placental cells are resistant to SARS-CoV-2 infection and replication.
Yoshida N, Thomas JR, Appios A, Brember MP, Aye ILMH, Edgar JR, Firth AE, Chung BYW, McGovern N, Stewart H. Yoshida N, et al. Wellcome Open Res. 2024 Nov 22;9:209. doi: 10.12688/wellcomeopenres.20514.2. eCollection 2024. Wellcome Open Res. 2024. PMID: 39640372 Free PMC article. - Public engagement to refine a whole-school intervention to promote adolescent mental health.
Bonell C, Hope S, Sundaram N, Lloyd-Houldey O, Michalopoulou S, Scott S, Nicholls D, Viner R. Bonell C, et al. Public Health Res (Southampt). 2024 Dec 4:1-22. doi: 10.3310/JWGT4863. Online ahead of print. Public Health Res (Southampt). 2024. PMID: 39636228 - Dynamic Field Theory of Executive Function: Identifying Early Neurocognitive Markers.
McCraw A, Sullivan J, Lowery K, Eddings R, Heim HR, Buss AT. McCraw A, et al. Monogr Soc Res Child Dev. 2024 Dec;89(3):7-109. doi: 10.1111/mono.12478. Monogr Soc Res Child Dev. 2024. PMID: 39628288 Free PMC article. - Interventions for preventing weight gain after smoking cessation.
Hartmann-Boyce J, Theodoulou A, Farley A, Hajek P, Lycett D, Jones LL, Kudlek L, Heath L, Hajizadeh A, Schenkels M, Aveyard P. Hartmann-Boyce J, et al. Cochrane Database Syst Rev. 2021 Oct 6;10(10):CD006219. doi: 10.1002/14651858.CD006219.pub4. Cochrane Database Syst Rev. 2021. PMID: 34611902 Free PMC article. Review.
References
- Raffelsberger N., Hetland M.A.K., Svendsen K., Småbrekke L., Löhr I.H., Andreassen L.L.E., Brisse S., Holt K.E., Sundsfjord A., Samuelsen Ø., et al. Gastrointestinal carriage of Klebsiella pneumoniae in a general adult population: a cross-sectional study of risk factors and bacterial genomic diversity. Gut Microb. 2021;13 doi: 10.1080/19490976.2021.1939599. - DOI - PMC - PubMed
- Allegra A., Innao V., Allegra A.G., Ettari R., Pugliese M., Pulvirenti N., Musolino C. Role of the microbiota in hematologic malignancies. Neth. J. Med. 2019;77:67–80. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials