World Gastroenterology Organisation (WGO) (original) (raw)
Global Guardian of Digestive Health. Serving the World.
World Gastroenterology Organisation Global Guidelines
Irritable Bowel Syndrome:
a Global Perspective
Update September 2015


Review team
Eamonn M.M. Quigley (USA, Chair)
Michael Fried (Switzerland)
Kok-Ann Gwee (Singapore)
Igor Khalif (Russia)
Pali Hungin (United Kingdom)
Greger Lindberg (Sweden)
Zaigham Abbas (Pakistan)
Luis Bustos Fernandez (Argentina)
Shobna J. Bhatia (India)
Max Schmulson (Mexico)
Carolina Olano (Uruguay)
Anton Le Mair (The Netherlands)
 Contents
Contents
(Click to expand section)
1. WGO Cascades
With this guideline, the World Gastroenterology Organisation (WGO) is aiming to guide health providers in the best management of irritable bowel disease (IBS) through a concise document with recommendations based on the latest evidence and resulting from our global expert consensus process based on best current practice.
A standardized, global approach to the diagnosis and management of IBS may not be feasible, since neither the epidemiology nor the clinical presentation of the condition, nor the availability of diagnostic or therapeutic resources, are sufficiently uniform throughout the world to support the provision of a single, gold standard approach.
This Global WGO Guideline, therefore, includes a set of “cascades” to provide context-sensitive and resource-sensitive options for the diagnosis and management of IBS. The WGO cascades are intended to serve as a “global” complement to, rather than a replacement for, the “gold standard” guidelines produced by regional groups and national societies. With their diagnostic and treatment cascades, WGO guidelines provide a resource-sensitive and context-sensitive approach.
WGO cascades: a hierarchical set of diagnostic, therapeutic, and management options for dealing with risk and disease, ranked by the resources available.
WGO guidelines and cascades are intended to highlight appropriate, context-sensitive and resource-sensitive management options for all geographical areas, regardless of whether they are considered to be “developing,” “semi-developed,” or “developed.” WGO cascades are context-sensitive and the context is not necessarily defined solely by resource availability.
N.B.: The context in which the following cascades were constructed is described in the relevant sections on the diagnosis and management of IBS.
1.1 Cascade options for resource-sensitive IBS diagnosis
High resource levels
- History, physical examination, exclusion of alarm symptoms, consideration of psychological factors.
- Full blood count (FBC), erythrocyte sedimentation rate (ESR) or C-reactive protein (CRP), stool studies (white blood cells, ova, parasites, occult blood).
- Selenium homocholic acid taurine (tauroselcholic acid) test (SeHCAT; incorporating selenium-75) for the investigation of bile acid malabsorption and measurement of bile acid pool loss. This test may have limited availability, even in areas with high resources.
- Thyroid function.
- Tissue transglutaminase (TTG) antibody to screen for celiac disease.
- Esophagogastroduodenoscopy (EGD) and distal duodenal biopsy in patients with diarrhea, to rule out celiac disease, tropical sprue, giardiasis, and in patients in whom abdominal pain and discomfort is located more in the upper abdomen.
- Colonoscopy and biopsy.*
- Fecal inflammation marker (e.g., calprotectin or lactoferrin) to distinguish IBS from inflammatory bowel disease where the latter is prevalent.
- Hydrogen breath test for lactose intolerance and small-intestinal bacterial overgrowth (SIBO).
Medium resource levels
- History, physical examination, exclusion of alarm symptoms, consideration of psychological factors
- FBC, ESR or CRP, stool studies, thyroid function
- Sigmoidoscopy*
Low resource levels
- History, physical examination, exclusion of alarm symptoms, consideration of psychological factors
- FBC, ESR, and stool examination
* N.B.: Even in “wealthy” countries, not all patients need colonoscopy, which should be reserved in particular for those with alarm symptoms or signs and those over the age of 50. The need for investigations and for sigmoidoscopy and colonoscopy, in particular, should also be dictated by the characteristics of the patient (presenting features, age, etc.) and the geographical location (i.e., whether or not in an area of high prevalence for inflammatory bowel disease, celiac disease, colon cancer, or parasitosis). One could argue, for example, that a 21-year-old woman with symptoms of IBS with diarrhea and no alarm features merits, at most, celiac serology and thyroid evaluation (where appropriate). In general, the diagnosis is “safer” in patients with constipation, whereas in patients with severe diarrhea, there is a greater need to consider tests to exclude organic pathology.
1.2 Cascade options for resource-sensitive IBS management
High resource levels
- Reassurance, dietary and lifestyle review, and counseling.
- Try a quality probiotic with proven efficacy.
- Symptomatic treatment of:
- Pain, with a locally available antispasmodic; for more severely affected patients, a low-dose tricyclic antidepressant or selective serotonin reuptake inhibitor (SSRI) should be added.
- Constipation with dietary measures and fiber supplementation, progressing to osmotic laxatives such as lactulose.
- Although the evidence to support their use is weak, it may be worth addressing diarrhea with simple antidiarrheals.
- Psychological approaches (hypnotherapy, psychotherapy, group therapy) should be considered and consultation with a dietitian, where indicated.
- Add specific pharmacological agents, where approved:
- Lubiprostone or linaclotide for IBS with constipation (IBS-C)
- Rifaximin for diarrhea and bloating
- Alosetron and eluxadoline for IBS with diarrhea (IBS-D)
Medium resources
- Reassurance, dietary and lifestyle review, and counseling.
- Add a quality probiotic with proven efficacy.
- Symptomatic treatment of:
- Pain, with a locally available antispasmodic; for more severely affected patients, a low-dose tricyclic antidepressant should be added.
- Constipation with dietary measures and fiber supplementation.
- Although the evidence to support their use is weak, it may be worth addressing diarrhea with bulking agents and simple antidiarrheals.
Low resources
- Reassurance, dietary and lifestyle review, and counseling.
- Symptomatic treatment of:
- Pain, with a locally available antispasmodic.
- Constipation, with dietary measures and fiber supplementation.
- Although the evidence to support their use is weak, it may be worth addressing diarrhea with bulking agents and simple antidiarrheals.
2. Introduction
Irritable bowel syndrome is a relapsing functional bowel disorder defined by symptom-based diagnostic criteria, in the absence of detectable organic causes. The symptomatic array is not specific for IBS, as such symptoms may be experienced occasionally by almost every individual. To distinguish IBS from transient gut symptoms, experts have underscored the chronic and relapsing nature of IBS and have proposed diagnostic criteria based on the occurrence rate of symptoms and their duration.
Definition. Irritable bowel syndrome (IBS) is a functional bowel disorder in which abdominal pain or discomfort is associated with defecation and/or a change in bowel habit. Sensations of discomfort (bloating), distension, and disordered defecation are commonly associated features. In some languages, the words “bloating” and “distension” may be represented by the same term.
Some characteristics of IBS are:
- It is not known to be associated with an increased risk for the development of cancer or inflammatory bowel disease, or with increased mortality.
- It generates significant direct and indirect health-care costs.
- No universal pathophysiological substrate has been demonstrated in IBS.
- Visceral hypersensitivity is generally accepted as being relevant to IBS [1].
- A transition of IBS to, and overlap with, other symptomatic gastrointestinal disorders (e.g., gastroesophageal reflux disease, dyspepsia, and functional constipation) may occur.
- The condition usually causes long-term symptoms:
- These may occur in episodes.
- Symptoms vary and are often associated with food intake and, characteristically, with defecation.
- Symptoms interfere with daily life and social functioning in many patients.
- Symptoms sometimes seem to develop as a consequence of an intestinal infection (postinfectious IBS) or to be precipitated by major life events, or occur during a period of considerable stress.
- Symptoms may develop following abdominal and/or pelvic surgery.
- Symptoms may be precipitated by antibiotic treatment.
2.1 IBS subclassification
According to the Rome III criteria, IBS may be subtyped or subclassified on the basis of the patient’s stool characteristics, as defined by the Bristol Stool Scale:
- IBS with diarrhea (IBS-D):
- Loose stools > 25% of the time and hard stools < 25% of the time
- Up to one-third of cases
- More common in men
- IBS with constipation (IBS-C):
- Hard stools > 25% of the time and loose stools < 25% of the time
- Up to one-third of cases
- More common in women
- IBS with mixed bowel habits or cyclic pattern (IBS-M):
- Both hard and soft stools > 25% of the time
- One-third to one-half of cases
- Un-subtyped IBS
- Insufficient abnormality of stool consistency to meet criteria IBS-C or M
It must be remembered, however, that:
- Patients commonly transition between these subtypes.
- The symptoms of diarrhea and constipation are commonly misinterpreted in IBS patients. Thus, many IBS patients who complain of “diarrhea” are referring to the frequent passage of formed stools and, in the same patient population, “constipation” may refer to any one of a variety of complaints associated with the attempted act of defecation and not simply to infrequent bowel movements.
- In addition, bowel habit must be evaluated without using antidiarrheals or laxatives.
On clinical grounds, other sub-classifications may be developed:
- Based on symptoms:
- IBS with predominant bowel dysfunction
- IBS with predominant pain
- IBS with predominant bloating
- Based on precipitating factors:
- Postinfectious (PI-IBS)
- Food-induced (meal-induced)
- Stress-related
However, with the exception of PI-IBS, which is quite well characterized, the relevance of any of these other classifications to the prognosis or response to therapy in patients with IBS remains to be defined.
It must also be remembered that the Rome III criteria are not commonly used in clinical practice. Furthermore, cultural issues may inform symptom reporting. In India, for example, a patient who reports straining or passing hard stools (often with a feeling of incomplete evacuation) is likely to complain of constipation even if he or she passes stools more than once daily.
There is considerable overlap and a tendency to transition between IBS-C and functional constipation.
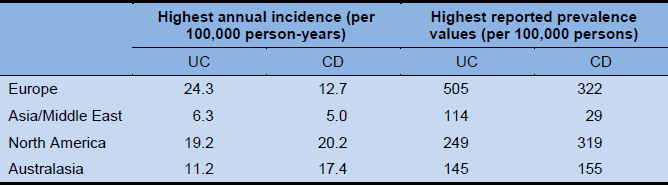
2.2 Global prevalence and incidence
The global picture of the prevalence of IBS is far from complete, as no data are available from several regions. In addition, comparisons of data from different regions are often problematic due to the use of different diagnostic criteria (in general, the “looser” the criteria, the higher the prevalence), as well as the influence of other factors such as population selection, the inclusion or exclusion of comorbid disorders (e.g., anxiety), access to health care, and cultural influences. In Mexico, for example, the prevalence of IBS in the general population, measured using the Rome II criteria, was 16%, but the figure increased to 35% among individuals in a university-based community. What is remarkable is that the available data suggest that the prevalence is quite similar across many countries, despite substantial lifestyle differences.
- The prevalence of IBS in Europe and North America is estimated to be 10–15%. In Sweden, the most commonly cited figure is 13.5%.
- The prevalence of IBS is increasing in countries in the Asia–Pacific region, particularly in those with developing economies. Estimates of the prevalence of IBS (using the Rome II diagnostic criteria) vary widely in the Asia–Pacific region. Studies from India showed that the Rome I criteria for IBS identified more patients than the Rome II criteria. Reported prevalence rates included 0.82% in Beijing, 5.7% in southern China, 6.6% in Hong Kong, 8.6% in Singapore, 14% in Pakistan, and 22.1% in Taiwan. A study in China found that the prevalence of IBS, as defined by the Rome III criteria, in individuals attending outpatient clinics was 15.9%.
- Generally, data from South America are scarce, but this may be related to a publication bias, as many studies are not published in English [2] or are not cited in commonly used search databases (e.g., Medline). In Uruguay, for example, one study reported an overall prevalence of 10.9% (14.8% in women and 5.4% in men)—58% with IBS-C and 17% with IBS-D. In 72% of the cases, the age of onset was < 45 years. Also, a study from Venezuela reported an IBS prevalence of 16.8%, with 81.6% of those affected being women and 18.4% men [3]. Studies on indigenous populations in Latin America revealed a high prevalence of IBS, which was similar to that in the rest of the population [4].
- Data from Africa are very scarce. A study in a Nigerian student population found a 26.1% prevalence, based on the Rome II criteria. A study among outpatients in the same country, based on the same criteria, reported a prevalence of 33%.
2.3 Other observations on IBS epidemiology
- IBS mainly occurs between the ages of 15 and 65 years.
- The first presentation of patients to a physician is usually in the 30–50-year-old age group.
- In some cases, symptoms may date back to childhood.
- The prevalence is greater in women—although this result is not reproduced in some studies from India, for example.
- There is a decrease in reporting frequency among older individuals.
- The estimated prevalence of IBS in children is similar to that in adults.
- Typical IBS symptoms are common in “healthy” population samples.
2.4 IBS demographics, East–West differences in presenting features
- As in the case of prevalence data, global information regarding presenting features also varies, and comparisons of studies based on community data, outpatient clinic data, and hospital statistics are fraught with difficulties.
- Typical IBS symptoms are common in healthy population samples, but the majority of sufferers with IBS are not actually medically diagnosed. This may explain apparent differences between countries in the reported prevalence. Most studies only count diagnosed IBS and not community prevalence.
- A study in China showed that the prevalence of IBS in south China was higher than that reported in Beijing, but lower than that reported in Western countries.
- Some studies in non-Western countries indicate:
- A close association between marked distress and IBS in men, in a manner similar to that found in women in Western studies.
- Greater frequency of upper abdominal pain.
- Lower impact of defecatory symptoms on a patient’s daily life.
- Several studies suggest that among African-Americans, in comparison to their white compatriots:
- Stool frequency is lower.
- The prevalence of constipation is higher.
- In Latin America, except in Argentina, constipation predominance is more frequent than diarrhea predominance.
- Stool frequency appears to be greater in the Indian community as a whole—99% passed stools once or more per day.
- In Mexico, 70% of patients have anxiety, 46% depression, and 40% both.
- In Mexico, IBS has a significant economic impact, as it leads to high use of medical resources.
- Clinical overlap between functional dyspepsia and IBS, defined according to the Rome III criteria, is very common in China. However, this may be related to the fact that IBS patients in that country commonly report their pain as being located in the epigastric region and not in the lower abdomen.
- Psychological distress, life events, and negative coping style may play important roles in the pathogenesis of IBS. These factors may also influence the individual’s illness behavior and the clinical outcome.
3. Diagnosis of IBS
3.1 Clinical history
Although it is currently described as a single coherent entity, it is most likely that the disorder termed “IBS” comprises a number of discrete pathophysiological entities, which have not as yet been defined. Thus, a number of pathological processes that we now recognize as quite distinct entities (microscopic colitis, carbohydrate intolerance, and bile acid malabsorption, for example) would formerly have been included within IBS.
In assessing the patient with IBS, it is important not only to consider the primary presenting symptoms, but also to identify precipitating factors and other associated gastrointestinal and extragastrointestinal symptoms. It is vital also to seek out and directly question for the presence of alarm symptoms and to consider, in the relevant context, other explanations for the patient’s symptoms (e.g., bile acid diarrhea, carbohydrate intolerance, microscopic colitis). Thus, the history is critical and involves both the identification of those features regarded as typical of IBS and also the recognition of “red flags,” or other features that suggest alternative diagnoses. Accordingly, the patient should be asked about the following (features marked with an asterisk* are compatible with IBS):
The pattern of abdominal pain or discomfort:
- Chronic duration.*
- Type of pain: intermittent* or continuous.
- Previous pain episodes.*
- Location of pain. In some individuals, pain may be well-localized (to the lower left quadrant of the abdomen, for example), while in others the pain location tends to move around.
- Relief with defecation or passing of flatus.*
- Nocturnal pain is unusual in IBS and is considered a warning sign.
Other abdominal symptoms:
- Bloating*
- Distension*
- Borborygmi
- Flatulence
N.B.: Distension can be measured; bloating is a subjective feeling. As defined in English, bloating and distension may not share the same pathophysiology and should not be regarded as equivalent and interchangeable terms, although in other languages they may be represented by a single word, or there may be no expression for bloating, as in Spanish. Nor does either necessarily imply that intestinal gas production is increased.
Nature of the associated bowel disturbance:
- Constipation
- Diarrhea
- Alternation
Abnormalities of defecation:
- Diarrhea for > 2 weeks (N.B.: one should always strive to understand exactly what the patient means by “diarrhea” and “constipation”)
- Mucus in the feces
- Urgency of defecation
- Feeling of incomplete defecation/evacuation (this symptom has been reported as particularly important in recent studies in Asian populations—51% in Singapore, 71% in India, 54% in Taiwan)
Other information from the patient’s history and important warning signs:
- Unintended weight loss
- Blood in stool
- Family history of:
- Colorectal malignancy
- Celiac disease
- Inflammatory bowel disease
- Fever accompanying lower abdominal pain
- Relation to menstruation
- Relation to:
- Drug therapy
- Consumption of foods that are known to cause intolerance (especially milk), artificial sweeteners, dieting products, or alcohol
- Visiting the (sub-)tropics
- Abnormal eating habits:
- Irregular or inadequate meals
- Insufficient fluid intake
- Excessive fiber intake
- Obsession with dietary hygiene
- Family history of IBS: IBS clearly aggregates within families, although its genetics are poorly understood and the mode of transmission is unclear.
- Nature of onset (sudden onset in relation to exposure to gastroenteritis suggests PI-IBS)
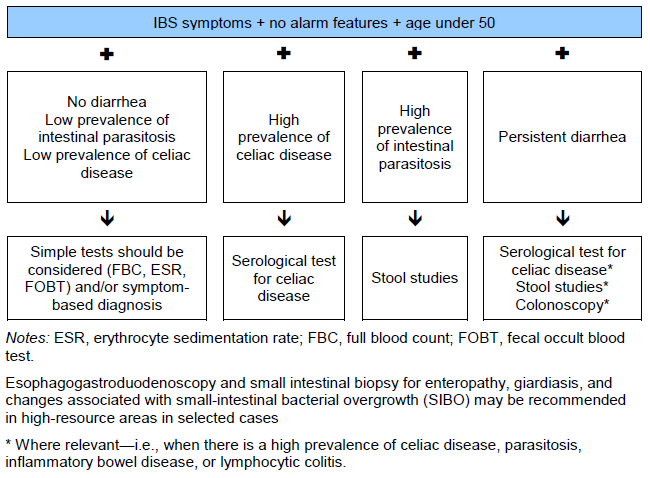
- Persistent diarrhea: the presence of persistent true diarrhea, especially if relatively painless, should prompt more extensive investigations for other causes of diarrhea, such as celiac disease, microscopic colitis (especially in a middle-aged or older woman), bile-acid diarrhea (due to impaired absorption of bile acids) or carbohydrate intolerance.
3.2 Psychological assessment
Psychological factors have not been shown to cause or influence the onset of IBS. IBS is not a psychiatric or psychological disorder. However, psychological factors may:
- Play a role in the persistence and perceived severity of abdominal symptoms.
- Contribute to impairment of quality of life and excessive use of health-care services.
For these reasons, coexisting psychological conditions are common in referral centers and may include:
- Anxiety
- Depression
- Somatization
- Hypochondriasis
- Symptom-related fears
- Catastrophizing
The following may be useful in providing an objective assessment of psychological features:
- Hospital Anxiety and Depression Scale (HADS). This is a simple 14-item questionnaire for measuring the level of anxiety and depression.
- The Sense of Coherence (SOC) test can be used to identify patients with a low SOC who respond to cognitive behavioral therapy.
- The Patient Health Questionnaire (PHQ-15). This is a 15-item questionnaire that helps identify the presence of multiple somatic symptoms (somatization). The PHQ-15 should be validated in a given country before it is used in clinical practice in that location.
3.3 Physical examination
- A physical examination reassures the patient and helps detect possible organic causes.
- A general examination is carried out for signs of systemic disease.
- Abdominal examination:
- Inspection
- Palpation
- Auscultation
- Examination of the perianal region:
- Digital rectal examination
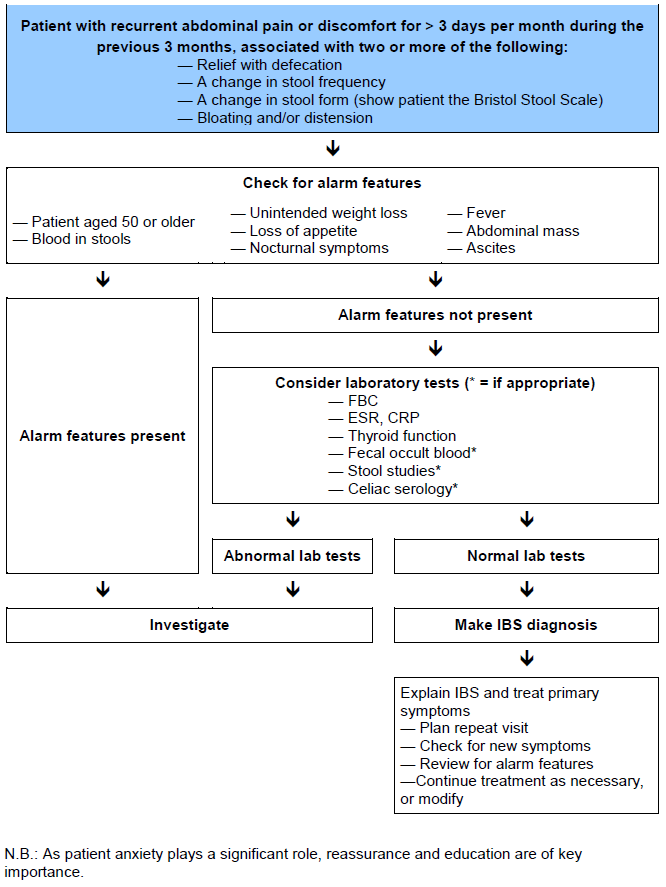
3.4 IBS diagnostic algorithm
Fig. 1 Algorithm for diagnosing irritable bowel syndrome (IBS).
4. Evaluation of IBS
A diagnosis of IBS is usually suspected on the basis of the patient’s history and physical examination, without additional tests. Confirmation of the diagnosis of IBS requires the confident exclusion of organic disease in a manner dictated by an individual patient’s presenting features and characteristics. In many instances (e.g., in young patients with no alarm features), a secure diagnosis can be made on clinical grounds alone.
There is a lack of robust evidence and prospective studies regarding the appropriate use of radiological imaging in patients with IBS-like symptoms [5].
4.1 Diagnostic criteria (Rome III)
Table 1 Rome III criteria for diagnosing IBS
A systematic review (2012) of the diagnostic criteria for IBS demonstrated low validity and utilization of the Rome III criteria, and suggested that the Manning criteria were more widely validated and may be more clinically applicable [6]. It is now 24 years since the first Rome meeting, and there have been several changes to the Rome criteria defining IBS. The upcoming Rome IV version should become available in 2016.
In clinical practice, whether in the setting of primary or specialist care, clinicians usually base a diagnosis of IBS on their evaluation of the whole patient (often over time) and consider a multiplicity of features that support the diagnosis (apart from pain and discomfort associated with defecation, or change in stool frequency or form).
Symptoms common in IBS and supportive of the diagnosis:
- Bloating
- Abnormal stool form (hard and/or loose)
- Abnormal stool frequency (less than three times per week or over three times per day)
- Straining at defecation
- Urgency
- Feeling of incomplete evacuation
- Passage of mucus per rectum
Behavioral features helpful in recognizing IBS in general practice:
- Symptoms present for > 6 months
- Stress aggravating symptoms
- Frequent consultations for nongastrointestinal symptoms
- History of previous medically unexplained symptoms
- Aggravation after meals
- Associated anxiety and/or depression
Noncolonic complaints that often accompany IBS:
- Dyspepsia—reported in 42–87% of IBS patients
- Nausea
- Heartburn
Associated non-gastrointestinal symptoms:
- Lethargy, fatigue
- Backache and other muscle and joint pains
- Fibromyalgia
- Headache
- Urinary symptoms:
- Nocturia
- Frequency and urgency of micturition
- Incomplete bladder emptying
- Dyspareunia, in women
- Insomnia
- Low tolerance to medications in general
4.2 Additional tests or investigations
In the majority of cases of IBS, no additional tests or investigations are required. An effort to keep investigations to a minimum is recommended in straightforward cases of IBS, and especially in younger individuals.
Additional tests or investigations should be considered if warning signs (“red flags”) are present:
- Onset of symptoms after 50 years of age
- Short history of symptoms
- Change in the bowel habit pattern
- Unintended weight loss
- Nocturnal symptoms
- Family history of colon cancer, celiac disease, inflammatory bowel disease
- Anemia
- Rectal bleeding
- Recent antibiotic use
- Abdominal/rectal masses
- Raised inflammatory markers
- Fever
The following tests (although commonly performed) are indicated only if supported by the clinical history and where locally relevant:
- Full blood counts
- Serum biochemistry
- Thyroid function tests
- Stool testing for occult blood and ova and parasites
Additional tests or investigations may also be considered if:
- The patient has persistent symptoms or is anxious despite treatment.
- A major qualitative change in chronic symptoms has occurred.
- A new coexisting condition should be considered.
4.3 Differential diagnosis
Bile acid malabsorption
- Adult-onset bile acid malabsorption (BAM) is now recognized as an important cause of an IBS-D–type presentation. A recent review study [7] found evidence that more than 25% of patients with IBS-D have bile acid malabsorption.
- Etiologic factors that appear to contribute to the onset and persistence of chronic diarrhea symptoms are alterations in the enterohepatic circulation, accelerated intestinal transit, an increase in the bile acid pool, and low levels of fibroblast growth factor-19 (FGF19) [8].
- Diagnostic tools that help in diagnosing BAM and differentiating it from IBS-D are assays of fecal bile acid concentration, 23-seleno-25-homo-taurocholic acid (SeHCAT) testing, and high-performance liquid chromatography for serum 7-α-OH-4-cholesten-3-one (C4)—in addition to the use of therapeutic trials (with the bile acid sequestering agents cholestyramine and colesevelam), and heightened awareness of the likelihood of bile acid malabsorption [9].
Celiac disease
Main symptoms and/or findings:
- Chronic diarrhea
- Failure to thrive (in children)
- Fatigue
- Estimated to affect approximately 1% of all Indo-European wheat-eating populations
- Should be considered in the differential diagnosis in regions of high prevalence [10]
N.B.: Many patients with celiac disease do not have classical features and may present with “IBS-type” symptoms, including bloating and constipation, along with iron deficiency. A low threshold for investigation should therefore be maintained in high-prevalence regions (those with a prevalence > 1% in the general population).
Lactose intolerance
Main symptoms and/or findings:
- Symptoms (bloating, flatulence, diarrhea) acutely related to consumption of milk and dairy products.
- Although genetic testing can now detect lactase deficiency, this is not necessarily predictive of intolerance, which is best tested using the lactose hydrogen breath test. Indeed, a substantial proportion of individuals who lack lactase can tolerate oral lactose despite bacterial fermentation.
In countries with a high prevalence of lactase deficiency, inappropriately labeling IBS patients as lactose-intolerant should be avoided, unless they are consuming substantial amounts of milk and/or milk products, as this could deprive the community of a cheap nutritious source of protein and nutrition in countries such as India. In all parts of the world, the prevalence of lactose malabsorption on breath tests has been consistently similar between IBS and non-IBS subjects.
Inflammatory bowel disease (Crohn’s disease, ulcerative colitis)
Main symptoms and/or findings:
- Significant variations in prevalence worldwide.
- Diarrhea has persisted for > 2 weeks.
- Rectal bleeding.
- Inflammatory mass, weight loss, perianal disease, fever.
- In areas in which it is endemic, intestinal tuberculosis should also be considered, as its presentation may be similar to that of inflammatory bowel disease (IBD): diarrhea, weight loss, abdominal distension, and fevers.
Colorectal carcinoma
Main symptoms and/or findings:
- Older patients who develop IBS-type symptoms for the first time
- Passage of blood in the feces
- Unintended weight loss
- Pain may be of an obstructive type for left-sided lesions
- Anemia or iron deficiency for right-sided lesions
Microcytic (lymphocytic and collagenous) colitis
- Accounts for 20% of unexplained diarrhea in patients over the age of 70
- Typically painless
- Most common in middle-aged females (M : F = 1 : 15)
- Diagnosed on colonic biopsies
Acute or chronic diarrhea due to protozoa or bacteria
Main symptoms and/or findings:
- Acute onset of diarrhea
- Stool examination or duodenal biopsy
A review [11] on the role of intestinal protozoa in IBS concluded that there was “a possible role for protozoan parasites, such as Blastocystis hominis and _Dientamoeba fragilis_” in the etiology of IBS.
- Dientamoeba fragilis is known to cause IBS-like symptoms and has a propensity to cause chronic infections. It can be detected using nested polymerase chain reaction (PCR) [12], where available, or alternatively using microscopy.
- The role of B. hominis as an etiological agent in IBS remains unclear, due to contradictory reports and the controversial nature of B. hominis as a human pathogen. The role of B. hominis may be genotype-related [13].
- Although Entamoeba histolytica infections occur predominantly in developing regions of the world, the clinical diagnosis of amebiasis is often difficult, as symptoms in patients with IBS may closely mimic those in patients with nondysenteric amebic colitis.
- Clinical manifestations of Giardia intestinalis infection also vary from asymptomatic carriage to acute and chronic diarrhea with abdominal pain.
While stool testing for Giardia and Amoeba is recommended in India, self-medication with imidazoles is common, rendering the results difficult to interpret.
N.B.: It is essential that all patients with IBS in relevant areas should undergo parasitological investigations in order to rule out the presence of protozoan parasites. It is equally important that these tests are appropriately interpreted and that overtreatment is avoided.
Small-intestinal bacterial overgrowth (SIBO)
- SIBO is rare unless the patient has a primary or secondary motility disorder, has been operated on (in particular with ileocecal resection or bariatric surgery), or has impaired immunity (such as immunoglobulin A deficiency).
- The classical features of SIBO are those of maldigestion and malabsorption.
- Some of the symptoms of SIBO (bloating, diarrhea) overlap with those of IBS, which has led to the suggestion that SIBO is related to IBS. However, it is generally believed that SIBO is not a common cause of IBS-like symptoms.
Tropical sprue
- Tropical sprue should be considered in returning travelers with persistent diarrhea.
- The symptoms and histologic findings of tropical sprue may resemble those of celiac disease (CD). A diagnosis of CD is unlikely in the absence of anti-endomysium or anti-tissue transglutaminase antibodies, but conversely their absence increases the likelihood of tropical sprue [14].
Diverticulitis
The relationship between IBS and so-called “painful diverticular disease” is unclear; is painful diverticular disease no more than IBS in a patient who has diverticula? In diverticulitis, the classical symptoms and/or findings are episodic and acute to subacute during an episode, featuring:
- Left-sided abdominal pain
- Fever
- Tender inflammatory mass in the left lower quadrant
However, it is now evident that afflicted patients may have more chronic symptoms in between discrete episodes/attacks, and that left-sided and bilateral, but not right-sided diverticular disease, may increase the risk for IBS [15].
Endometriosis
Main symptoms and/or findings:
- Cyclical lower abdominal pain
- Enlarged ovaries or nodules dorsal to the cervix (on digital vaginal examination)
Pelvic inflammatory disease
Main symptoms and/or findings:
- Chronic lower abdominal pain
- Fever
- Upward pressure pain or adnexal tenderness and swollen adnexa (on digital vaginal examination)
Ovarian cancer
In women over the age of 40, ovarian cancer should be considered in the differential diagnosis. In one survey, the following symptoms were more common among women with ovarian cancer:
- Increased abdominal girth
- Bloating
- Urinary urgency
- Pelvic pain
The combination of bloating, increased abdominal girth, and urinary symptoms was found in 43% of women with ovarian cancer, but in only 8% of a control population.
Other considerations for the differential checklist
- Colitis associated with nonsteroidal anti-inflammatory drugs (NSAIDs). This may account for diarrhea in elderly patients who are receiving treatment from neurologists and rheumatologists.
4.4 Comorbidity with other diseases
Patients with overlap syndromes tend to have more severe IBS.
- Fibromyalgia in 20–50% of IBS patients (although there is no evidence of this in China, for example)
- IBS is common in several other chronic pain disorders:
- Present in 51% of patients with chronic fatigue syndrome
- Temporomandibular joint disorder: 64%
- Chronic pelvic pain: 50%
- Nonulcer dyspepsia, biliary dyskinesia
In a meta-analysis, the prevalence of biopsy-proven celiac disease was found to be more than four times higher in patients who met the diagnostic criteria for IBS than in control individuals without IBS [16].
There is a significantly higher prevalence of chronic idiopathic constipation (CIC) in patients with IBS. Distinguishing between IBS-C and CIC may be difficult in clinical practice; several recent studies have called into question the appropriateness and feasibility of creating what appears to be an artificial division between these two functional gastrointestinal disorders [17].
The prevalence of gastroesophageal reflux-type symptoms in patients with IBS is four times higher than in those without IBS. There is an overlap between the two conditions in up to 25% of individuals. It is recommended that when physicians encounter patients with symptoms of IBS, they should routinely screen for coexistent gastroesophageal reflux symptoms [18].
Symptoms compatible with IBS have been reported to be significantly higher in patients with inflammatory bowel disease (IBD) in comparison with non-IBD controls, even among those thought to be in remission. IBS-type symptoms were also found to be significantly more common in patients with Crohn’s disease (CD) than in those with ulcerative colitis (UC), and in those with active disease [19]. Of course, a diagnosis of IBS would not be appropriate in a patient with active IBD.
5. Management of IBS
5.1 Introduction
Figure 2 provides a general outline of a management scheme for patients presenting with IBS-type symptoms.
Fig. 2 Management of patients with symptoms of irritable bowel syndrome.
Given that there is no general agreement on the cause of IBS, it comes as no surprise that no single treatment is currently regarded throughout the world as being universally applicable to the management of all IBS patients.
Given also the common association between IBS symptoms and such factors as diet, stress, and psychological factors, attention should be given to adopting measures that may alleviate, if not eliminate, such precipitants. Dietary differences between different countries and ethnic groups would be expected to have a significant influence on the prevalence of symptoms of IBS, but little information is available.
Recent data on disturbances in the intestinal flora (microbiota) in IBS, as well as the suggestion mentioned above (albeit a controversial one) that SIBO may be a factor, have spurred interest in novel approaches: probiotics, prebiotics, and antibiotics. Recent meta-analyses confirm a role for probiotics in IBS, but also make it clear that the effects of probiotics in IBS, as elsewhere, are highly strain-specific. Variability and the formulation of specific strains vary dramatically around the world. For example, Bifidobacterium infantis 35624, which currently has the best evidence base for efficacy in IBS, is at present available only in the United States, Canada, the United Kingdom, and Ireland. Issues of quality control also continue to complicate recommendations in this area.
IBS patients commonly have recourse to a variety of alternative/complementary therapies throughout the world. In India (in Ayurvedic medicine) and China, for example, herbal remedies are widely available and commonly used for IBS. However, their efficacy is difficult to assess, as the concentrations of active ingredients vary considerably depending on the extraction process. Few “alternative” therapies have been subjected to the rigors of a randomized trial in IBS.
A recent systematic review, although noting limitations related to trial design in many instances, provided evidence to support the use of antidepressants (both tricyclic antidepressants and selective serotonin reuptake inhibitors, SSRIs) in IBS.
Nonpharmacological factors are often ignored, but are of paramount importance in the management of IBS. The physician–patient relationship is critical and should include attention to the following, both during the initial assessment and in the subsequent follow-up:
- Identifying and exploring the patient’s concerns. A positive patient–physician relationship should be established, with the patient’s symptoms and distress being accepted as real.
- Appreciating the impact of symptoms.
- Discussing the patient’s anxieties related to symptoms and possible diagnoses, with the aim being to eliminate unnecessary worries.
- Identifying and helping to resolve stressful factors.
- Reducing avoidance behavior. Patients may avoid activities that they fear are causing the symptoms, but avoidance behavior has a negative influence on the prognosis.
- General guidance on diet and activity: fiber-rich diets (where appropriate), regular mealtimes, intake of sufficient fluids, and sufficient physical activity may have (general) beneficial effects, but, with the exception of fiber (see below), there is no adequate proof that these directly influence the outcome in IBS.
5.2 Diet
Specialized diets may improve symptoms in some IBS patients [20].
Fibers
- A fiber-rich diet or a bulk-former (e.g., psyllium) combined with sufficient intake of fluids would appear to be a logical approach in IBS, but the general status of fiber in IBS is not straightforward [20]. Insoluble fibers may exacerbate symptoms and provide little relief—adverse events and bloating, distension, flatulence and cramping, in particular, may limit the use of insoluble fiber, especially if increases in fiber intake are not introduced gradually. Soluble fibers such as psyllium (ispaghula), on the other hand, provide relief in IBS [21].
- Diets low in fermentable oligo-, di-, monosaccharides and polyols (FODMAPs) reduce abdominal pain and bloating, and improve the stool pattern [21], but long-term outcomes and the safety of low-FODMAP diets remain to be demonstrated. It is also still unclear whether the low-FODMAP intervention diet is beneficial to all IBS patients [21].
- Although they are widely employed, especially in North America and Europe, the status of wheat-free or gluten-free diets in IBS is uncertain.
Probiotics
Some probiotics provide global relief of symptoms in IBS, and others alleviate individual symptoms such as bloating and flatulence [20,22]. However, the duration of these benefits and the nature of the most effective species are not clear [23]. The efficacy of probiotics is difficult to interpret, as different strains, doses, formulations, and methods of delivery have been used in various studies [21]. Furthermore, most randomized controlled studies of probiotics in IBS have been of short duration, have not used an appropriate study design, and have not adequately reported adverse events [22].
There is at present insufficient evidence for a general recommendation of prebiotics or synbiotics in patients with IBS [20]. A recent consensus statement provides guidance on the use of specific probiotics in the management of IBS [24].
5.3 Drug therapy
A variety of agents are used throughout the world for the treatment of individual symptoms in IBS, as follows:
- Antispasmodics for pain.
- Laxatives, fiber, and bulking agents for constipation. The chloride-channel agonist lubiprostone (2 × 8 μg/day) has been approved by the Food and Drugs Administration (FDA) in the United States for chronic constipation and constipation-predominant IBS, and the guanylate cyclase agonist linaclotide has been approved in the United States for chronic constipation and constipation-predominant IBS and in several European countries for constipation-predominant IBS. The precise positioning of such agents in the overall management of IBS remains to be established.
- Fiber, bulking agents, and anti-diarrheals for diarrhea. Very recently, the poorly absorbable antibiotic rifaximin (at a dosage of 550 mg t.i.d. for 14 days) and eluxadoline, a mu opioid receptor agonist and delta opioid receptor antagonist, were approved in the United States for diarrhea-predominant IBS.
- Charcoal resins, antiflatulents, and other agents are widely used, although without supporting evidence, for bloating, distension, and flatulence.
It is important to note that the range of agents available and their formulations vary considerably between countries, and it is imperative that the prescribing physician be knowledgeable regarding the efficacy and risk profile of any agent that he or she is about to prescribe, rather than extrapolating from evidence derived from other agents in the same class or agents that have similar modes of action.
Overall symptoms—first-line therapy
- Certain antispasmodics (otilonium, hyoscine, cimetropium, pinaverium, dicyclomine and mebeverine) provide symptomatic short-term relief in IBS. Adverse events are more common with antispasmodics than with a placebo [20].
- Peppermint oil is superior to placebo in improving IBS symptoms [20,25]. The risk of adverse events is no greater with peppermint oil than with a placebo [20].
Overall symptoms—second-line therapy
- Laxatives.
- Antidiarrheals.
- Tricyclic antidepressants (TCAs) and selective serotonin reuptake inhibitors (SSRIs) are effective for symptom relief in IBS [20,21,26]. Adverse effects are common, with drowsiness and dizziness the most common [26], and may limit patient tolerance [20]. TCAs are associated with significant adverse effects in treating IBS-D and should be avoided in IBS-C; clinicians should expect one adverse effect for every three patients who benefit from therapy [27].
- SSRIs may be considered in resistant IBS-C, although it is not currently recommended that SSRIs should be routinely prescribed for IBS in patients without comorbid psychiatric conditions, because of conflicting and limited data regarding efficacy, safety, and long-term outcomes [28].
Overall symptoms—other therapeutic options
- Rifaximin is effective in reducing overall symptoms in IBS-D [20,29]. Rifaximin may be considered as a second-line therapy [21]. Older patients and women were found to have higher response rates [29]. Rifaximin is well tolerated [30], but its efficacy and safety have not been established beyond 16 weeks [29]. However, re-treatment efficacy and safety has been recently reported [31]. It has also been reported that 846 patients benefit for each adverse effect [27].
- Alosetron is useful for second-line therapy of IBS-D [20,21]. However, it has been associated with an increased risk of ischemic colitis and may cause severe constipation [21]. Clinicians should expect one adverse effect for every three patients who benefit from therapy [27].
- Lubiprostone is safe and effective for treatment of IBS-C [20,27]. Nausea has been the major side effect limiting use.
- Linaclotide is safe and effective for treatment of IBS-C [20,32,33]. Diarrhea is the major adverse effect of linaclotide; further studies are needed to evaluate its long-term efficacy and safety [33].
- There is insufficient evidence to recommend loperamide for use in IBS [20].
- Mixed 5-HT4 agonists/5-HT3 antagonists are no more effective than placebo at improving symptoms of IBS-C [20].
- Renzapride and cisapride have no benefit in IBS [34].
- There is no evidence that polyethylene glycol (PEG) improves overall symptoms in patients with IBS, but it may relieve constipation [20].
- Ondansetron was found to improve urgency, diarrhea, and bloating in IBS-D, but did not provide any benefits in relation to pain. Ramosetron, where available, should also be considered as second-line therapy in IBS-D; it has also been shown to be effective in IBS-D and appears to be devoid of serious adverse effects such as severe constipation and ischemic colitis [21].
Specific symptoms—pain
- If an analgesic is required, paracetamol is preferable to nonsteroidal anti-inflammatory drugs (NSAIDs). Opiates are to be avoided at all costs, as dependence and addiction are a significant risk in such a chronic condition. NSAIDs and opiates also have undesirable side effects on the gastrointestinal tract.
- The probiotic strain Bifidobacterium infantis 35624 (one capsule per day) has been shown to reduce pain, bloating, and defecatory difficulty and to normalize stool habit in IBS patients, regardless of predominant bowel habit, but is currently available only in the United States, Canada, the United Kingdom and Ireland.
- Antispasmodics:
- The availability of compounds varies tremendously throughout the world.
- Antispasmodics, including peppermint oil, are still considered to represent a first-line treatment for abdominal pain in patients with IBS [21].
- Tricyclic antidepressants—e.g.:
- Amitriptyline, with a starting dose of 10 mg/day, target dose 25–50 mg/day, at bedtime.
- Desipramine, starting dose 50 mg/day, target dose 100–150 mg/day, at bedtime.
- These tend to be constipating and should be avoided among constipated patients.
- Selective serotonin reuptake inhibitors (SSRIs)—e.g.:
- Paroxetine, 10–60 mg/day.
- Citalopram, 5–20 mg/day.
- Linaclotide reduces abdominal pain in IBS-C [21].
- There is no evidence that PEG improves pain [20], but it improves constipation-related symptoms in patients with IBS-C.
Specific symptoms—constipation
- For remarks on a fiber-rich diet or bulk-former, see section 5.2 above.
- The probiotic strain Bifidobacterium lactis DN-173010 has been shown to accelerate gastrointestinal transit and to increase stool frequency among IBS patients with constipation.
- Osmotic laxatives are often useful; few have been formally tested in IBS.
- Lubiprostone:
- For the treatment of IBS with constipation in women aged 18 and over.
- To be taken twice a day in 8-μg doses with food and water.
- Improves the stool pattern in laxative-resistant IBS-C [21].
- Linaclotide:
- For the treatment of IBS with constipation in women aged 18 and over.
- To be taken once daily in a dose of 290 μg 30 minutes before food.
Specific symptoms—diarrhea
- Loperamide (2 mg every morning or twice a day) is no more effective than a placebo in reducing pain, bloating, and global symptoms of IBS, but it is an effective agent for the treatment of diarrhea, reducing stool frequency and improving stool consistency. Because of the lack of effects on pain, the cardinal symptom of IBS, there is insufficient evidence to recommend loperamide for use in IBS [20].
- Alosetron, a 5-hydroxytryptamine-3 (5-HT3) receptor antagonist:
- Indicated only for women with severe IBS-D with symptoms > 6 months and no response to antidiarrheal agents. May rarely cause ischemic colitis.
- Eluxadoline and rifaximin have recently been approved in the United States for IBS-D; it is difficult, at this early stage, to define their position in IBS management.
Specific symptoms—bloating and distension
- Diets that produce less gas, such as the low-FODMAP diet, may be helpful in some patients.
- There is no evidence to support the use of activated charcoal-containing products, “antiflatulents,” simethicone, and other agents in IBS.
- Probiotics: some specific strains, such as Bifidobacterium lactis DN-173010 and the probiotic cocktail VSL#3, have clinical trial evidence of efficacy for bloating, distension, and flatulence. Others, such as Bifidobacterium infantis 35624, reduce bloating as well as the other cardinal symptoms of IBS.
- Antibiotic treatment with rifaximin 3 × 550 mg/day has been shown to reduce bloating in some IBS patients. In countries where the 550 mg preparation is not available, 3 × 400 mg/day may be used. Older patients and women have been found to have higher response rates [29]. Rifaximin is well tolerated and it has now been shown to be safe and effective when re-treating patients who have relapsed after a first effective treatment [31].
5.4 Psychological and other treatments
General nonpharmacological recommendations
- Discuss the patient’s anxieties. This reduces complaints; aim to eliminate unnecessary worries.
- Aim to reduce avoidance behavior. Patients may avoid activities that they fear are causing the symptoms, but avoidance behavior has a negative influence on the prognosis.
- Discuss fear of cancer.
- Discuss and aim to resolve stressful factors.
- Regular mealtimes, the intake of sufficient fluids, and sufficient physical activity may have (general) beneficial effects, but there is no adequate proof that these influence IBS.
Psychological interventions
Apart from the general approaches described above for governing the conduct of the doctor–patient relationship in IBS, more formal psychological interventions may be contemplated in certain circumstances and depending on the availability of appropriate resources and expertise. Such approaches may include:
- Cognitive behavioral therapy (CBT), in group or individual sessions. CBT has shown excellent results, but its limited availability and labor-intensive nature limit routine use [21,26]. Behavioral techniques are aimed at modifying dysfunctional behaviors through:
- Relaxation techniques
- Contingency management (by rewarding healthy behavior)
- Assertion training
- Hypnosis: Gut-directed hypnosis should be recommended for patients with IBS refractory to conventional (drug) treatment [35]. It has a high level of safety and tolerability, and there is evidence of sustained efficacy, in contrast to drug therapy [35]. It should be offered by licensed hypnotherapists with specialist training in the technique [35]. Group treatment is more time-efficient than individual sessions and at least as effective [35]. Daily practice by patients, supported by audio recordings, boosts efficacy; training and experiences should regularly be discussed with patients [35]. However, there is limited evidence from randomized controlled trials (RCTs). Future RCTs are needed that use strict diagnostic criteria, have follow-up periods of at least 1 year, and include newly diagnosed and treatment-resistant patients [36]. The limited availability and labor-intensive nature of hypnotherapy limits routine use [21].
The American College of Gastroenterology (ACG) Task Force [37] concluded that psychological therapies, including cognitive therapy, dynamic psychotherapy, and hypnotherapy, but not relaxation therapy, are more effective than usual care in relieving the global symptoms of IBS. However, Ford et al. [20] found that the quality of evidence was very low and that the results were only slightly superior to usual care or waiting-list control. With the exception of a single study, these therapies have not been shown to be superior to placebo. The sustainability of their effect is questionable.
With regard to herbal therapies and acupuncture, the ACG Task Force concluded that the available randomized controlled trials, mostly testing unique Chinese herbal mixtures, appeared to show a benefit. It was not possible to combine these studies into a meaningful meta-analysis, however, and overall, any benefit of Chinese herbal therapy in IBS continues to be potentially confounded by the variable components used and their purity. Also, there are significant concerns about toxicity, especially liver failure, with the use of any Chinese herbal mixture. A systematic review of trials of acupuncture was inconclusive due to heterogeneous outcomes. Further research is needed before any recommendations on acupuncture or herbal therapy can be made.
5.5 Prognosis
For most patients with IBS, symptoms are likely to persist, but not worsen. Symptoms will deteriorate in a smaller proportion, and some patients will recover completely.
Factors that may negatively affect the prognosis include:
- Avoidance behavior related to IBS symptoms
- Anxiety about certain medical conditions
- Impaired function as a result of symptoms
- A long history of IBS symptoms
- Chronic ongoing life stress
- Psychiatric comorbidity
Approaches by the physician that positively affect the treatment outcome:
- Acknowledging the disease
- Educating the patient about IBS
- Reassuring the patient
5.6 Follow-up
In mild cases, there is generally no medical need for follow-up consultations in the long term, unless:
- Symptoms persist, with considerable inconvenience or dysfunction.
- The patient is seriously worried about the condition.
- Persistent diarrhea > 2 weeks.
- Constipation persists and does not respond to therapy.
- Warning signs for possibly serious gastrointestinal disease developing:
- Rectal bleeding
- Anemia
- Unintended weight loss
- Family history of colon cancer
- Fever
- A major change in the symptom pattern
- One should beware of eating disorders developing:
- Most patients with IBS try some form of dietary manipulation.
- This can lead to nutritionally inadequate diets or ingestion of abnormal amounts of fruit, caffeine, dairy products, and dietary fiber.
- The tendency for eating disorders to develop is more common in female IBS patients.
6. Appendix: useful resources
- 2014 American College of Gastroenterology monograph on the management of irritable bowel syndrome and chronic idiopathic constipation:
Ford AC, Moayyedi P, Lacy BE, Lembo AJ, Saito YA, Schiller LR, et al. American College of Gastroenterology monograph on the management of irritable bowel syndrome and chronic idiopathic constipation. Am J Gastroenterol 2014;109 Suppl 1:S2–26; quiz S27. doi: 10.1038/ajg.2014.187. - 2012 British Dietetic Association evidence-based guidelines for the dietary management of irritable bowel syndrome in adults:
McKenzie YA, Alder A, Anderson W, Wills A, Goddard L, Gulia P, et al. British Dietetic Association evidence-based guidelines for the dietary management of irritable bowel syndrome in adults. J Hum Nutr Diet 2012;25:260–74. doi: 10.1111/j.1365-277X.2012.01242.x. - 2010 Asian consensus (Asian Neurogastroenterology and Motility Association) on irritable bowel syndrome:
Gwee KA, Bak YT, Ghoshal UC, Gonlachanvit S, Lee OY, Fock KM, et al. Asian consensus on irritable bowel syndrome. J Gastroenterol Hepatol 2010;25:1189–205. doi: 10.1111/j.1440-1746.2010.06353.x. - 2007 British Society of Gastroenterology guidelines on mechanisms and practical management in irritable bowel syndrome:
Spiller R, Aziz Q, Creed F, Emmanuel A, Houghton L, Hungin P, et al. Guidelines on the irritable bowel syndrome: mechanisms and practical management. Gut 2007;56:1770–98. Erratum in: Gut 2008;57:1743. doi: 10.1136/gut.2007.119446.
References
- Azpiroz F, Bouin M, Camilleri M, Mayer EA, Poitras P, Serra J, et al. Mechanisms of hypersensitivity in IBS and functional disorders. Neurogastroenterol Motil 2007;19(1 Suppl):62–88.
- Porras R, López-Colombo A, Schmulson M. Increase in Mexican and Latin American scientific articles on irritable bowel syndrome. Rev Gastroenterol Mex 2015;80:228–35.
- Veitia G, Pernalete B, Cachima L, Manuitt J, La Cruz M, Da Farias A, et al. Prevalencia del síndrome intestino irritable en la población adulta venezolana. Rev GEN 2013;67:139–44.
- Bujanda L, Gutiérrez-Stampa MA, Caballeros CH, Alkiza ME. [Gastrointestinal disorders in Guatemala and their relation with parasitic infections]. An Med Interna 2002;19:179–82.
- O’Connor OJ, McSweeney SE, McWilliams S, O’Neill S, Shanahan F, Quigley EMM, et al. Role of radiologic imaging in irritable bowel syndrome: evidence-based review. Radiology 2012;262:485–94.
- Dang J, Ardila-Hani A, Amichai MM, Chua K, Pimentel M. Systematic review of diagnostic criteria for IBS demonstrates poor validity and utilization of Rome III. Neurogastroenterol Motil 2012;24:853–e397.
- Slattery SA, Niaz O, Aziz Q, Ford AC, Farmer AD. Systematic review with meta-analysis: the prevalence of bile acid malabsorption in the irritable bowel syndrome with diarrhoea. Aliment Pharmacol Ther 2015;42:3–11.
- Barkun AN, Love J, Gould M, Pluta H, Steinhart H. Bile acid malabsorption in chronic diarrhea: pathophysiology and treatment. Can J Gastroenterol 2013;27:653–9.
- Wedlake L, A’Hern R, Russell D, Thomas K, Walters JRF, Andreyev HJN. Systematic review: the prevalence of idiopathic bile acid malabsorption as diagnosed by SeHCAT scanning in patients with diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther 2009;30:707–17.
- Olano C, Rodriguez X, Aleman A, Rodriquez N, Pigni S, Cabrera G, et al. P1461/ Functional gastrointestinal disorders (FD/IBS/etc.) Symptoms of irritable bowel syndrome in celiac patients; relationship to disease activity [poster presentation]. J Gastroenterol Hepatol 2013;28(Suppl 3):574.
- Stark D, van Hal S, Marriott D, Ellis J, Harkness J. Irritable bowel syndrome: a review on the role of intestinal protozoa and the importance of their detection and diagnosis. Int J Parasitol 2007;37:11–20.
- Sarafraz S, Farajnia S, Jamali J, Khodabakhsh F, Khanipour F. Detection of Dientamoeba fragilis among diarrheal patients referred to Tabriz health care centers by nested PCR. Trop Biomed 2013;30:113–8.
- Yakoob J, Jafri W, Beg MA, Abbas Z, Naz S, Islam M, et al. Irritable bowel syndrome: is it associated with genotypes of Blastocystis hominis? Parasitol Res 2010;106:1033–8.
- Langenberg MCC, Wismans PJ, van Genderen PJJ. Distinguishing tropical sprue from celiac disease in returning travellers with chronic diarrhoea: a diagnostic challenge? Travel Med Infect Dis 2014;12:401–5.
- Yamada E, Inamori M, Uchida E, Tanida E, Izumi M, Takeshita K, et al. Association between the location of diverticular disease and the irritable bowel syndrome: a multicenter study in Japan. Am J Gastroenterol 2014;109:1900–5.
- Ford AC, Chey WD, Talley NJ, Malhotra A, Spiegel BMR, Moayyedi P. Yield of diagnostic tests for celiac disease in individuals with symptoms suggestive of irritable bowel syndrome: systematic review and meta-analysis. Arch Intern Med 2009;169:651–8.
- Suares NC, Ford AC. Prevalence of, and risk factors for, chronic idiopathic constipation in the community: systematic review and meta-analysis. Am J Gastroenterol 2011;106:1582–91; quiz 1581, 1592.
- Lovell RM, Ford AC. Prevalence of gastro-esophageal reflux-type symptoms in individuals with irritable bowel syndrome in the community: a meta-analysis. Am J Gastroenterol 2012;107:1793–801; quiz 1802.
- Halpin SJ, Ford AC. Prevalence of symptoms meeting criteria for irritable bowel syndrome in inflammatory bowel disease: systematic review and meta-analysis. Am J Gastroenterol 2012;107:1474–82.
- Ford AC, Moayyedi P, Lacy BE, Lembo AJ, Saito YA, Schiller LR, et al. American College of Gastroenterology monograph on the management of irritable bowel syndrome and chronic idiopathic constipation. Am J Gastroenterol 2014;109 Suppl 1:S2–26; quiz S27.
- Vanuytsel T, Tack JF, Boeckxstaens GE. Treatment of abdominal pain in irritable bowel syndrome. J Gastroenterol 2014;49:1193–205.
- Brenner DM, Moeller MJ, Chey WD, Schoenfeld PS. The utility of probiotics in the treatment of irritable bowel syndrome: a systematic review. Am J Gastroenterol 2009;104:1033–49; quiz 1050.
- Moayyedi P, Ford AC, Talley NJ, Cremonini F, Foxx-Orenstein AE, Brandt LJ, et al. The efficacy of probiotics in the treatment of irritable bowel syndrome: a systematic review. Gut 2010;59:325–32.
- Hungin APS, Mulligan C, Pot B, Whorwell P, Agréus L, Fracasso P, et al. Systematic review: probiotics in the management of lower gastrointestinal symptoms in clinical practice—an evidence-based international guide. Aliment Pharmacol Ther 2013;38:864–86.
- Khanna R, MacDonald JK, Levesque BG. Peppermint oil for the treatment of irritable bowel syndrome: a systematic review and meta-analysis. J Clin Gastroenterol 2014;48:505–12.
- Ford AC, Talley NJ, Schoenfeld PS, Quigley EMM, Moayyedi P. Efficacy of antidepressants and psychological therapies in irritable bowel syndrome: systematic review and meta-analysis. Gut 2009;58:367–78.
- Shah E, Kim S, Chong K, Lembo A, Pimentel M. Evaluation of harm in the pharmacotherapy of irritable bowel syndrome. Am J Med 2012;125:381–93.
- Bundeff AW, Woodis CB. Selective serotonin reuptake inhibitors for the treatment of irritable bowel syndrome. Ann Pharmacother 2014;48:777–84.
- Menees SB, Maneerattannaporn M, Kim HM, Chey WD. The efficacy and safety of rifaximin for the irritable bowel syndrome: a systematic review and meta-analysis. Am J Gastroenterol 2012;107:28–35; quiz 36.
- Iorio N, Malik Z, Schey R. Profile of rifaximin and its potential in the treatment of irritable bowel syndrome. Clin Exp Gastroenterol 2015;8:159–67.
- Schoenfeld P, Pimentel M, Chang L, Lembo A, Chey WD, Yu J, et al. Safety and tolerability of rifaximin for the treatment of irritable bowel syndrome without constipation: a pooled analysis of randomised, double-blind, placebo-controlled trials. Aliment Pharmacol Ther 2014;39:1161–8.
- Wensel TM, Luthin DR. Linaclotide: a novel approach to the treatment of irritable bowel syndrome. Ann Pharmacother 2011;45:1535–43.
- Atluri DK, Chandar AK, Bharucha AE, Falck-Ytter Y. Effect of linaclotide in irritable bowel syndrome with constipation (IBS-C): a systematic review and meta-analysis. Neurogastroenterol Motil 2014;26:499–509.
- Ford AC, Brandt LJ, Young C, Chey WD, Foxx-Orenstein AE, Moayyedi P. Efficacy of 5-HT3 antagonists and 5-HT4 agonists in irritable bowel syndrome: systematic review and meta-analysis. Am J Gastroenterol 2009;104:1831–43; quiz 1844.
- Schaefert R, Klose P, Moser G, Häuser W. Efficacy, tolerability, and safety of hypnosis in adult irritable bowel syndrome: systematic review and meta-analysis. Psychosom Med 2014;76:389–98.
- Rutten JMTM, Reitsma JB, Vlieger AM, Benninga MA. Gut-directed hypnotherapy for functional abdominal pain or irritable bowel syndrome in children: a systematic review. Arch Dis Child 2013;98:252–7.
- American College of Gastroenterology Task Force on Irritable Bowel Syndrome, Brandt LJ, Chey WD, Foxx-Orenstein AE, Schiller LR, Schoenfeld PS, et al. An evidence-based position statement on the management of irritable bowel syndrome. Am J Gastroenterol 2009;104 Suppl 1:S1–35.
Share
X
Global Guidelines Survey
