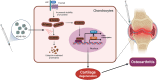
MSAB limits osteoarthritis development and progression through inhibition of β-catenin-DDR2 signaling - PubMed (original) (raw)
MSAB limits osteoarthritis development and progression through inhibition of β-catenin-DDR2 signaling
Ke Lu et al. Bioact Mater. 2024.
Abstract
The aberrant activation of the canonical Wnt/β-catenin signaling has been identified as a significant contributor to the pathogenesis of osteoarthritis (OA), exacerbating OA symptoms and driving OA progression. Despite its potential as a therapeutic target, clinical translation is impeded by the lack of a targeting delivery system and effective drug candidate that can modulate steady-state protein levels of β-catenin at post-translational level. Our study addresses these challenges by offering a new approach for OA treatment. To overcome these challenges, we introduced a novel delivery system using human serum albumin (HSA) to deliver a small molecule β-catenin inhibitor, Methyl-Sulfonyl AB (MSAB). This system is designed to enhance the bioavailability of MSAB, ensuring its accumulation inside the joint space, and facilitating the degradation of β-catenin protein. We have demonstrated that MSAB, when delivered via HSA, not only effectively inhibits cartilage damage but also ameliorates OA-related pain in an OA mouse model. We then performed proteomic analysis and biochemical studies to determine the molecular mechanisms underlying the therapeutic effects of MSAB. We identified that discoidin domain receptor 2 (DDR2), a critical mediator in OA pathology, is a downstream molecule of β-catenin signaling and β-catenin/TCF7 directly controls DDR2 gene transcription. MSAB suppressed the DDR2 expression in chondrocytes. MSAB ameliorated OA progression and OA-associated pain through inhibition of β-catenin-DDR2 signaling. This study underscores the efficacy of MSAB/HSA in OA treatment, providing new insights into its molecular mechanism of OA. It suggests that targeted therapies with MSAB/HSA could be a new OA management strategy.
Keywords: Cartilage degeneration; DDR2; MSAB; OA pain; Osteoarthritis; β-catenin.
© 2024 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
Graphical abstract
Fig. 1
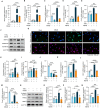
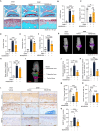
MSAB alleviates TNF-α-induced chondrocytes injury through inhibition of β-catenin signaling. (A–E) qRT-PCR was performed to examine changes in mRNA expression of Mmp3 (A), Mmp13 (B), Adamts4 (C), Adamts5 (D) and Col-X (E) in ATDC5 cells as indicated (n = 3). (F) Expression of MMP13, ADAMTS4 and ADAMTS5 proteins was detected by Western blotting in ATDC5 cells treated with TNF-α or MSAB as indicated (n = 3). (G–I) Representative IF images (G) showing active β-catenin (non-phosphorylated β-catenin) and total β-catenin expression. Quantification of fluorescence intensity of active β-catenin (H) and total β-catenin (I) in ATDC5 cells treated with TNF-α or MSAB (n = 6). (J–M) The mRNA expression of Runx2 (J), Axin2 (K), Dkk1 (L) and Ccnd1 (M) in ATDC5 cells was detected by qRT-PCR (n = 3). (N–Q) Representative immunoblots (N) and quantification of active β-catenin (O), AXIN2 (P), and MMP3 (Q) expression in ATDC5 cells treated with TNF-α or MSAB as indicated (n = 3). ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001, N.S.: no significant difference. Data are represented as mean ± SD. Statistical differences were analyzed by one-way ANOVA followed by the Tukey post-hoc test (A-E, H-M and O-Q).
Fig. 2
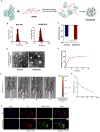
Serum albumin nanoparticles efficiently target chondrocytes. (A) A schematic diagram illustrating HSA loaded with MSAB. (B) The size distribution and (C) apparent Zeta potential of HAS-NPs and MSAB/HSA. (D) The representative images of transmission electron microscopy of HAS-NPs and MSAB/HSA (E) Cumulative drug release of MSAB delivered by HSA in PBS (n = 3) (F and G) Tracking HSA-NP-ICG after intra-articular injection with in vivo imaging (F) and quantification (G). HSA-NP-ICG was detected after intra-articular injection by in vivo imaging in the time points as indicated. (H) Representative IF images showing HSA-NP-ICG enter into the ATDC5 cells and are located in the cytoplasm. Purified HSA-NP were labeled with Purified from the dye ICG. Dil was used to stain the cell membranes. DAPI was used to stain the nuclei. Data were analyzed by two-tailed Student's _t_-test analysis (C).
Fig. 3
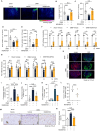
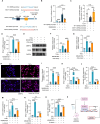
MSAB/HSA improves OA related pain in OA mouse model. (A–E) The representative images of the motion track record of the mice (A), average speed (B), distance (C), rearing duration (D), and climb duration (E) in mouse groups as indicated (n = 8). (F) The von Frey test showed the sensitivity to mechanical allodynia in the mice with Sham operation or DMM surgery treated with Vehicle or MSAB/HAS in the time points as indicated (n = 8). (G) The hotplate test showed the sensitivity to thermal hyperalgesia in the mice with Sham operation or DMM surgery treated with Vehicle or MSAB/HSA in the time points as indicated (n = 8). (H, I and J) Representative IF images (H) showed CGRP and NGF expression and quantitative fluorescence intensity of CGRP (I) and NGF (J) in dorsal root ganglia (DRG) in the mice with Sham operation or DMM surgery treated with Vehicle or MSAB/HSA (n = 6). (K) qRT-PCR was performed to examine changes in the relative mRNA expression of Ccl2 (K) in different groups of ATDC5 cells (n = 3) as indicated. (L) Secreted CCL2 level in ATDC-5 cell treated with TNF-α or MSAB/HSA as indicated (n = 4) (M–N) Representative IHC images (M) (scale bar 50 μm) showing expression of CCL2+ (N) cells in cartilage area of in the mice with Sham operation or DMM surgery treated with Vehicle or MSAB/HSA (n = 4). ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001, N.S.: no significant difference. Data are represented as mean ± SD. Statistical differences were analyzed by one-way (or two-way) ANOVA followed by the Tukey post-hoc test (B-G, I-K and N) and by two-tailed Student's _t_-test (L).
Fig. 4
MSAB/HSA alleviates cartilage degradation in DMM-induced OA mouse model. (A–F) Representative Safranin O/Fast green staining images (A) (scale bar 100 μm) and analysis of OARSI scores (B), cartilage area (C), synovitis scores (D), osteophyte size (E) and osteophyte maturity (F) in the mice with Sham operation or DMM surgery treated with Vehicle or MSAB/HSA (n = 8). (G and H) Micro-CT representative images (G), and bone volume of calcified meniscus and synovium (Purple markers) quantifications (H) in the mice with Sham operation or DMM surgery treated with Vehicle or MSAB/HSA (n = 5). (I) A schematic diagram showed the region of interest (ROI) selected to quantify the relevant indicators. ROI: medial subchondral bone (yellow); trabecular bone (green); cortical bone (pink). (J and K) Quantification of medial subchondral bone mineral density (J) and medial subchondral bone volume per tissue volume (K) of the mice with Sham operation or DMM surgery treated with Vehicle or MSAB/HSA (n = 6). (L–P) Representative IHC images (L) (scale bar 25 μm) showing expression of COL-2+ (M) cells, ACAN+ (N) area and MMP13+ (O) cells in cartilage area of in the mice with Sham operation or DMM surgery treated with vehicle or MSAB/HSA (n = 6). ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001, N.S.: no significant difference. Data are represented as mean ± SD. Statistical differences were analyzed by two-way ANOVA followed by the Tukey post-hoc test (B-F, H, J, K, M − O).
Fig. 5
MSAB decreases DDR2 expression in OA. (A–C) The number (A), location (B) and EggNOG analysis (C) of changed proteins in primary chondrocytes treated with TNF-α+MSAB/HSA vs TNF-α+Vehicle. (D)Volcano plot analysis showed DDR2 was down-regulated in primary chondrocytes treated with TNF-α+MSAB/HSA compare with the cells treated with TNF-α+Vehicle. (E) Fold changes in DDR2 expression in MSAB treated group compared with PBS treated group (n = 2 paired biological replicates). (F) qRT-PCR was performed to examine the relative Ddr2 mRNA levels in different groups of ATDC5 cells treated as indicated (n = 3). (G) Representative Western blot data of DDR2 expression in ATDC5 cells treated with MSAB. (G and H) Representative IHC images (H) (scale bar 25 μm) showing expression of DDR2+ (I) cells in the cartilage area of the mice with Sham operation or DMM surgery treated with Vehicle or MSAB/HSA (n = 6). ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001, N.S.: no significant difference. Data are represented as mean ± SD. Two group of data were analyzed by two-tailed Student's _t_-test (E). Comparisons of data with multiple groups were analyzed by two-way ANOVA followed by the Tukey post-hoc test (F and I).
Fig. 6
MSAB decreases DDR2 expression by inhibiting β-catenin signaling. (A) A schematic diagram indicated the mutation sites of DDR2 luciferase reporter constructs. (B and C) DDR2 luciferase activity of different groups as indicated, with the level in the blank group arbitrarily set to 1. (D) qRT-PCR was performed to examine the relative Mmp1_3 mRNA levels of different groups of ATDC5 cells as indicated (n = 3). (E, F and G) Representative immunoblots (E) and quantification of DDR2 (F) and MMP13 (G) expression in ATDC5 cells with CMV-DDR2 infection (n = 3). (H and I) Representative IF images (H) showing MMP13 and quantification of MMP13 fluorescence intensity (I) in ATDC5 cells with CMV-DDR2 infection (n = 6). (K and L) qRT-PCR was performed to examine changes in the relative mRNA expression of Mmp13 (K) and Adamts5 (L) in ATDC5 cells treated as indicated. (M–O) qRT-PCR was performed to examine changes in the relative mRNA expression of Ddr2 (M), Mmp13 (N) and Adamts4 (O) in ATDC5 cells treated as indicated (n = 3) (P) A schematic diagram showed the mechanism by which MSAB decreases cartilage degradation. ∗_P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001, N.S.: no significant difference Data are represented as mean ± SD. Statistical differences were analyzed by two-way ANOVA followed by the Tukey post-hoc test (B-D, F, G, I-L and M − O).
Similar articles
- MicroRNA-320c inhibits development of osteoarthritis through downregulation of canonical Wnt signaling pathway.
Hu S, Mao G, Zhang Z, Wu P, Wen X, Liao W, Zhang Z. Hu S, et al. Life Sci. 2019 Jul 1;228:242-250. doi: 10.1016/j.lfs.2019.05.011. Epub 2019 May 8. Life Sci. 2019. PMID: 31075235 - Discoidin domain receptor 2: An emerging pharmacological drug target for prospective therapy against osteoarthritis.
Kumar A, Dutta Choudhury M, Ghosh P, Palit P. Kumar A, et al. Pharmacol Rep. 2019 Jun;71(3):399-408. doi: 10.1016/j.pharep.2019.01.007. Epub 2019 Jan 15. Pharmacol Rep. 2019. PMID: 31003149 Review. - Verapamil protects against cartilage degradation in osteoarthritis by inhibiting Wnt/β-catenin signaling.
Takamatsu A, Ohkawara B, Ito M, Masuda A, Sakai T, Ishiguro N, Ohno K. Takamatsu A, et al. PLoS One. 2014 Mar 21;9(3):e92699. doi: 10.1371/journal.pone.0092699. eCollection 2014. PLoS One. 2014. PMID: 24658359 Free PMC article. - Inhibition of Wnt/β-catenin signaling ameliorates osteoarthritis in a murine model of experimental osteoarthritis.
Lietman C, Wu B, Lechner S, Shinar A, Sehgal M, Rossomacha E, Datta P, Sharma A, Gandhi R, Kapoor M, Young PP. Lietman C, et al. JCI Insight. 2018 Feb 8;3(3):e96308. doi: 10.1172/jci.insight.96308. eCollection 2018 Feb 8. JCI Insight. 2018. PMID: 29415892 Free PMC article. - Wnt/β-catenin Signaling in Osteoarthritis and in Other Forms of Arthritis.
Zhou Y, Wang T, Hamilton JL, Chen D. Zhou Y, et al. Curr Rheumatol Rep. 2017 Sep;19(9):53. doi: 10.1007/s11926-017-0679-z. Curr Rheumatol Rep. 2017. PMID: 28752488 Free PMC article. Review.
Cited by
- Cathepsin K Inhibitors as Potential Drugs for the Treatment of Osteoarthritis.
Brizuela L, Buchet R, Bougault C, Mebarek S. Brizuela L, et al. Int J Mol Sci. 2025 Mar 22;26(7):2896. doi: 10.3390/ijms26072896. Int J Mol Sci. 2025. PMID: 40243480 Free PMC article. Review. - Advancing Osteoarthritis Research: Insights from Rodent Models and Emerging Trends.
Rajalekshmi R, Agrawal DK. Rajalekshmi R, et al. J Orthop Sports Med. 2025;7(1):110-128. doi: 10.26502/josm.511500187. Epub 2025 Mar 14. J Orthop Sports Med. 2025. PMID: 40264810 Free PMC article.
References
- Tong L., Wijnen A.J.v., Wang H., Chen D. Advancing bone biology: the mutual promotion of biology and pioneering technologies. The Innovation Life. 2024;2(3)
- Xia C., Wang P., Fang L., Ge Q., Zou Z., Dong R., Zhang P., Shi Z., Xu R., Zhang L., Luo C., Ying J., Xiao L., Shen J., Chen D., Tong P., Jin H. Activation of beta-catenin in Col2-expressing chondrocytes leads to osteoarthritis-like defects in hip joint. J. Cell. Physiol. 2019;234(10):18535–18543. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials