Recommended Immunization Schedules for Persons Aged 0--18 Years --- United States, 2008 (original) (raw)


Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail.
Please note: An erratum has been published for this article. To view the erratum, please click here.
The recommended immunization schedules for persons aged 0--18 years and the catch-up immunization schedule for 2008 have been approved by the Advisory Committee on Immunization Practices, the American Academy of Pediatrics, and the American Academy of Family Physicians. The standard MMWR footnote format has been modified for publication of this schedule.
Suggested citation: Centers for Disease Control and Prevention. Recommended immunization schedules for persons aged 0--18 years---United States, 2008. MMWR 2007;56(51&52):Q1--Q4.
The Advisory Committee on Immunization Practices (ACIP) annually publishes a recommended immunization schedule for persons aged 0--18 years to reflect changes in vaccine formulations and current recommendations for the use of licensed vaccines. Changes to the previous schedule (1) are as follows:
- The pneumococcal conjugate vaccine (PCV) footnote reflects updated recommendations for incompletely vaccinated children aged 24--59 months, including those with underlying medical conditions (2).
- Recommendations for use of the live attenuated influenza vaccine (LAIV) now include healthy children aged as young as 2 years. LAIV should not be administered to children aged <5 years with recurrent wheezing (3). Children aged <9 years who are receiving influenza vaccine for the first time or who were vaccinated for the first time last season, but only received 1 dose, should have 2 doses of vaccine, at least 4 weeks apart. Other updates are included (4).
- For meningococcal vaccines, changes affect certain children aged 2--10 years (5). Vaccinating with meningococcal conjugate vaccine (MCV4) is preferred to meningococcal polysaccharide vaccine (MPSV4) for children at increased risk for meningococcal disease, including children who are traveling to or residents of countries in which the disease is hyperendemic or epidemic, children who have terminal complement component deficiencies, and children who have anatomic or functional asplenia. The catch-up schedule for youths aged 13--18 years has been updated. MPSV4 is an acceptable alternative for short-term (i.e., 3--5 years) protection against meningococcal disease for persons aged 2--18 years (6).
- The tetanus and diphtheria toxoids/tetanus and diphtheria toxoids and acellular pertussis vaccine (Td/Tdap) catch-up schedule for persons aged 7--18 years who received their first dose before age 12 months now indicates that these youths should receive 4 doses, with at least 4 weeks (not 8 weeks) between doses 2 and 3.
- The catch-up bars for hepatitis B and Haemophilus influenzae type b conjugate vaccine have been deleted on the routine schedule for persons aged 0--6 years (Figure 1). The figure title refers users to the catch-up schedule (Table) for patients who fall behind or start late with vaccinations.
The National Childhood Vaccine Injury Act requires that health-care providers provide parents or patients with copies of Vaccine Information Statements before administering each dose of the vaccines listed in the schedule. Additional information is available from state health departments and from CDC at http://www.cdc.gov/vaccines/pubs/vis/default.htm.
Detailed recommendations for using vaccines are available from package inserts, ACIP statements (available at http://www.cdc.gov/vaccines/pubs/acip-list.htm), and the 2006 Red Book (7). Guidance regarding the Vaccine Adverse Event Reporting System form is available at http://www.vaers.hhs.gov or by telephone, 800-822-7967.
References
- CDC. Recommended childhood and adolescent immunization schedule---United States. MMWR 2007;55(51&52):Q1--Q4.
- CDC. Revised recommendations of the Advisory Committee on Immunization Practices (ACIP) for the prevention of pneumococcal disease. Atlanta, GA: US Department of Health and Human Services, CDC; 2007. Available athttp://www.cdc.gov/vaccines/recs/acip/downloads/min_oct07.pdf
- CDC. Expansion of use of live attenuated influenza vaccine (FluMist®) to children aged 2--4 years and other FluMist changes for the 2007--08 influenza season. MMWR 2007;56:1217--9.
- CDC. Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 2007;56(No. RR-6).
- CDC. Recommendation from the Advisory Committee on Immunization Practices (ACIP) for use of quadrivalent meningococcal conjugate vaccine (MCV4) in children aged 2--10 years at increased risk for invasive meningococcal disease. MMWR 2007;56:1265--6.
- CDC. Revised recommendations of the Advisory Committee on Immunization Practices (ACIP) to vaccinate all persons aged 11--18 years with meningococcal conjugate vaccine. MMWR 2007;56:794--5.
- American Academy of Pediatrics. Active and passive immunization. In: Pickering LK, ed. 2006 red book: report of the Committee on Infectious Diseases. 27th ed. Elk Grove Village, IL: American Academy of Pediatrics; 2006.
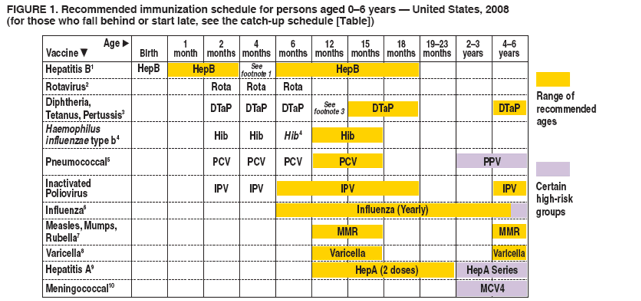
FIGURE 1. Recommended immunization schedule for persons aged 0--6 years --- United States, 2008 (for those who fall behind or start late, see the catch-up schedule [Table])
This schedule indicates the recommended ages for routine administration of currently licensed childhood vaccines, as of December 1, 2007, for children aged 0--6 years. Additional information is available at http://www.cdc.gov/vaccines/recs/schedules. Any dose not administered at the recommended age should be administered at any subsequent visit, when indicated and feasible. Additional vaccines may be licensed and recommended during the year. Licensed combination vaccines may be used whenever any components of the combination are indicated and other components of the vaccine are not contraindicated and if approved by the Food and Drug Administration for that dose of the series. Providers should consult the respective Advisory Committee on Immunization Practices statement for detailed recommendations, including for high-risk conditions: http://www.cdc.gov/vaccines/pubs/acip-list.htm. Clinically significant adverse events that follow immunization should be reported to the Vaccine Adverse Event Reporting System (VAERS). Guidance about how to obtain and complete a VAERS form is available at http://www.vaers.hhs.gov or by telephone, 800-822-7967.
1. Hepatitis B vaccine (HepB). (Minimum age: birth)
At birth:
- Administer monovalent HepB to all newborns before hospital discharge.
- If mother is hepatitis surface antigen (HBsAg)-positive, administer HepB and 0.5 mL of hepatitis B immune globulin (HBIG) within 12 hours of birth.
- If mother's HBsAg status is unknown, administer HepB within 12 hours of birth. Determine the HBsAg status as soon as possible and if HBsAg-positive, administer HBIG (no later than age 1 week).
- If mother is HBsAg-negative, the birth dose should only be delayed, in rare cases, with health-care--provider's order and a copy of the mother's negative HBsAg laboratory report documented in the infant's medical record. After the birth dose:
- The HepB series should be completed with either monovalent HepB or a combination vaccine containing HepB. The second dose should be administered at age 1--2 months. The final dose should be administered no earlier than age 24 weeks. Infants born to HBsAg-positive mothers should be tested for HBsAg and antibody to HBsAg after completion of at least 3 doses of a licensed HepB series, at age 9--18 months (generally at the next well-child visit). 4-month dose:
- It is permissible to administer 4 doses of HepB when combination vaccines are administered after the birth dose. If monovalent HepB is used for doses after the birth dose, a dose at age 4 months is not needed. 2. Rotavirus vaccine (Rota). (Minimum age: 6 weeks)
- Administer the first dose at age 6--12 weeks.
- Do not start the series later than age 12 weeks.
- Administer the final dose in the series by age 32 weeks. Do not administer a dose later than age 32 weeks.
- Data on safety and efficacy outside of these age ranges are insufficient.
3. Diphtheria and tetanus toxoids and acellular pertussis vaccine (DTaP).
(Minimum age: 6 weeks) - The fourth dose of DTaP may be administered as early as age 12 months, provided 6 months have elapsed since the third dose.
- Administer the final dose in the series at age 4--6 years.
4. Haemophilus influenzae type b conjugate vaccine (Hib).
(Minimum age: 6 weeks) - If PRP-OMP (PedvaxHIB® or ComVax® [Merck]) is administered at ages 2 and 4 months, a dose at age 6 months is not required.
- TriHiBit® (DTaP/Hib) combination products should not be used for primary immunization but can be used as boosters after any Hib vaccine in children aged >12 months. 5. Pneumococcal vaccine. (Minimum age: 6 weeks for pneumococcal conjugate vaccine [PCV]; 2 years for pneumococcal polysaccharide vaccine [PPV])
- Administer 1 dose of PCV to all healthy children aged 24--59 months having any incomplete schedule.
- Administer PPV to children aged >2 years with underlying medical conditions. 6. Influenza vaccine. (Minimum age: 6 months for trivalent inactivated influenza vaccine [TIV]; 2 years for live, attenuated influenza vaccine [LAIV])
- Administer annually to children aged 6--59 months and to all eligible close contacts of children aged 0--59 months.
- Administer annually to children aged >5 years with certain risk factors, to other persons (including household members) in close contact with persons in groups at higher risk, and to any child whose parents request vaccination.
- For healthy persons (i.e., those who do not have underlying medical conditions that predispose them to influenza complications) aged 2--49 years, either LAIV or TIV may be used.
- Children receiving TIV should receive 0.25 mL if aged 6--35 months or 0.5 mL if aged>3 years.
- Administer 2 doses (separated by >4 weeks) to children aged <9 years who are receiving influenza vaccine for the first time or who were vaccinated for the first time last season but only received 1 dose. 7. Measles, mumps, and rubella vaccine (MMR).(Minimum age: 12 months)
- Administer the second dose of MMR at age 4--6 years. MMR may be administered before age 4--6 years, provided >4 weeks have elapsed since the first dose. 8. Varicella vaccine. (Minimum age: 12 months)
- Administer the second dose of varicella vaccine at age 4--6 years; may be administered>3 months after first dose.
- Do not repeat second dose if administered >28 days after first dose. 9. Hepatitis A vaccine (HepA). (Minimum age: 12 months)
- Administer to all children aged 1 year (i.e., aged 12--23 months). Administer the 2 doses in the series at least 6 months apart.
- Children not fully vaccinated by age 2 years can be vaccinated at subsequent visits.
- HepA is recommended for certain other groups of children, including in areas where vaccination programs target older children. 10. Meningococcal vaccine. (Minimum age: 2 years for meningococcal conjugate vaccine [MCV4] and for meningococcal polysaccharide vaccine [MPSV4])
- Administer MCV4 to children aged 2--10 years with terminal complement deficiencies or anatomic or functional asplenia and certain other high-risk groups. MPSV4 also is acceptable.
- Administer MCV4 to persons who received MPSV4 >3 years previously and remain at increased risk for meningococcal disease.
The Recommended Immunization Schedules for Persons Aged 0--18 Years are approved by the Advisory Committee on Immunization Practices (http://www.cdc.gov/nip/acip), the American Academy of Pediatrics (http://www.aap.org), and the American Academy of Family Physicians (http://www.aafp.org).
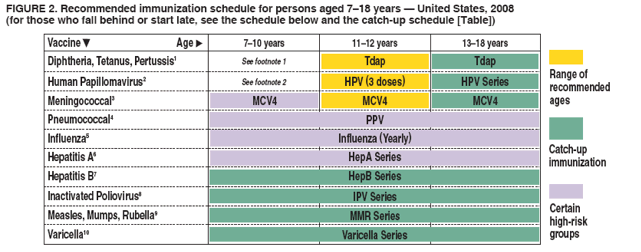
FIGURE 2. Recommended immunization schedule for persons aged 7--18 years --- United States, 2008 (for those who fall behind or start late, see the schedule below and the catch-up schedule [Table])
This schedule indicates the recommended ages for routine administration of currently licensed childhood vaccines, as of December 1, 2007, for children aged 7--18 years. Additional information is available at http://www.cdc.gov/vaccines/recs/schedules. Any dose not administered at the recommended age should be administered at any subsequent visit, when indicated and feasible. Additional vaccines may be licensed and recommended during the year. Licensed combination vaccines may be used whenever any components of the combination are indicated and other components of the vaccine are not contraindicated and if approved by the Food and Drug Administration for that dose of the series. Providers should consult the respective Advisory Committee on Immunization Practices statement for detailed recommendations, including for high-risk conditions: http://www.cdc.gov/vaccines/pubs/acip-list.htm. Clinically significant adverse events that follow immunization should be reported to the Vaccine Adverse Event Reporting System (VAERS). Guidance about how to obtain and complete a VAERS form is available at http://www.vaers.hhs.gov or by telephone, 800-822-7967.
1. Tetanus and diphtheria toxoids and acellular pertussis vaccine (Tdap). (Minimum age: 10 years for BOOSTRIX® and 11 years for ADACEL™)
- Administer at age 11--12 years for those who have completed the recommended childhood DTP/DTaP vaccination series and have not received a tetanus and diphtheria toxoids vaccine (Td) booster dose.
- Adolescents aged 13--18 years who missed the 11--12 year Tdap dose or received Td only are encouraged to receive 1 dose of Tdap 5 years after the last Td/DTaP dose. 2. Human papillomavirus vaccine (HPV). (Minimum age: 9 years)
- Administer the first dose of the HPV vaccine series to females at age 11--12 years.
- Administer the second dose 2 months after the first dose and the third dose 6 months after the first dose.
- Administer the HPV vaccine series to females at age 13--18 years if not previously vaccinated. 3. Meningococcal vaccine.
- Administer meningococcal conjugate vaccine (MCV4) at age 11--12 years and at age 13--18 years if not previously vaccinated. Meningococcal polysaccharide vaccine (MPSV4) is an acceptable alternative.
- Administer MCV4 to previously unvaccinated college freshmen living in dormitories.
- MCV4 is recommended for children aged 2--10 years with terminal complement deficiencies or anatomic or functional asplenia and certain other groups at high risk.
- Persons who received MPSV4 >3 years previously and remain at increased risk for meningococcal disease should be vaccinated with MCV4. 4. Pneumococcal polysaccharide vaccine (PPV).
- Administer to certain groups at high risk. 5. Influenza vaccine.
- Administer annually to all close contacts of children aged 0--59 months.
- Administer annually to person with certain risk factors, health-care workers, and other persons (including household members) in close contact with persons in groups at higher risk.
- Administer 2 doses (separated by >4 weeks) to children aged <9 years who are receiving influenza vaccine for the first time or who were vaccinated for the first time last season but only received 1 dose.
- For healthy nonpregnant persons (i.e., those who do not have underlying medical conditions that predispose them to influenza complications) aged 2--49 years, either LAIV or TIV may be used. 6. Hepatitis A vaccine (HepA).
- Administer 2 doses in the series at least 6 months apart.
- HepA is recommended for certain other groups of children, including in areas where vaccination programs target older children. 7. Hepatitis B vaccine (HepB).
- Administer the 3-dose series to those who were not previously vaccinated.
- A 2-dose series of Recombivax HB® is licensed for children aged 11--15 years. 8. Inactivated poliovirus vaccine (IPV).
- For children who received an all-IPV or all-oral poliovirus (OPV) series, a fourth dose is not necessary if the third dose was administered at age >4 years.
- If both OPV and IPV were administered as part of a series, a total of 4 doses should be administered, regardless of the child's current age. 9. Measles, mumps, and rubella vaccine (MMR).
- If not previously vaccinated, administer 2 doses of MMR during any visit, with>4 weeks between the doses. 10. Varicella vaccine.
- Administer 2 doses of varicella vaccine to persons aged <13 years at least 3 months apart. Do not repeat the second dose, if administered >28 days after the first dose.
- Administer 2 doses of varicella vaccine to persons aged>13 years at least 4 weeks apart.
The Recommended Immunization Schedules for Persons Aged 0--18 Years are approved by the Advisory Committee on Immunization Practices (http://www.cdc.gov/nip/acip), the American Academy of Pediatrics (http://www.aap.org), and the American Academy of Family Physicians (http://www.aafp.org).
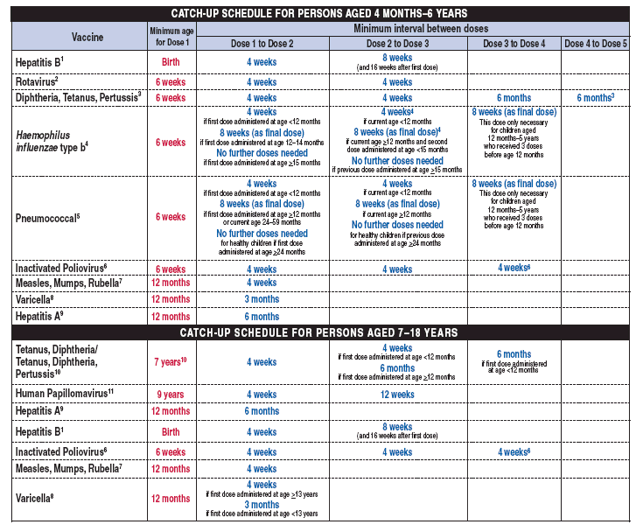
TABLE. Catch-up immunization schedule for persons aged 4 months--18 years who start late or who are >1 month behind --- United States, 2008
The table below provides catch-up schedules and minimum intervals between doses for children whose vaccinations have been delayed. A vaccine series does not need to be restarted, regardless of the time that has elapsed between doses. Use the section appropriate for the child's age.
1. Hepatitis B vaccine (HepB).
- Administer the 3-dose series to those who were not previously vaccinated.
- A 2-dose series of Recombivax HB® is licensed for children aged 11--15 years. 2. Rotavirus vaccine (Rota).
- Do not start the series later than age 12 weeks.
- Administer the final dose in the series by age 32 weeks.
- Do not administer a dose later than age 32 weeks.
- Data on safety and efficacy outside of these age ranges are insufficient. 3. Diphtheria and tetanus toxoids and acellular pertussis vaccine (DTaP).
- The fifth dose is not necessary if the fourth dose was administered at age >4 years.
- DTaP is not indicated for persons aged >7 years. 4. Haemophilus influenzae type b conjugate vaccine (Hib).
- Vaccine is not generally recommended for children aged >5 years.
- If current age <12 months and the first 2 doses were PRP-OMP (PedvaxHIB® or ComVax® [Merck]), the third (and final) dose should be administered at age 12--15 months and at least 8 weeks after the second dose.
- If first dose was administered at age 7--11 months, administer 2 doses separated by 4 weeks plus a booster at age 12--15 months. 5. Pneumococcal conjugate vaccine (PCV).
- Administer 1 dose of PCV to all healthy children aged 24--59 months having any incomplete schedule.
- For children with underlying medical conditions, administer 2 doses of PCV at least 8 weeks apart if previously received <3 doses or 1 dose of PCV if previously received 3 doses. 6. Inactivated poliovirus vaccine (IPV).
- For children who received an all-IPV or all-oral poliovirus (OPV) series, a fourth dose is not necessary if third dose was administered at age >4 years.
- If both OPV and IPV were administered as part of a series, a total of 4 doses should be administered, regardless of the child's current age.
- IPV is not routinely recommended for persons aged >18 years. 7. Measles, mumps, and rubella vaccine (MMR).
- The second dose of MMR is recommended routinely at age 4--6 years but may be administered earlier if desired.
- If not previously vaccinated, administer 2 doses of MMR during any visit with >4 weeks between the doses. 8. Varicella vaccine.
- The second dose of varicella vaccine is recommended routinely at age 4--6 years but may be administered earlier if desired.
- Do not repeat the second dose in persons aged <13 years if administered >28 days after the first dose. 9. Hepatitis A vaccine (HepA).
- HepA is recommended for certain groups of children, including in areas where vaccination programs target older children. See MMWR 2006;55(No. RR-7). 10. Tetanus and diphtheria toxoids vaccine (Td) and tetanus and diphtheria toxoids and acellular pertussis vaccine (Tdap).
- Tdap should be substituted for a single dose of Td in the primary catch-up series or as a booster if age appropriate; use Td for other doses.
- A 5-year interval from the last Td dose is encouraged when Tdap is used as a booster dose. A booster (fourth) dose is needed if any of the previous doses were administered at age <12 months. See MMWR 2006;55(No. RR-3). 11. Human papillomavirus vaccine (HPV).
- Administer the HPV vaccine series to females at age 13--18 years if not previously vaccinated.
Information about reporting reactions after immunization is available online at http://www.vaers.hhs.gov or by telephone via the 24-hour national toll-free information line 800-822-7967. Suspected cases of vaccine-preventable diseases should be reported to the state or local health department. Additional information, including precautions and contraindications for immunization, is available from the National Center for Immunization and Respiratory Diseases at http://www.cdc.gov/vaccines or telephone, 800-CDC-INFO (800-232-4636).
Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of Health and Human Services.
References to non-CDC sites on the Internet are provided as a service to MMWR readers and do not constitute or imply endorsement of these organizations or their programs by CDC or the U.S. Department of Health and Human Services. CDC is not responsible for the content of pages found at these sites. URL addresses listed in MMWR were current as of the date of publication.
Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices.
**Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.
Date last reviewed: 1/10/2008