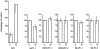Activation of the urokinase plasminogen activator/urokinase plasminogen activator receptor system and redistribution of E-cadherin are associated with hepatocyte growth factor-induced motility of pancreas tumor cells overexpressing Met - PubMed (original) (raw)
Activation of the urokinase plasminogen activator/urokinase plasminogen activator receptor system and redistribution of E-cadherin are associated with hepatocyte growth factor-induced motility of pancreas tumor cells overexpressing Met
R Paciucci et al. Am J Pathol. 1998 Jul.
Abstract
Because hepatocyte growth factor (HGF) is a potent mitogen for normal human exocrine pancreas cells (NPCs) in vitro, we have analyzed the expression of HGF and its receptor, Met, in NPC and pancreas cancer cells and studied its effects in vitro. Using immunohistochemistry, Northern blotting, and reverse transcription-polymerase chain reaction, we examined the expression of HGF and Met in normal pancreas and pancreas cancer. Scatter assays, wound-healing assays, and migration through transwell filters were used to study HGF-stimulated motility of IMIM-PC-2 cancer cells. In tumors, HGF is mainly detected in stromal cells, whereas Met is overexpressed in cancer cells with an unpolarized distribution. In vitro, HGF stimulates motogenesis but not proliferation in cancer cells. Cell motility is accompanied by a rapid decrease in the cytoskeleton-bound E-cadherin, an acceleration of cellular adhesion to the substrate, an up-regulation of urokinase plasminogen activator (u-PA) RNA and protein, and a change in the solubility and proteolysis of the u-PA receptor. Cell motility is significantly reduced by inhibitors of u-PA proteolytic activity such as antibodies neutralizing u-PA activity, plasminogen activator inhibitor 1, and amiloride. These results show that a paracrine loop of HGF activation may participate in the development or progression of pancreas cancer. In vitro, the HGF-stimulated motogenesis of pancreas cancer cells involves the activation of the u-PA/u-PA receptor proteolytic system, suggesting its role in the invasive stages of tumor progression.
Figures
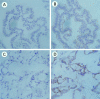
Figure 1.
Met expression in normal pancreas and pancreas cancer tissues. In normal pancreas ducts, Met is apically distributed (A and B). In pancreas cancers, Met is mainly distributed in the cytoplasm and is overexpressed (C and D). A and C: Preimmune rabbit serum; B and D: met-7 serum. Original magnification: ×400.
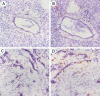
Figure 2.
HGF expression in normal pancreas and pancreas cancer tissues. In normal pancreas, few HGF-expressing cells are present in the mesenchyme surrounding the ducts (A and B). In pancreas cancer tissues, strong HGF expression is detected in stromal areas (C and D). A and C: Anti-HGF mouse mAb 10C11 preincubated with HGF (100 μg/ml); A through D: mAb 10C11. Original magnification: ×200.
Figure 3.
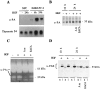
Met expression in pancreas cancer cell lines. A: met transcript levels in normal pancreatic tissue (NPT), NPCs, and pancreas cancer cell lines. In IMIM-PC-2 cells, expression was analyzed in preconfluent (p), 2 days postconfluent (2), and 10 days postconfluent cultures (10). Photographs of 28 S ribosomal RNA in ethidium bromide-stained gels are shown below each autoradiograph to normalize RNA deposits. B: Western blotting analysis of met using membrane fractions from preconfluent (p) and 10 days postconfluent (10) pancreas cancer cells. MKN45 and AsPC-1 cell membranes were obtained from preconfluent cultures. Equal amounts of protein were loaded in each lane (50 μg), and met was detected with mAb 19S. Two bands corresponding to 145 kd and 170 kd are detected. Normalization of protein deposits was performed by Ponceau red staining and wheat germ agglutinin lectin blotting (not shown).
Figure 4.
Effect of HGF on proliferation of NPC and pancreas cancer cells. Cells were plated in the absence (C) or in the presence (H) of HGF (10 ng/ml), and [3H]thymidine incorporation was determined as described in Materials and Methods. Results are expressed as the mean ± SD of triplicate cultures. Data are from one representative experiment of several performed.
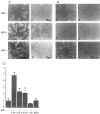
Figure 5.
HGF stimulates IMIM-PC-2 pancreas tumor cell motility through activation of u-PA. Motogenesis was evaluated by scattering assays (A), wound-healing assays (B), and migration through polycarbonate transwell filters (C), performed as described in Materials and Methods. Cells grown in DMEM supplemented with normal FBS (a, b, and c) or in DMEM containing plasminogen-depleted FBS (d, e, and f) were serum starved for 24 h and incubated with HGF (20 ng/ml) (HGF+) overnight (A and B) or for 48 h (C). Control cells were incubated in DMEM alone (HGF−) or DMEM supplemented with other compounds (Plg+; plasminogen was added at 2 μg/ml). Inhibition of the HGF-stimulated motility was evaluated by incubating cells with neutralizing rabbit anti-u-PA antibodies (150 μg/ml) (a u-PA+), PAI-1 (50 μg/ml) (PAI-1+), amiloride (0.2 mmol/L) (Am.), and EACA (50 mmol/L). A and B show an experiment representative of several performed with similar results; C, data from one of two independent experiments (in triplicate samples). Original magnifications: A, frames a through c, ×200; A, frames d through f, and B, ×100.
Figure 6.
HGF affects the levels of expression of u-PA and the localization of u-PAR in pancreas cells. Cells were serum starved for 24 h and treated with HGF (20 ng/ml) for the time periods shown, in the absence or in the presence of amiloride (Am) (0.2 mmol/L) or EACA (50 mmol/L), conditions not permissive for scatter. A: Northern blotting with total RNA (15 μg) from NPCs and IMIM-PC-2 pancreas cancer cells was hybridized to u-PA cDNA probe and subsequently to a thymosin β4 cDNA probe to normalize for RNA deposits. Filters were exposed for 48 h to autoradiography. B: Equal amounts of protein (50 μg) from cell lysates were analyzed by Western blotting with anti-u-PA antibody. C: Twenty-four hour conditioned medium (20 μl) from the same experiment shown in B was analyzed by gelatin zymography in the presence of plasminogen as a substrate. D: Equal amounts of 1% Triton X-100-soluble proteins (s) (50 μg) and equivalent amounts of the insoluble fractions (i) were analyzed by Western blotting using anti-u-PAR antibody. Mobility markers correspond to ovalbumin (53 kd) and carbonic anhydrase (35 kd).
Figure 7.
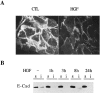
HGF affects the subcellular distribution of E-cadherin. Twenty-four hour serum-starved IMIM-PC-2 cells were treated with HGF (20 ng/ml) for different time periods. A: Unpermeabilized, 4% paraformaldehyde-fixed cells were incubated with anti-E-cadherin antibody. Rhodamine-coupled secondary antibody was used to reveal immunoreactivity, and photographs were taken by confocal microscopy. CTL, control cells; HGF, cells treated with HGF for 24 hours. B: Equal amounts of 1% Triton X-100-soluble (s) (50 μg) proteins and equivalent amounts of the insoluble (i) fractions from untreated or HGF-treated cells for the indicated time points were analyzed by Western blotting using anti-E-cadherin antibody.
Similar articles
- Simultaneous up-regulation of urokinase-type plasminogen activator (uPA) and uPA receptor by hepatocyte growth factor/scatter factor in human glioma cells.
Moriyama T, Kataoka H, Hamasuna R, Yoshida E, Sameshima T, Iseda T, Yokogami K, Nakano S, Koono M, Wakisaka S. Moriyama T, et al. Clin Exp Metastasis. 1999;17(10):873-9. doi: 10.1023/a:1006729611241. Clin Exp Metastasis. 1999. PMID: 11089886 - The geldanamycins are potent inhibitors of the hepatocyte growth factor/scatter factor-met-urokinase plasminogen activator-plasmin proteolytic network.
Webb CP, Hose CD, Koochekpour S, Jeffers M, Oskarsson M, Sausville E, Monks A, Vande Woude GF. Webb CP, et al. Cancer Res. 2000 Jan 15;60(2):342-9. Cancer Res. 2000. PMID: 10667586 - The plasminogen activator system in pancreas cancer: role of t-PA in the invasive potential in vitro.
Paciucci R, Torà M, Díaz VM, Real FX. Paciucci R, et al. Oncogene. 1998 Feb 5;16(5):625-33. doi: 10.1038/sj.onc.1201564. Oncogene. 1998. PMID: 9482108 - Regulation and interactions in the activation of cell-associated plasminogen.
Myöhänen H, Vaheri A. Myöhänen H, et al. Cell Mol Life Sci. 2004 Nov;61(22):2840-58. doi: 10.1007/s00018-004-4230-9. Cell Mol Life Sci. 2004. PMID: 15558213 Review. - Hepatocyte growth factor in invasive growth of carcinomas.
Desiderio MA. Desiderio MA. Cell Mol Life Sci. 2007 Jun;64(11):1341-54. doi: 10.1007/s00018-007-7050-x. Cell Mol Life Sci. 2007. PMID: 17415522 Free PMC article. Review.
Cited by
- Targeting HGF/c-MET Axis in Pancreatic Cancer.
Pothula SP, Xu Z, Goldstein D, Pirola RC, Wilson JS, Apte MV. Pothula SP, et al. Int J Mol Sci. 2020 Dec 1;21(23):9170. doi: 10.3390/ijms21239170. Int J Mol Sci. 2020. PMID: 33271944 Free PMC article. Review. - Simultaneous up-regulation of urokinase-type plasminogen activator (uPA) and uPA receptor by hepatocyte growth factor/scatter factor in human glioma cells.
Moriyama T, Kataoka H, Hamasuna R, Yoshida E, Sameshima T, Iseda T, Yokogami K, Nakano S, Koono M, Wakisaka S. Moriyama T, et al. Clin Exp Metastasis. 1999;17(10):873-9. doi: 10.1023/a:1006729611241. Clin Exp Metastasis. 1999. PMID: 11089886 - Molecular mechanism of pancreatic cancer--understanding proliferation, invasion, and metastasis.
Mihaljevic AL, Michalski CW, Friess H, Kleeff J. Mihaljevic AL, et al. Langenbecks Arch Surg. 2010 Apr;395(4):295-308. doi: 10.1007/s00423-010-0622-5. Epub 2010 Mar 18. Langenbecks Arch Surg. 2010. PMID: 20237938 Review. - Invasion and metastasis in pancreatic cancer.
Keleg S, Büchler P, Ludwig R, Büchler MW, Friess H. Keleg S, et al. Mol Cancer. 2003 Jan 22;2:14. doi: 10.1186/1476-4598-2-14. Mol Cancer. 2003. PMID: 12605717 Free PMC article. Review.
References
- Gherardi E, Stoker M: Hepatocytes and scatter factor. Nature 1991, 346:228 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous