Preventing Pneumococcal Disease Among Infants and Young Children
([original](https://www.cdc.gov/mmwr/preview/mmwrhtml/rr4909a1.htm)) ([raw](?raw))

Recommendations of the Advisory Committee on Immunization Practices (ACIP)
Advisory Committee on Immunization Practices
Membership List, June 2000
CHAIR
John F. Modlin, M.D.
Professor of Pediatrics and Medicine
Dartmouth Medical School
Lebanon, New Hampshire
EXECUTIVE SECRETARY
Dixie E. Snider, Jr., M.D., M.P.H.
Associate Director for Science
Centers for Disease Control and Prevention
Atlanta, Georgia
MEMBERS
Dennis A. Brooks, M.D., M.P.H.
Johnson Medical Center
Baltimore, Maryland
Richard D. Clover, M.D.
University of Louisville School of Medicine
Louisville, Kentucky
Fernando A. Guerra, M.D.
San Antonio Metropolitan Health District
San Antonio, Texas
Charles M. Helms, M.D., Ph.D.
University of Iowa Hospital and Clinics
Iowa City, Iowa
David R. Johnson, M.D., M.P.H.
Michigan Department of Community Health
Lansing, Michigan
Chinh T. Le, M.D.
Kaiser Permanente Medical Center
Santa Rosa, California
Paul A. Offit, M.D.
Children's Hospital of Philadelphia
Philadelphia, Pennsylvania
Margaret B. Rennels, M.D.
University of Maryland School of Medicine
Baltimore, Maryland
Lucy S. Tompkins, M.D., Ph.D.
Stanford University Medical Center
Stanford, California
Bonnie M. Word, M.D.
State University of New York
Stony Brook, New York
EX-OFFICIO MEMBERS
James E. Cheek, M.D., M.P.H.
Indian Health Service
Albuquerque, New Mexico
William Egan, Ph.D.
Food and Drug Administration
Rockville, Maryland
Geoffrey S. Evans, M.D.
Health Resources and Services Administration
Rockville, Maryland
T. Randolph Graydon
Health Care Financing Administration
Baltimore, Maryland
Carole Heilman, M.D.
National Institutes of Health
Bethesda, Maryland
Martin G. Myers, M.D.
Centers for Disease Control and Prevention
Atlanta, Georgia
Kristin Lee Nichol, M.D., M.P.H.
VA Medical Center
Minneapolis, Minnesota
David H. Trump, M.D., M.P.H.
Office of the Assistant Secretary of Defense (Health Affairs)
Falls Church, Virginia
LIAISON REPRESENTATIVE
American Academy of Family Physicians
Williams R. Phillips, M.D., M.P.H.
Seattle, Washington
Richard Zimmerman, M.D.
Pittsburgh, Pennsylvania
American Academy of Pediatrics
Larry Pickering, M.D.
Atlanta, Georgia
Jon Abramson, M.D.
Winston-Salem, North Carolina
American Association of Health Plans
Eric K. France, M.D.
Denver, Colorado
American College of Obstetricians and Gynecologists
Stanley A. Gall, M.D.
Louisville, Kentucky
American College of Physicians
Pierce Gardner, M.D.
Stony Brook, New York
American Hospital Association
William Schaffner, M.D.
Nashville, Tennessee
American Medical Association
H. David Wilson, M.D.
Grand Forks, North Dakota
Association of Teachers of Preventive Medicine
W. Paul McKinney, M.D.
Louisville, Kentucky
Biotechnology Industry Organization
Vacant
Canadian National Advisory Committee on Immunization
Victor Marchessault, M.D.
Cumberland, Ontario, Canada
Hospital Infection Control Practices Advisory Committee
Jane D. Siegel, M.D.
Dallas, Texas
Infectious Diseases Society of America
Samuel L. Katz, M.D.
Durham, North Carolina
National Immunization Council and Child Health Program, Mexico
Jose Ignacio Santos, M.D.
Mexico City, Mexico
National Medical Association
Rudolph E. Jackson, M.D.
Atlanta, Georgia
National Vaccine Advisory Committee
Georges Peter, M.D.
Providence, Rhode Island
Pharmaceutical Research and Manufacturers of America
Barbara J. Howe, M.D.
Collegeville, Pennsylvania
The following CDC staff members prepared this report:
Chris A. Van Beneden, M.D., M.P.H.
Cynthia G. Whitney, M.D., M.P.H.
Orin S. Levine, Ph.D.
Division of Bacterial and Mycotic Diseases
National Center for Infectious Diseases
Benjamin Schwartz, M.D.
Division of Epidemiology and Surveillance
National Immunization Program
in collaboration with the Advisory Committee on Immunization Practices Working Group on Pneumococcal Conjugate Vaccines
Robert F. Breiman, M.D.
National Vaccine Program Office
Office of the Director, CDC
Jay C. Butler, M.D.
Arctic Investigations Program
Orin S. Levine, Ph.D.
Chris A. Van Beneden, M.D., M.P.H.
Cynthia G. Whitney, M.D., M.P.H.
Division of Bacterial and Mycotic Diseases
National Center for Infectious Diseases, CDC
Benjamin Schwartz, M.D.
Division of Epidemiology and Surveillance
National Immunization Program
Stanley A. Gall, M.D.
University of Louisville School of Medicine
Louisville, Kentucky
Mary P. Glode, M.D.
Children's Hospital and University of Colorado Health Sciences Center
Denver, Colorado
Neal Halsey, M.D.
Johns Hopkins University School of Public Health
Baltimore, Maryland
Charles M. Helms, M.D., Ph.D.
University of Iowa College of Medicine
Iowa City, Iowa
David R. Johnson, M.D., M.P.H., Working Group Chair
Michigan Department of Community Health
Lansing, Michigan
Gary D. Overturf, M.D.
University of New Mexico School of Medicine
Albuquerque, New Mexico
Karen Midthun, M.D.
R. Douglas Pratt, M.D., M.P.H.
Food and Drug Administration
Rockville, Maryland
William Schaffner, M.D.
Vanderbilt University School of Medicine
Nashville, Tennessee
Jane D. Siegel, M.D.
University of Texas Southwestern Medical Center
Dallas, Texas
Richard K. Zimmerman, M.D., M.P.H.
University of Pittsburgh
Pittsburgh, Pennsylvania
Summary
In February 2000, a 7-valent pneumococcal polysaccharide-protein conjugate vaccine (Prevnar,™ marketed by Wyeth Lederle Vaccines) was licensed for use among infants and young children. CDC's Advisory Committee on Immunization Practices (ACIP) recommends that the vaccine be used for all children aged 2--23 months and for children aged 24--59 months who are at increased risk for pneumococcal disease (e.g., children with sickle cell disease, human immunodeficiency virus infection, and other immunocompromising or chronic medical conditions). ACIP also recommends that the vaccine be considered for all other children aged 24--59 months, with priority given to a) children aged 24--35 months, b) children who are of Alaska Native, American Indian, and African-American descent, and c) children who attend group day care centers. This report includes ACIP's recommended vaccination schedule for infants at ages 2, 4, 6, and 12--15 months. This report also includes a pneumococcal vaccination schedule for infants and young children who are beginning their vaccination series at an older age and for those who missed doses. In addition, this report updates earlier recommendations for use of 23-valent pneumococcal polysaccharide vaccine among children aged >2 years. Among children aged 24--59 months for whom polysaccharide vaccine is already recommended, ACIP recommends vaccination with the new conjugate vaccine followed, >2 months later, by 23-valent polysaccharide vaccine. Conjugate vaccine has not been studied sufficiently among older children or adults to make recommendations for its use among persons aged >5 years. Persons aged >5 years who are at increased risk for serious pneumococcal disease should continue to receive 23-valent polysaccharide vaccine in accordance with previous ACIP recommendations.
INTRODUCTION
Streptococcus pneumoniae (pneumococcus) remains a leading cause of serious illness among young children worldwide and is the most frequent cause of pneumonia, bacteremia, sinusitis, and acute otitis media (AOM). In the United States, S. pneumoniae causes approximately 17,000 cases/year of invasive disease among children aged <5 years, including 700 cases of meningitis and 200 deaths (CDC's Active Bacterial Core Surveillance [ABCs]/Emerging Infections Program [EIP] Network, unpublished data, 2000).*
CDC's Advisory Committee on Immunization Practices (ACIP) previously recommended 23-valent pneumococcal polysaccharide vaccines for use among children aged >2 years who have high rates of disease, including those with sickle cell disease (SCD), chronic underlying diseases, human immunodeficiency virus (HIV) infection, or others who are immunocompromised. The new 7-valent pneumococcal conjugate vaccine (PCV7;** Prevnar,™ licensed in February 2000 and marketed by Wyeth Lederle Vaccines) should be a key addition to existing pneumococcal disease prevention measures. Although the previously licensed 23-valent pneumococcal polysaccharide vaccines (PPV23; PNU-IMUNE® 23 marketed by Wyeth-Ayerst Laboratories and Pneumovax® 23 by Merck and Company) are effective in preventing invasive pneumococcal disease among older children and adults, these vaccines do not protect children aged <2 years, the age group with the highest rate of disease (1--3). Furthermore, PPV23 does not decrease nasopharyngeal carriage, a substantial source of transmission of pneumococci (4). In contrast, conjugate vaccine decreases colonization, and PCV7 prevents pneumococcal disease among children aged <2 years (5--7). This report provides ACIP's recommendations for pneumococcal vaccination of children aged <5 years.
BACKGROUND
Incidence of Invasive Disease Among Children
The highest rates of invasive pneumococcal disease (e.g., bacteremia, meningitis, or other infection of a normally sterile site) occur among young children, especially those aged <2 years. In 1998, estimated incidence in the United States of invasive pneumococcal infections among children aged <12 months and 12--23 months were 165 and 203 cases/100,000 population, respectively, with peak incidence occurring among children aged 6--11 months (235/100,000). In contrast, incidence among persons of all ages and among persons aged >65 years were 24 and 61/100,000, respectively (Figure 1) (8).
In the United States, the most common manifestation of invasive pneumococcal disease among young children is bacteremia without a known site of infection, which accounts for approximately 70% of invasive pneumococcal cases among children aged <2 years. Only 12%--16% of patients with invasive pneumococcal disease among this age group have pneumonia (ABCs/EIP Network, unpublished data, 2000). With the success of conjugate vaccines in preventing invasive Haemophilus influenzae type b (Hib) disease, S. pneumoniae has become the leading cause of bacterial meningitis in the United States. Children aged <1 year have the highest incidence of pneumococcal meningitis, which is approximately 10/100,000 population (9).
Occurrence of Noninvasive Syndromes
S. pneumoniae also contributes substantially to noninvasive respiratory infections and is the most common bacterial cause of community-acquired pneumonia, AOM, and sinusitis among young children (10--16). In two prospective studies of community-acquired pneumonia among young children, 17%--28% of cases were diagnosed as pneumococcal (10,11). However, these and other studies probably underestimate the actual proportion of pneumococcal pneumonia cases as a result of low sensitivity of routine diagnostic testing.
Studies using diagnostic tympanocentesis among children with AOM have found S. pneumoniae in 28%--55% of all middle ear aspirates (12--15). Otitis media is the most frequent reason for pediatric office visits in the United States, resulting in >15 million visits/year (17--19). By age 12 months, 62% of children have had at least one episode of AOM; peak incidence of AOM occurs during ages 6 months--1year (20). Although serious complications are rare, economic costs of otitis media are estimated at >$3.5 billion/year in the United States (21). During 1996, approximately 500,000 tympanostomy tubes were placed in children's ears in the United States (22). AOM is also the leading reason for prescribing antibiotics during childhood, and use of antibiotics for treatment of AOM and sinusitis contributes substantially to increased antimicrobial resistance (23--25).
Children at Increased Risk for Pneumococcal Infections
Racial and Ethnic Populations
In the United States, higher rates of invasive pneumococcal disease occur among African-Americans, Alaska Natives, and specific American Indian populations, compared with whites (Table 1) (26--32). Incidence of pneumococcal bacteremia and meningitis among Alaska Native children aged <5 years ranges from 598 cases/100,000 population among children aged 6--11 months to 56 cases/100,000 population for children aged 36--47 months, which is approximately four times that of similarly aged non-Alaska Native/non-American Indian children (30). The highest rates for any ethnic group in the United States are found among Navajo and Apache populations living on reservations in the southwestern United States. Incidence among children aged 1--2 years in these populations is 557--2,396/100,000 (31,32). Among children aged <5 years, incidence of invasive pneumococcal disease among African-American children in the United States is 2--3 times higher than the rate among white children of the same age (8,27,29,33). In a case-control study of risk factors for invasive disease among young children, the association of race with disease risk was not statistically significant in an analysis that controlled for socioeconomic status (34). However, other studies have reported persistence of increased risk when controlling for income (27,33,35--37). Reasons for increased rates of disease among Alaska Natives, American Indians, and African-Americans are unclear but probably multifactorial.
Children with Functional or Anatomic Asplenia
Other children at high risk for invasive pneumococcal disease include patients with SCD, other sickle hemoglobinopathies (e.g., hemoglobin S-C disease or S-ß-thalassemia), and those who are otherwise functionally or anatomically asplenic (38--40). Although rates of pneumococcal infection among children with hemoglobin S-C disease are lower than rates among persons with SCD, rates are higher than among healthy children (41,42). Rates of overall bacterial sepsis in other hemoglobinopathies (e.g., S-ß-thalassemia) have not been calculated but are estimated to be intermediate between those for hemoglobin S-C and hemoglobin SS diseases (40). High risk for pneumococcal infections among persons with SCD is thought to be caused by the combination of low levels of circulating antibodies, splenic dysfunction, and complement deficiency, resulting in decreased clearance of encapsulated bacteria from the bloodstream (43,44). The protective effect of pneumococcal polysaccharide vaccine among SCD patients, initially reported in a study published in 1977, has not been reproduced consistently by subsequent prospective or case-control studies (45--49). Although use of prophylactic penicillin has reduced the risk for pneumococcal disease, children aged <5 years with SCD still have increased rates of invasive disease (range: 1,230--1,500/100,000 population), probably reflecting noncompliance with and failure of penicillin prophylaxis combined with suboptimal protection by pneumococcal polysaccharide vaccine (38,39,47,49).
HIV-Infected Children
Children infected with HIV have a markedly increased risk for pneumococcal infection compared with those who are not HIV-infected (50,51). In two prospective cohort studies, HIV-infected children had rates of invasive pneumococcal disease that were 2.8 and 12.6 times the rate among HIV-negative control subjects for children aged <5 and <3 years, respectively (52,53). Incidence of invasive pneumococcal disease is 6.1 cases/100 patient-years among HIV-infected children through age 7 years (54).
Children in Day Care
Out-of-home day care increases the risk for invasive pneumococcal disease and AOM among children (34,55,56). In a study of risk factors for invasive pneumococcal disease among children in the United States, attendance at a group day care center*** during the preceding 3 months was associated with an approximately 2.3-fold increase in invasive disease among children aged 12--23 months, and 3.2-fold increased risk among children aged 24--59 months (34). Moreover, in a recent population-based case-control study, nonelderly adults (i.e., persons aged 18--64 years) who lived in a household that included children who attended day care were at greater risk for acquiring invasive pneumococcal infections than adults who did not (multivariate odds ratio [OR] = 3.0) (57).
In studies of otitis media resulting from all causes, risk for AOM was higher among children who attended day care outside the home compared with family day care (58,59), and risk for middle ear effusions increased with exposure to larger numbers of children in day care settings (60). Younger age when starting day care also increases risk for experiencing recurrent AOM (59). Day care attendance is also a risk factor for other acute upper respiratory tract infections among children aged <5 years (61).
Antimicrobial Resistance
Treatment of pneumococcal disease among young children is complicated by emergence of pneumococcal strains resistant to penicillin and other antibiotics (12,62). Among a national sample of invasive pneumococcal isolates, resistance to penicillin (i.e., minimum inhibitory concentration [MIC] > 2.0 µg/ml) has increased substantially during the past decade, from 1.3% in 1992 to 13.6% in 1997 (62,63). In certain areas of the United States, approximately 35% of invasive isolates are penicillin-nonsusceptible (i.e., intermediate susceptibility [MIC = 0.12--1.0 µg/ml] or resistant [MIC > 2.0 µg/ml]) (63). In one nasopharyngeal carriage study among children in a rural Kentucky community, 59% of children attending day care centers carried penicillin-nonsusceptible S. pneumoniae(64).
Risk factors associated with infection with penicillin-resistant pneumococci include younger age, attendance at a day care center, higher socioeconomic status, recent (i.e., <3 months) antibiotic use, and recurrent AOM (26,34,65,66). Recent day care attendance and recent antibiotic treatment are associated independently with invasive disease as a result of penicillin-resistant pneumococci (34). Penicillin resistance has been associated with treatment failures in AOM and meningitis (12,67--69); these failures could be because of difficulty in achieving high antimicrobial concentrations in middle ear fluid and cerebrospinal fluid (CSF) (70). Additional research is needed to determine the association between penicillin resistance and treatment failure in pneumococcal pneumonia or bacteremia among children (12,71--73).
Pneumococcal Serotypes
The capsule of the Streptococcus pneumoniae bacterium consists of polysaccharides and constitutes a major virulence factor for the bacterium (74). Antibodies directed against the capsular polysaccharide protect against infection; type-specific antibodies bind capsular antigens; and opsonization facilitates phagocytosis (75). Knowledge of the distribution of pneumococcal serotypes causing disease is fundamental to evaluating the potential impact of a pneumococcal vaccine. Currently, 90 serotypes of S. pneumoniae have been identified on the basis of antigenic differences in their capsular polysaccharides (76). The majority of serotypes cause serious disease, yet a relatively limited number of serotypes cause the majority of invasive pneumococcal infections. The 10 most common serotypes are estimated to account for approximately 62% of invasive disease worldwide; however, ranking and serotype prevalence differ by age group and country (77). In the United States, the seven most common serotypes isolated from the blood or CSF of children aged <6 years (i.e., 14, 6B, 19F, 18C, 23F, 4, and 9V) account for 80% of infections and are the serotypes in the licensed PCV7 (Figure 2). These same seven serotypes, by comparison, account for only 50% of isolates among persons aged >6 years in the United States (78,79). Distribution of serotype coverage also differs among Alaska Natives and American Indians in the United States; the proportion of vaccine serotypes causing invasive pneumococcal disease among children aged <5 years is less among Alaska Natives and American Indians than among non-Alaska Natives/non-American Indians (30,31). Types 1 and 5 account for only a limited percentage of invasive isolates in the United States, but are more common in Western Europe and in certain developing countries (77,80,81). Among children with AOM in rural Kentucky, the seven PCV7 serotypes accounted for 71% of infections among children aged <24 months (82). Among 414 S. pneumoniae isolates obtained from middle ear fluid cultures among children with AOM in Finland, 250 (60%) were caused by serotypes contained in PCV7 (83).
Antimicrobial resistance is detected most frequently among serotypes included in PCV7. According to 1998 surveillance data from eight states, the PCV7 serotypes accounted for 80% of 312 penicillin-nonsusceptible isolates (MIC > 0.1 µg/ml) collected from normally sterile sites among children aged <6 years (ABCs/EIP Network, unpublished data, 1999). A similar serotype distribution of penicillin-nonsusceptible isolates was identified during a study of nasopharyngeal carriage among 216 children aged <6 years in Memphis, Tennessee; 78% of resistant isolates were of the same seven serotypes (65).
PNEUMOCOCCAL CONJUGATE VACCINE
Advantages of Pneumococcal Conjugate Vaccine: Immunologic Theory
Bacterial polysaccharides, including those present on the pneumococcal capsule, are T-independent antigens. T-independent antigens stimulate mature B-lymphocytes but not T-lymphocytes. This type of antigen induces an immune system response that is neither long-lasting nor characterized by an anamnestic (i.e., booster) response upon subsequent challenge with native polysaccharides (84,85). Polysaccharide vaccines fail to elicit a protective immune system response among infants and very young children because these children respond poorly to T-independent antigens. Although antibody response to T-dependent antigens is present soon after birth, immune system responses to T-independent antigens develop during the first years of life. Certain serotypes that cause the majority of pneumococcal disease among children (i.e., 6A, 14, 19F, and 23F) remain poorly immunogenic until approximately age 5 years (86).
Conjugation of polysaccharides to proteins changes the nature of the antipolysaccharide response from T-independent to T-dependent. This antigen complex stimulates a T-helper--cell response, leading to a substantial primary response among infants and a strong booster response at reexposure (84). Success of Hib conjugate vaccine in reducing by 95% incidence of invasive Hib disease among young children after the vaccine's introduction for use among infants in 1990 is one example of the potential efficacy of bacterial polysaccharide-protein conjugate vaccines. Declines in Hib disease occurred among children aged <1 year before the vaccine was licensed for use among this age group (87). This herd-immunity effect was consistent with later observations that Hib conjugate vaccine interrupts transmission by reducing acquisition of carriage. Pneumococcal conjugate vaccines, in contrast with polysaccharides, has been reported to reduce carriage and might lead to population effects beyond direct protection.
Vaccine Composition
The 7-valent pneumococcal conjugate vaccine (Prevnar) includes seven purified capsular polysaccharides of S. pneumoniae, each coupled with a nontoxic variant of diphtheria toxin, CRM197 (CRM, cross-reactive material). The vaccine contains approximately 2 µg each of capsular polysaccharide from serotypes 4, 9V, 14, 19F, and 23F, and oligosaccharide from 18C, 4 µg of serotype 6B, 20 µg of the carrier protein CRM197, and 0.125 mg of aluminum/0.5-ml dose as an aluminum phosphate adjuvant. Prevnar contains no thimerosal or other preservative. Serotypes included in PCV7 and potentially cross-reactive serotypes (i.e., 6A, 9A, 9L, 18B, and 18F) accounted for 86% of bacteremia, 83% of meningitis, and 65% of AOM among children aged <6 years occurring in the United States during the period 1978--1994 (79).
Immunogenicity
Polysaccharide-protein conjugate vaccine induces type-specific antibodies that bind to polysaccharide on the surface of bacteria and enhance opsonization, phagocytosis, and killing of pneumococci. The amount of antibody required to prevent either pneumococcal carriage or disease is unknown. Additionally, quantitative measurement of total antibody concentration, as measured in the majority of pneumococcal antibody assays, might not correlate with the level of functional immune system response. Functional measurements (e.g., opsonophagocytic activity) might be more appropriate than serum antibody concentrations for evaluating clinically relevant responses to pneumococcal vaccination (88).
Although specific levels of antibody or opsonophagocytic activity that correlate with protection against pneumococcal disease remain unknown, presence of type-specific antibody to capsular polysaccharide is associated with protection among adults. Studies of Hib conjugate vaccine have reported that levels of anticapsular antibody >0.15 µg/ml are protective against subsequent Hib infection; thus, researchers have hypothesized that this minimum level might also be protective against pneumococcal disease (89--91). Through measurement of total antibody concentrations, researchers have demonstrated that pneumococcal conjugate vaccines using several protein carriers are more immunogenic than PPV23 among young children (92--98).
Immunogenicity Studies Among Healthy Infants and Children
In one study, 212 healthy infants were randomized to receive either PCV7 or an investigational meningococcal conjugate vaccine at ages 2, 4, 6, and 12--15 months. Vaccination with PCV7 resulted in substantial increases in serum antibody concentrations to all seven serotypes compared with baseline concentrations. After three doses of PCV7, from 92% (serotypes 6B and 23F) to 100% (serotype 4) of children had >0.15 µg/ml of type-specific antibody, and from 51% (serotype 9V) to 90% (serotype 19F) achieved a level of >1.0 µg/ml against the vaccine serotypes. The fourth dose resulted in an anamnestic response to each of the seven serotypes (95).
Children with SCD
In a study of children and young adults with SCD, children aged >2 years received either PPV23 only (n = 12) or PCV7 (two doses, 8 weeks apart) followed by PPV23 8 weeks later (n = 11) (93). When measured 3--6 weeks after administration of PPV23, serum antibody geometric mean concentrations (GMCs) were higher among the PCV7 plus PPV23 group than among the PPV23-only group for all of the PCV7 serotypes, reaching statistical significance for serotypes 14 and 19F. Serum antibody GMCs were similar between the two groups for serotypes 1 and 15B, two serotypes contained in PPV23 but not in PCV7, demonstrating that PCV7 did not interfere with the immune system response to PPV23. After administration of PPV23, 4 of 11 subjects in the PCV7 plus PPV23 group and 2 of 11 in the PPV23-only group reported fever. Rates and severity of local reactions to PPV23 did not differ between the two groups.
In a study of 34 infants aged <24 months with SCD who were vaccinated with PCV7 at ages 2, 4, and 6 months, GMCs of type-specific immunoglobulin G (IgG) antibodies measured by enzymelinked immunoabsorbent assay (ELISA) increased from <0.1 µg/ml at baseline to >2.0 µg/ml 1 month after the third dose for all seven vaccine serotypes (97,99). Among 27 study participants who remained in the study and who were vaccinated with PPV23 at age 24 months, a substantial booster response (GMC > 9.0 µg/ml) occurred after administration of PPV23 for all serotypes in the PCV7 vaccine. Both serum opsonic activity and total IgG antibodies were measured for two serotypes (i.e., 6B and 14), and both tests demonstrated substantial increases after the PPV23 booster.
HIV-Infected Children
Data are limited regarding use of PCV7 among HIV-infected children. However, in two studies using 5-valent formulations of greater antigen content (i.e., 10 µg each of serotypes 6B, 14, 18C, 19F, and 23F oligosaccharides) and the same carrier protein, conjugate vaccine was demonstrated to be immunogenic among HIV-infected children (94,100). In a study among 60 children aged >2 years, 5-valent pneumococcal conjugate vaccine was more immunogenic for all five serotypes than was PPV23 among both HIV-infected (n = 30) and HIVuninfected (n = 30) children. Among HIV-infected children, 60% demonstrated a >4fold rise in IgG level after one dose of conjugate vaccine, compared with 31% after one dose of PPV23 (P < 0.05) (94). Prevaccination CDC HIV classification and CD4 counts of the HIV-infected children were similar among the conjugate vaccine and PPV23 groups.
In a second study using the same conjugate vaccine among younger children, antibody response to three doses of 5-valent pneumococcal conjugate vaccine was compared between 18 HIVinfected children aged <2 years and 33 children without HIV infection (_100_). The vaccine was found to be immunogenic among both groups. Antibody levels achieved by the HIVinfected infants and toddlers were higher than those reported after one dose of 5-valent pneumococcal conjugate vaccine in the study of children aged >2 years discussed previously (94). In both studies, no substantial difference in frequency of local or systemic reactions was reported among children who received the conjugate vaccine compared with control groups.
Alaska Natives and American Indians
Researchers have studied the immunogenicity of a pneumococcal conjugate vaccine using a different carrier protein among Alaska Native, American Indian, and non-Alaska Native/non-American Indian infants aged <2 years (101). A 7-valent pneumococcal vaccine consisting of serotypes 4, 6B, 9V, 14, 18C, 19F, and 23F linked with the outer membrane protein complex of Neisseria meningitidis was administered to Alaska Native, American Indian (i.e., Apache and Navajo), and non-Alaska Native/non-American Indian infants aged 2, 4, and 6 months, with a booster dose at age 15 months. Response after three primary doses of vaccine was similar among all three groups of infants, except for serotypes 14 and 23F. However, 1 month after the booster dose, serotype-specific GMCs increased to all seven serotypes among the three groups of infants when compared with the pneumococcal IgG GMCs before the booster dose. Although a different conjugate-carrier protein was used, this study indicated that PCV7 immunogenicity among Alaska Natives and American Indians will likely be similar to immune system response among non-Alaska Natives/non-American Indians. Prevnar is under evaluation in an efficacy study among American Indians, and study results are pending.
Efficacy
Data from a trial using PCV7 provide evidence of the vaccine's efficacy against invasive pneumococcal disease, as well as its effectiveness against clinical pneumonia and AOM among healthy children aged <2 years. The results are summarized in the following section. Other clinical efficacy trials using 7-, 9- and 11-valent pneumococcal conjugate vaccines are currently being conducted or are planned in the United States, South Africa, and certain other countries. Outcomes to be measured in these studies include protective efficacy against overall mortality, pneumonia, invasive disease, AOM, and nasopharyngeal carriage of pneumococci.
Efficacy Trials
In a prospective double-blind study among patients of a health maintenance organization in northern California, a total of 37,830 healthy children (i.e., without immune system disorders or serious chronic disease) were randomly assigned to receive either PCV7 or a control (meningococcal C conjugate) vaccine. Vaccinations were administered at ages 2, 4, 6, and 12--15 months. Routine, licensed vaccines**** were administered concurrently according to schedule. Initially, diphtheria toxoid-tetanus toxoid whole-cell pertussis (DTwP) vaccine was included among the childhood vaccinations; however, during the study, use of acellular pertussis vaccine became routine. As of March 2000, >50% of study participants had been followed for >36 months (7).
Invasive pneumococcal disease was defined as an acute illness consistent with pneumococcal disease in a child from whom S. pneumoniae was cultured from a normally sterile site (e.g., blood or CSF). Cases were identified through active surveillance. At the time of the primary efficacy analysis in August 1998, PCV7 was 100% efficacious against vaccine serotypes among children who were either fully vaccinated (i.e., completed the three-dose primary series) or partially vaccinated (i.e., received >1 doses) (95% confidence interval [CI] = 80.5%--100% and 85.4%--100%, respectively). Additional cases have been identified since completion of the primary analysis. When all cases of invasive disease were evaluated during follow-up analysis in April 1999, PCV7 was 97.4% (95% CI = 82.7%--99.9%) and 93.9% (95% CI = 79.6%--98.5%) efficacious against vaccine serotypes among children who were fully or partially vaccinated, respectively (Table 2). Statistically significant serotype-specific protection also was reported for four of the seven vaccine serotypes (i.e., 19F, 14, 18C, and 23F). The study's ability to evaluate efficacy for the remaining three serotypes was limited because too few cases were caused by these serotypes in either treatment group (7). No evidence existed of an increase in invasive disease caused by nonvaccine serotypes.
To examine effectiveness of PCV7 in preventing pneumonia of any etiology among the study population as a secondary outcome, investigators reviewed hospital, outpatient, and emergency room records of the children in the study. Monitored outcomes included clinical diagnoses of pneumonia, clinical pneumonia with abnormal chest X-rays, and clinical pneumonia with consolidation of >2.5-cm found on chest X-rays. Among children who received >1 doses of study vaccine (intent-to-treat analysis), use of PCV7 resulted in 11.4% (95% CI = 1.3%--20.5%) fewer episodes of clinical pneumonia, regardless of X-ray or culture result. Cases of clinical pneumonia accompanied by an X-ray with any evidence of an infiltrate were reduced by 33.0% (95% CI = 7.3%--51.5%). Among children who had clinically diagnosed pneumonia and X-ray evidence of an area of consolidation of >2.5 cm as read by both a pediatrician and a radiologist, efficacy of PCV7 was 73.1% (95% CI = 3.0%--88.3%) (102).
Effectiveness of PCV7 in preventing health-care visits for AOM from all causes, including risk for first AOM episode, frequent AOM episodes, and tympanostomy tube placement, was assessed as a secondary outcome in the Northern California Kaiser Permanente efficacy trial. Episodes of physician-diagnosed AOM among the study population were identified retrospectively through computerized databases of clinic and emergency room encounters. To exclude return visits related to one episode of AOM, a case of AOM was defined as a visit for otitis media, with no visits for otitis media made during the previous 21 days, or if the visit had occurred during the previous 21--42 days, the appointment had been made <3 days in advance. Frequent AOM was defined as >3 episodes within 6 months or >4 episodes within 1 year.
A substantial reduction in episodes of AOM was found. Vaccine impact was greatest for frequent otitis and tympanostomy tube placement (Table 3) (7). Compared with children who received control vaccine, children who received PCV7 had 6.4% (95% CI = 3.9%--8.7%) fewer episodes of AOM, 9.1% (95% CI = 4.1%--13.8%) fewer episodes of frequent AOM (defined as >3 episodes in 6 months or >4 in 1 year), and they underwent 20.3% (95% CI = 3.6%--34.1%) fewer tympanostomy tube placements (Table 3). Among a subset of study children with spontaneously ruptured tympanic membranes, S. pneumoniae was cultured from the draining ears of 6 children vaccinated with PCV7 and 17 children who received the control vaccine (vaccine efficacy was 65% for AOM caused by vaccine-serotype pneumococci [P = 0.035]).
The ability of PCV7 to protect children against AOM was also evaluated in an efficacy trial conducted in Finland (103). Children (N = 1,662) were randomized to receive either PCV7 or hepatitis B (control) vaccine at ages 2, 4, 6, and 12 months. Outcomes measured included AOM with concurrent myringotomy to establish bacterial diagnosis. Investigators reported 2,596 AOM episodes during the follow-up period in perprotocol analysis. Of these, 357 episodes were caused by vaccine-serotype pneumococci. A total of 107 episodes occurred among the PCV7 group, and 250 among the control group, for an estimated efficacy of 57% (95% CI = 44%--67%) against culture-confirmed AOM caused by vaccine serotypes. The estimated percent efficacy was lower for outcomes that included nonvaccine pneumococcal serotypes or other pathogens causing AOM. For prevention of AOM caused by pneumococci of any serotype, efficacy was estimated to be 34% (95% CI = 21%--45%), and efficacy against AOM irrespective of etiology was 6% (95% CI = -4%--16%) (83). Further analysis revealed an efficacy against vaccine-related serotypes (i.e., 6A, 9N, 18B, 19A, and 23A) of 51% (95% CI = 27%--67%). An increase of 33% in the rate of AOM episodes caused by nonvaccine serotypes occurred among the group who received PCV7 compared with the control group. However, in spite of the increase in disease caused by nonvaccine serotypes, the net effect regarding pneumococcal AOM was a reduction of 34%.
Effect on Antimicrobial Use
Prevention of pneumococcal infections among young children after widespread use of PCV7 might result in decreased use of antibiotics, as reported in the Northern California Kaiser Permanente vaccine efficacy trial where a 5.3% reduction was reported among the group of children who received PCV7 (104). A reduction in antibiotic use might slow or reverse the trend of increasing prevalence of antimicrobial resistance among pneumococci.
Other Studies of Vaccine Impact
Hib conjugate vaccines reduce acquisition of nasopharyngeal carriage of the bacterium among infants and children, an effect that is thought to contribute to the success of Hib vaccination programs by providing herd immunity (105). A 40% and 50% reduction of vaccine-serotype S. pneumoniae carriage has been reported in studies of a 9-valent CRM197 pneumococcal conjugate vaccine conducted in Israel and South Africa, respectively (5,6). In both studies, vaccination resulted in a reduction in nasopharyngeal carriage of vaccine-type pneumococci and a simultaneous increase in detection of carriage of pneumococci of nonvaccine serotypes. Whether this represents true replacement or unmasking of serotypes that were also colonizing the nasopharynx is unknown. Additional information regarding the impact of PCV7 on nasopharyngeal carriage is expected from a future efficacy trial among Navajo and Apache populations.
In a double-blind, randomized study of a 9-valent pneumococcal conjugate vaccine containing the CRM197 carrier among healthy toddlers attending day care centers in Israel, conjugate vaccine or control (meningococcal C conjugate) vaccine was administered to children in two age groups, 12--17 months (two doses) and 18--35 months (one dose). Primary outcomes were carriage rates of vaccine serotype- and penicillin-resistant pneumococci. After 21 months of follow-up, carriage rates of vaccine-serotype pneumococci were substantially lower among toddlers who were vaccinated with 9-valent pneumococcal conjugate vaccine compared with the control group. For both vaccine-serotype and penicillin-resistant S. pneumoniae, differences in carriage persisted for children aged <36 months and those >36 months, although the difference was greatest for children aged <36 months. The study also reported herd immunity within families; siblings of 9-valent pneumococcal conjugate vaccine recipients were substantially less likely to carry antibiotic-resistant pneumococci compared with siblings of control-vaccine recipients (106).
Duration of Protection
Duration of protection after vaccination with PCV7 is unknown. However, immunologic memory does occur. Among infants aged <20 months who received primary vaccination with two or three doses of PCV7, administration of PPV23 <18 months later resulted in a booster response. A similar response was elicited among children aged 2--3 years when administered PPV23 <20 months after a bivalent (i.e., 6A plus 23F) CRM197 conjugate vaccine (92,96). Additional studies of PCV7 with longer follow-up periods are needed. Evaluation of duration of protection will be critical for older children at high risk for disease (e.g., those with SCD or HIV infection).
Cost-Benefit Analysis
Costs and benefits of a routine PCV7 program for healthy U.S. infants and children were evaluated in a study using current estimates of pneumococcal disease burden (i.e., meningitis, bacteremia, pneumonia, and AOM episodes), clinical outcomes, vaccine efficacy, and health-care costs (107). Sources of clinical outcomes and costs included published and unpublished data, expert consensus, and computerized databases from Kaiser Permanente of Northern California. For each annual U.S. birth cohort, routine PCV7 vaccination is estimated to prevent approximately 12,000 (78% of potential) cases of pneumococcal meningitis and bacteremia; 53,000 (69% of potential) pneumococcal pneumonia cases; and >1 million (8% of potential) episodes of clinically diagnosed otitis media. Vaccination of healthy infants would result in net savings to society if vaccine costs were <$46/dose. Net savings to health insurers would result if the vaccine costs were <$18/dose. A program in which one dose of vaccine was administered to children aged 24--59 months to bring them up-to-date with their vaccinations would result in societal cost savings at a vaccine price of <$80 for children aged 24--35 months and <$50 for those aged 48--59 months. From the health-care--payer perspective, savings would result if vaccine costs were <$40 and <$20 when administered to children aged 24--35 months and 48--59 months, respectively. Results of this study demonstrate that cost-effectiveness of vaccination of infants and toddlers is most influenced by vaccine price, especially among children aged >24 months. In a recent reanalysis of cost-effectiveness of PCV7 using additional and updated information (including newly available safety data, national costs, and rates of tympanostomy tube placement), the same investigators found that break-even costs for vaccination of infants from societal and health-care--payer perspective were 40and40 and 40and17, respectively (108).
Vaccine Safety
Safety of Administration of PCV7 Series Among Infants
Rates and types of adverse events associated with PCV7 administered at ages 2, 4, 6, and 12--15 months are acceptable when compared with the demonstrated benefits of vaccination. In the Northern California Kaiser Permanente efficacy trial, rates of adverse events were compared between children who received PCV7 and those who received the control vaccine (investigational group C meningococcal conjugate). Routine childhood vaccines were administered concurrently with PCV7 and control vaccines.***** To assess vaccine safety, information regarding local and systemic reactions was collected at 48--72 hours and 14 days after each dose. Using telephone interviews, investigators collected adverse-event histories for two subsets of the study population --- initially a group receiving DTwP (N = 6,000) and later a group receiving diphtheria toxoid-tetanus toxoid acellular pertussis (DTaP) vaccine (N = 1,500). Frequency of uncommon events requiring medical attention after vaccination was evaluated for the entire study cohort and included emergency room and outpatient clinic visits occurring <30 days and hospitalizations occurring <60 days after receiving the study vaccines.
PCV7 vaccination resulted in less frequent local reactions than DTwP vaccine, but more frequent local reactions than DTaP vaccine and the control vaccine. Except for erythema, no pattern of increasing local reactogenicity with subsequent doses of PCV7 was reported (Table 4).
Fever >100.4 F (>38 C) <48 hours after vaccination was more common among children who received PCV7 concomitantly with DTaP vaccine and other recommended vaccines than among those who received the control vaccine. This difference was statistically significant after each dose of vaccine in the primary series but not the fourth dose (Table 5). Rates of fever >102.2 F (>39 C) were substantially greater among those who received PCV7 after dose two of the primary series (i.e., 2.5% versus 0.8%).
Febrile seizures after vaccination were slightly more common in the PCV7 group; however, the majority of events occurred when whole-cell pertussis vaccine was administered concurrently with PCV7. Using hospitalizations, emergency room visits, and data from all other sources, seizures occurring <3 days after vaccination were reported for four children who received control vaccine and eight who received PCV7. Of the eight PCV7 recipients who had a seizure, seven had received concurrent DTwP vaccine. Two children who were vaccinated with concurrent DTaP vaccine had a seizure <3 days after vaccination, one in each of the PCV7 and control-vaccine groups (109). The seizure rate <3 days after vaccination with PCV7 and concurrent vaccinations was approximately 1/7,000 doses, below historic rates after whole-cell pertussis vaccinations (109,110).
As of April 20, 1999, a total of 32 children who were originally enrolled in the study had died (109); however, none of the deaths were reported by investigators to be related to the vaccine. A total of 12 cases of sudden infant death syndrome (SIDS) were observed among the study cohort <1 year after vaccination, 4 among the PCV7 group (0.2 cases/1,000 children) and 8 among the control group (0.4 cases/1,000 children). These rates are less than expected historically; 0.5 cases/1,000 children were observed in California in 1996 and 1997. One death attributable to SIDS occurred within the first week of vaccination in a child who received PCV7 concurrently with other vaccines. In an age- and season-adjusted analysis based on California SIDS data, 1.06 cases would have been expected <1 week after vaccination (7).
Safety of Administration of PCV7 Among Older Children
Safety data are available from four immunogenicity studies of PCV7 among older infants and children (109). Approximately 900 doses of PCV7 were administered to 560 children aged 7 months--9 years following the recommended schedule for vaccination administration for children who are beyond the age of the infant schedule. Comparisons across the studies demonstrate that, generally, frequency of local reactions was higher among older children than among children vaccinated at age <1 year. Frequency of fever of >100.4 F (>38 C) after one dose of PCV7 ranged from 6.8% to 36.7%. No distinct, age-related patterns of systemic reactions was evident. Fussiness was the most commonly observed systemic reaction (Tables 6,7).
Safety of Administration of PCV7 After PPV23
Minimal safety data are available regarding the sequence of PPV23 followed by PCV7. However, in one published study of children and young adults with SCD, 16 of the study participants had already received one dose of PPV23 3--15 years previously (persons who had received PPV23 within the previous 2 years were excluded). Of these 16 SCD patients, 9 who had received two doses of PCV7 followed by one dose of PPV23 8 weeks later were compared with 7 who had received one dose of PPV23. No severe reactions were reported; local reactions were similar between the two groups (93).
Safety of Administration of PPV23 After PCV7
Five studies have been completed in which 152 infants, children, or young adults received >1 doses of 2-, 5-, or 7-valent pneumococcal conjugate vaccine conjugated to CRM197 followed by a dose of PPV23. Populations studied included healthy infants and children (92,96), HIV-infected children and young adults (94), and infants and children with SCD (93,99). PPV23 was administered 6 weeks--20 months after pneumococcal conjugate vaccine. Adverse events were described in three of the five studies. No serious adverse events were identified after the dose of PPV23. No increase in adverse events was reported among HIV-infected persons who received 5-valent pneumococcal conjugate vaccine followed by PPV23 as compared with those who received PPV23 alone (94). Among children with SCD who received either PCV7 followed by PPV23 or PPV23 alone, fever occurred among 4 of 11 and 2 of 11 children, respectively. Local reactions did not differ between the two groups (93).
PPV23 Revaccination
No published studies have been designed specifically to examine adverse events among children who were administered a second dose of PPV23 after an earlier dose of PPV23. However, in the previously described study (93) in which young children and adults with SCD were vaccinated with either PCV7 and PPV23 consecutively or PPV23 alone, 16 of 23 enrolled patients had been vaccinated with one dose of PPV23 3--15 years earlier. All patients were randomized to receive either one dose of PPV23 or two doses of PCV7 followed by a booster dose of PPV23 8 weeks later. No severe reactions were reported after the second dose of PPV23.
Vaccine Administration
PCV7 is administered intramuscularly as a 0.5-ml dose. PCV7 is licensed for use among infants aged >6 weeks. PCV7 can be administered at the same time as other routine childhood vaccinations in a separate syringe at a separate injection site. In clinical studies of PCV7, when routine childhood vaccinations were administered simultaneously with PCV7 vaccine but at different sites, suppression of Hib response occurred after the fourth dose. However, >97% of children achieved antibody titers >1 µg/ml. Additionally, a limited decrease in antibody response to poliovirus Type 1 occurred; however, clinical significance of these decreased responses is uncertain (109,111).
Conjugate vaccines containing diphtheria toxoid or protein as carriers (e.g., CRM197) should not be considered immunizing agents against diphtheria. Thus, no change in the vaccination schedule for DTaP vaccine is currently recommended. Simultaneous administration of PCV7 with other vaccines at the 2-, 4-, and 6-month visits might necessitate five injections at each visit. Physicians might consider potential approaches to decreasing the number of simultaneous injections that are needed. One approach is to administer the first two doses of hepatitis B vaccine at birth and age 1 month. Alternatively, a Hib-hepatitis B combination product is available for use at ages 2, 4, and 12--15 months. An option to decrease the number of simultaneous injections when the fourth dose is administered would be to divide needed injections between visits at ages 12 months and 15 or 18 months. Combination vaccine products currently being evaluated by manufacturers might further decrease the need for multiple injections.
Precautions and Contraindications
Vaccination with PCV7 is contraindicated among persons known to have a hypersensitivity to any component of the vaccine. Health-care providers can choose to delay vaccination of children with moderate or severe illness until the child has recovered, although minor illnesses (e.g., mild upper-respiratory infection with or without low-grade fever) are not contraindications to vaccination. Any adverse event suspected to be associated with PCV7 vaccination should be reported to the Vaccine Adverse Events Reporting System (VAERS).****** Concurrent administration of PCV7 and PPV23 is not recommended because safety and efficacy of concurrent vaccination has not been studied.
RECOMMENDATIONS FOR USE OF PCV7
Children for Whom PCV7 Is Recommended
Children Aged <23 Months
All children aged <23 months should be vaccinated with PCV7 (Table 8). Infant vaccination provides the earliest possible protection, and children aged <23 months have the highest rates of pneumococcal infection. PCV7 is safe and highly efficacious in preventing invasive disease, and it is effective in preventing a portion of AOM cases and pneumonia among healthy infants and young children (Strength of evidence: children aged 2--6 months, A;******* children aged 7--23 months, B) (Table 9).
Vaccination Schedule. Infants receiving their first dose at age <6 months should receive three doses of PCV7 at intervals of approximately 2 months, followed by a fourth dose at age 12--15 months (Table 10). Newborns should begin the schedule at age 2 months, although PCV7 can be administered as young as age 6 weeks. Prematurely born infants (i.e., <37 weeks gestation) should receive PCV7 at the recommended chronologic age concurrent with other routine vaccinations. For infants with prolonged nursery stays, initiation of vaccination should begin during discharge planning. Children aged >7 months not previously vaccinated should also be vaccinated according to the recommended schedule (Table 10). The proposed vaccination schedule is the same for all children aged <23 months, regardless of the presence of underlying medical conditions (e.g., children with HIV infection, SCD or other asplenia, chronic disease, or who are otherwise immunocompromised). Interruption of the vaccination schedule does not require reinstitution of the entire series or the addition of extra doses (Table 11).
Children Aged 24--59 Months Who Are at High Risk for Pneumococcal Infection
Children aged 24--59 months should receive PCV7 vaccination if they are at high risk for pneumococcal infection caused by an underlying medical condition (Table 8). This recommendation applies to the following groups:
- children with SCD and other sickle cell hemoglobinopathies, including hemoglobin SS, hemoglobin S-C, or hemoglobin S-ß-thalassemia, or children who are functionally or anatomically asplenic;
- children with HIV infection;
- children who have chronic disease, including chronic cardiac and pulmonary disease (excluding asthma), diabetes mellitus, or CSF leak; and
- children with immunocompromising conditions, including a) malignancies (e.g., leukemia, lymphoma, Hodgkin's disease); b) chronic renal failure or nephrotic syndrome; c) those children receiving immunosuppressive chemotherapy, including long-term systemic corticosteroids; and d) those children who have received a solid organ transplant.********
Immunogenicity and safety studies have been conducted using PCV7 among children with SCD (93,97,99) and a 5-valent conjugate vaccine among children with HIV infection (94,100) (Strength of evidence: B). The efficacy of PCV7 among children with chronic disease or who are immunocompromised has not been evaluated, but effectiveness is anticipated on the basis of studies conducted in other groups (Strength of evidence: C).
Vaccination Schedule. For children aged 24--59 months with underlying medical conditions (Table 8), ACIP recommends two doses of PCV7, administered 2 months apart, followed by one dose of PPV23 administered >2 months after the second dose of PCV7 (Tables 10,12). The recommendation for two PCV7 doses is based on results of an immunogenicity study conducted among SCD patients. That study reported that a nonstatistically significant antibody response to serotype 6B after one dose of PCV7 increased statistically significantly after a second dose of PCV7 (93). Serotype 6B is one of the most common pneumococcal serotypes colonizing or causing invasive disease among SCD patients and healthy children (47,112--114).
Penicillin prophylaxis should be continued for children with SCD to age >5 years, regardless of vaccination with PCV7. Protective efficacy of PCV7 for children with SCD has not been studied, and the vaccine does not protect against all serotypes causing disease. However, penicillin prophylaxis substantially reduces the risk for invasive pneumococcal infections among SCD patients (112).
Other Children Who Might Benefit from Vaccination with PCV7
ACIP recommends that health-care providers consider PCV7 vaccination for all other children aged 24--59 months, with priority given to the following populations:
- children aged 24--35 months;
- children of Alaska Native or American Indian descent;
- children of African-American descent;
- children who attend group day care centers;*********
This recommendation is made on the basis of the moderate risk for pneumococcal disease, including antibiotic-resistant infections, among these populations and on potential cost-benefit. Data regarding efficacy and immunogenicity of PCV7 are limited for these specific risk and age groups. However, the vaccine is safe and immunogenic among all healthy children aged 24--59 months. Also, immunogenicity data are available regarding use of another pneumococcal conjugate vaccine among Apache, Navajo, and Alaska Native children (101), and efficacy data for children aged <23 months probably are relevant for healthy children aged 24--59 months (Strength of Evidence: B).
PPV23 is licensed for use among children aged >2 years who are at high risk for pneumococcal infections (e.g., those with SCD or HIV infection). However, the conjugate vaccine has advantages over PPV23, which include induction of immune system memory (possibly resulting in longer duration of protection), reduction in carriage, probable higher efficacy against serotypes causing most invasive disease, and probable effectiveness against noninvasive syndromes (e.g., nonbacteremic pneumonia and AOM). If pneumococcal vaccine is to be used among healthy children aged 24--59 months, ACIP recommends that PCV7 be used.
Vaccination Schedules. ACIP recommends that one dose of PCV7 be considered for Alaska Native and American Indian children aged 24--59 months. Previously, ACIP recommended PPV23 for Alaska Natives and certain American Indian populations aged >2 years (1). However, use of PCV7 among these children offers multiple potential advantages over PPV23 as previously discussed. In contrast, PPV23 offers potentially broader serotype coverage. Recent studies demonstrate that only 68% and 57% of invasive infections among Alaska Natives and American Indians in the U.S. Southwest aged 24--59 months, respectively, were caused by serotypes included in the 7-valent conjugate vaccine, lower proportions than among non-Alaska Native/non-American Indian populations (115,116). Therefore, vaccination program personnel and other health-care providers might consider whether Alaska Native and American Indian children aged 24--59 months would benefit by the additional coverage provided by the 23-valent polysaccharide vaccine. Data are limited regarding safety and immunogenicity of PPV23 after PCV7. If additional serotype coverage is desired by parents and health-care providers, PPV23 should be administered >2 months after PCV7 (Strength of evidence: C). A community-randomized trial to evaluate the efficacy of PCV7 among Navajo and Apache children in preventing pneumococcal disease is underway. Future recommendations for use of PCV7 among Alaska Native and American Indian populations might be modified on the basis of that trial.
ACIP recommends that physicians consider administering one dose of PCV7 to their African-American pediatric patients aged 24--59 months because of their increased risk for pneumococcal infection. The proportion of invasive pneumococcal disease among African-American children that is caused by PCV7 serotypes does not differ from whites in the United States, and rates of invasive disease decline with age (ABCs/EIP Network, unpublished data, 2000). Therefore, no additional vaccination with PPV23 is recommended (Strength of evidence: B). Additionally, because of increased risk for invasive pneumococcal disease, colonization with antibiotic-resistant pneumococcal strains, and AOM, health-care providers should consider administering one dose of PCV7 to previously unvaccinated children aged 24--59 months who attend group day care centers.
Children Aged >5 Years and Adults Who Are At High Risk for Pneumococcal Infection
Data are limited regarding efficacy of PCV7 among children aged >5 years and adults. However, limited studies report that a) 5-valent pneumococcal conjugate vaccine is immunogenic among HIV-infected children aged 2--9 years (94); b) PCV7 is immunogenic among children aged 2--13 years with recurrent respiratory infections (117); and c) PCV7 is immunogenic among older children and adults aged 4--30 years with SCD (93). Administering PCV7 to older children with high-risk conditions is not contraindicated.
Studies among healthy adults aged >50 years (118) and among HIV-infected adults aged 18--65 years (119) did not demonstrate substantially greater ELISA antibody concentrations after administration of 5-valent pneumococcal conjugate vaccine compared with PPV23. Also, the proportion of invasive pneumococcal isolates covered by PCV7 is only 50%--60% among older children and adults, in contrast with 80%--90% coverage by PPV23 among this older group. Therefore, current data do not support a recommendation to replace PPV23 with PCV7 among older children and adults.
Recommendations for Use of PCV7 Among Children Previously Vaccinated with PPV23
Children aged 24--59 months who are at high risk for pneumococcal disease and who have already received PPV23 (i.e., children with SCD, HIV infection, or who have other immunocompromising illnesses or chronic diseases) could benefit from the immunologic priming and T-cell--dependent immune system response induced by PCV7. Thus, among children in these groups at high risk, sequential use of the two pneumococcal vaccines can provide additional protection. Health-care providers should vaccinate children aged 24--59 months at high risk who have not previously received PCV7 but who have already received PPV23 with two doses of PCV7 administered >2 months apart. Vaccination with PCV7 should be initiated >2 months after vaccination with PPV23. Providers should be aware that minimal safety data are available regarding this vaccine sequence.
Recommendations for Use of PPV23 Among Children Previously Vaccinated with PCV7
Administration of PCV7 Followed by PPV23 Among Children at High Risk for Pneumococcal Disease
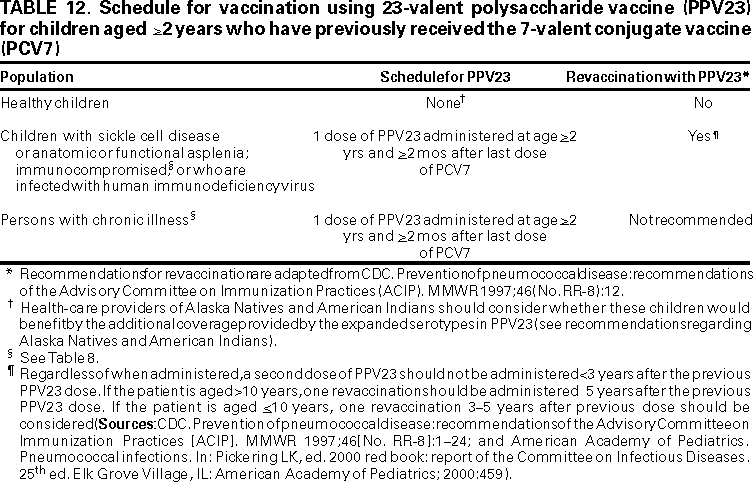
Children who have completed the PCV7 vaccination series before age 2 years and who are among risk groups for which PPV23 is already recommended should receive one dose of PPV23 at age 2 years (>2 months after the last dose of PCV7). These groups at high risk include children with SCD, children with functional or anatomic asplenia, children who are HIV-infected, and children who have immunocompromising or chronic diseases (1) (Table 8). Although data regarding safety of PPV23 administered after PCV7 are limited, the opportunity to provide additional serotype coverage among these children at very high risk justifies use of the vaccines sequentially. For children of Alaska Native or American Indian descent, addition of PPV23 after PCV7 can be considered.
Revaccination with PPV23
Immunocompromised children or children with SCD or functional or anatomic asplenia should be revaccinated with PPV23 as previously recommended (1) (Table 12). If the child is aged <10 years, one revaccination should be considered 3--5 years after the previous dose of PPV23 (1,120). Data are limited regarding adverse events related to a second dose of PPV23 administered after PCV7. Health-care providers should not administer a second dose of PPV23 any earlier than 3 years after the initial dose of PPV23.
AREAS FOR FUTURE RESEARCH
With recent licensure and introduction of PCV7, close monitoring of disease trends and long-term vaccine safety will be high priorities for public health organizations and health-care providers. Postlicensure surveillance will be necessary to a) detect potential changes in serotype distribution, including any increase in disease caused by serotypes not contained in PCV7; b) follow trends in antimicrobial resistance and antibiotic use; c) detect potential herd immunity induced by widespread use of PCV7; and d) track vaccine-related health events.
Areas where research is ongoing or necessary to determine the most effective use of PCV7 include the following:
- Optimal vaccination schedule for older children at high risk. Further studies are needed to identify optimal vaccination schedules for PCV7 and PPV23 among children aged >2 years who are at high risk for infection, including children with SCD, HIV infection, and other chronic diseases or immunocompromising conditions, particularly children who have received a bone marrow transplant. Research is critical regarding optimal scheduling of vaccination with PCV7 among children who have already received PPV23 and revaccination with PPV23 after vaccination with PCV7.
- Combined vaccines including PCV7 and other routine vaccines for infants. Reducing the number of required separate injections would improve the infant vaccination schedule. Additional research is needed to evaluate safety and immunogenicity of PCV7 when administered in combination with other antigens.
- Studies of safety, immunogenicity, and efficacy among adults at high risk for pneumococcal infection. Additional studies are needed to evaluate potential use of PCV7 among adults at increased risk for pneumococcal infection. Benefits and risks involved with using a 7-, 9-, 11-, or 15-valent pneumococcal conjugate vaccine in place of or in addition to PPV23 warrant further investigation. Rationale for additional study of a combined or sequential regimen includes potential benefits of conjugate pneumococcal vaccines (e.g., induction of immunologic memory with increased duration of protection, reduction of nasopharyngeal carriage of pneumococci, and potential impact on nonbacteremic pneumonia) along with the benefit of broader serotype coverage provided by PPV23.
- **Duration of protection.**For persons of all age groups, duration of protection after vaccination with PCV7 is unknown. Investigation of potential need for revaccination with PCV7 or PPV23 after primary vaccination is warranted.
- Identifying and defining immune system correlates of protection. Type-specific antibody concentration necessary to confer protection against pneumococcal infection is unknown. Researchers are attempting to determine and standardize the level of antibody, as measured by ELISA, that provides serotype-specific protection against pneumococcal disease. Functional tests of induced immune system response (e.g., opsonophagocytosis) are also being evaluated. A determination of immunologic markers that best correlate with clinical protection is needed. Validated immune system correlates of protection will accelerate evaluation and licensure of new vaccines against pneumococcal infection.
- Alternative pneumococcal vaccines. Research is ongoing regarding development of alternative pneumococcal vaccines. Investigators are evaluating possible roles of conserved pneumococcal proteins (e.g., pneumolysin, surface protein A, or surface adhesion A) as antigens that have potential to provide broad protection against disease caused by all pneumococcal serotypes (121). Use of other peptides or pneumococcal proteins as carriers in conjugate vaccines is also being studied. Additionally, alternative routes of delivery including intranasally and orally administered vaccines are under investigation (122,123).
Acknowledgments
The authors express their appreciation for contributions from the following persons: Donna M. Ambrosino, M.D.; Steven Black, M.D.; Ron Dagan, M.D.; Jill Hackell, M.D.; William P. Hausdorff, Ph.D.; James C. King, Jr., M.D.; Tracy A. Lieu, M.D., M.P.H.; Carlton K. Meschievitz, M.D., M.P.H.; John F. Modlin, M.D.; Deborah C. Molrine, M.D., M.P.H.; Katherine L. O'Brien, M.D., M.P.H.; Peter Paradiso, Ph.D.; G. Thomas Ray, M.B.A.; Mathuram Santosham, M.D., M.P.H.; Henry R. Shinefield, M.D.; Jeffrey L. Silber, M.D.; Louis Vernacchio, M.D.; and Catherine Wilfert, M.D.
We are also thankful for assistance from the following CDC staff: William L. Atkinson, M.D., M.P.H.; Sharon Balter, M.D.; Scott F. Dowell, M.D.; John R. Livengood, M.D.; Walter A. Orenstein, M.D.; Anne Schuchat, M.D.; Carolyn J. Wright; Elizabeth R. Zell, M.Stat.; and the Active Bacterial Core Surveillance/Emerging Infections Program Network staff.
References
- CDC. Prevention of pneumococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 1997;46(No. RR-8):1--24.
- Douglas RM, Miles HB. Vaccination against Streptococcus pneumoniae in childhood: lack of demonstrable benefit in young Australian children. J Infect Dis 1984;149:861--9.
- Sell SH, Wright PF, Vaughn WK, Thompson J, Schiffman G. Clinical studies of pneumococcal vaccines in infants; I: reactogenicity and immunogenicity of two polyvalent polysaccharide vaccines. Reviews of Infectious Diseases 1981;3 (suppl):S97--107.
- Douglas RM, Hansman D, Miles HB, Paton JC. Pneumococcal carriage and type-specific antibody. American Journal of Diseases of Children 1986;140:1183--5.
- Dagan R, Givon N, Yagupsky P, Porat N, Janco J, Chang I, et al. Effect of a 9-valent pneumococcal vaccine conjugated to CRM197 on nasopharyngeal (NP) carriage of vaccine type and non-vaccine type S. pneumoniae strains among day care center (DCC) attendees [Abstract G-52]. 38th Interscience Conference on Antimicrobial Agents and Chemotherapy, San Diego, CA 1998;29.
- Mbelle N, Huebner RE, Wasas AD, Kimura A, Chang I, Klugman K. Immunogenicity and impact on nasopharyngeal carriage of a nonavalent pneumococcal conjugate vaccine. J Infect Dis 1999;180:1171--6.
- Black S, Shinefield H, Fireman B, et al. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Pediatr Infect Dis J 2000;19:187--95.
- CDC. Active Bacterial Core Surveillance (ABCs) Report, Emerging Infections Program Network (EIP), Streptococcus pneumoniae, 1998. Atlanta, GA: US Department of Health and Human Services, CDC 1999. Available at <http://www.cdc.gov/ncidod/dbmd/abcs/spneu98.pdf>. Accessed August 4, 2000.
- Schuchat A, Robinson K, Wenger JD, et al. Bacterial meningitis in the United States in 1995. N Engl J Med 1997;337:970--6.
- Turner RB, Lande AE, Chase P, Hilton N, Weinberg D. Pneumonia in pediatric outpatients: cause and clinical manifestations. J Pediatr 1987;111:194--200.
- Heiskanen-Kosma T, Korppi M, Jokinen C, et al. Etiology of childhood pneumonia: serologic results of a prospective, population-based study. Pediatr Infect Dis J 1998;17:986--91.
- Dowell SF, Butler JC, Giebink GS, et al. Acute otitis media: management and surveillance in an era of pneumococcal resistance---a report from the Drug-Resistant Streptococcus pneumoniae Therapeutic Working Group. Pediatr Infect Dis J 1999;18:1--9.
- Heikkinen T, Thint M, Chonmaitree T. Prevalence of various respiratory viruses in the middle ear during acute otitis media. N Engl J Med 1999;340:260--4.
- Block SL. Causative pathogens, antibiotic resistance and therapeutic considerations in acute otitis media. Pediatr Infect Dis J 1997;16:449--56.
- Bluestone CD, Stephenson JS, Martin LM. Ten-year review of otitis media pathogens. Pediatr Infect Dis J 1992;11:S7--11.
- Wald ER, Milmoe GJ, Bowen A, Ledesma-Medina J, Salamon N, Bluestone CD. Acute maxillary sinusitis in children. N Engl J Med 1981;304:749--54.
- National Center for Health Statistics. National ambulatory medical care survey: 1998 summary. Hyattsville, MD: US Department of Health and Human Services, CDC, 2000. (Advance data, no. 315, July 19, 2000). Available at <http://www.cdc.gov/nchs/products/pubs/pubd/ad/311-320/311-320.htm>. Accessed August 9, 2000.
- National Center for Health Statistics. National hospital ambulatory medical care survey: 1998; outpatient department summary. Hyattsville, MD: US Department of Health and Human Services, CDC, 2000. (Advance data, no. 317, July 27, 2000). Available at <http://www.cdc.gov/nchs/products/pubs/pubd/ad/311-320/311-320.htm>. Accessed August 9, 2000.
- National Center for Health Statistics. Ambulatory health care visits by children: principal diagnosis and place of visit. Hyattsville, MD: US Department of Health and Human Services, CDC, 1998. (Vital and health statistics, series 13, no. 137).
- Teele DW, Klein JO, Rosner B, and the Greater Boston Otitis Media Study Group. Epidemiology of otitis media during the first seven years of life in children in greater Boston: a prospective cohort study. J Infect Dis 1989;160(1):83--94.
- Stool SE, Field MJ. Impact of otitis media. Pediatr Infect Dis J 1989;8:S11--4.
- National Center for Health Statistics. Ambulatory surgery in the United States, 1996. Hyattsville, MD: US Department of Health and Human Services, CDC, 1998. (Advance data from vital and health statistics, no. 300).
- Dagan R, Leibovitz E, Cheletz G, Leiberman A, Porat N, Yagupsky P. Antibiotic treatment (Tx) in acute otitis media (AOM) induces middle ear fluid (MEF) superinfection with preexisting nasopharyngeal (NP) antibiotic-resistant Streptococcus pneumoniae [Abstract 1183]. 39th Interscience Conference on Antimicrobial Agents and Chemotherapy, San Francisco, CA 1999;677.
- Block SL, Harrison CJ, Hedrick JA, et. al. Penicillin-resistant Streptococcus pneumoniae in acute otitis media: risk factors, susceptibility patterns and antimicrobial management. Pediatr Infect Dis J 1995;14:751--9.
- Dowell SF, Schwartz B. Resistant pneumococci: protecting patients through judicious use of antibiotics. Am Fam Physician 1997;55:1647--54.
- Hofmann J, Cetron MS, Farley MM, et al. Prevalence of drug-resistant Streptococcus pneumoniae in Atlanta. N Engl J Med 1995;333:481--6.
- Breiman RF, Spika JS, Navarro VJ, Darden PM, Darby CP. Pneumococcal bacteremia in Charleston County, South Carolina: a decade later. Arch Intern Med 1990;150:1401--5.
- Davidson M, Parkinson AJ, Bulkow LR, Fitzgerald MA, Peters HV, Parks DJ. Epidemiology of invasive pneumococcal disease in Alaska, 1986--1990---ethnic differences and opportunities for prevention. J Infect Dis 1994;170:368--76.
- Bennett NM, Buffington J, LaForce FM. Pneumococcal bacteremia in Monroe County, New York. Am J Public Health 1992;82:1513--6.
- Butler JC, Bulkow LR, Parks DJ, Parkinson AJ. Epidemiology of pneumococcal bacteremia and meningitis during the first 5 years of life in Alaska: implications for conjugate pneumococcal vaccine use [Abstract 1058]. 39th Interscience Conference on Antimicrobial Agents and Chemotherapy, San Francisco, CA, 1999;672.
- O'Brien KL, Croll J, Parkinson AJ, Reid R, Santosham M. Active laboratory-based surveillance for invasive Streptococcus pneumoniae (pneumococcus) among Navajo people in the American southwest, 1989--1996 [Abstract 1187]. 39th Interscience Conference on Antimicrobial Agents and Chemotherapy, San Francisco, CA, 1999;678.
- Cortese MM, Wolff M, Almeido-Hill J, Reid R, Ketcham J, Santosham M. High incidence rates of invasive pneumococcal disease in the White Mountain Apache population. Arch Intern Med 1992;152:2277--82.
- Harrison LH, Dwyer DM, Billmann L, Kolczak M, Schuchat A. Invasive pneumococcal infection in Baltimore, Md. Arch Intern Med 2000;160:89--94.
- Levine OS, Farley M, Harrison LH, et al. Risk factors for invasive pneumococcal disease in children: a population-based case-control study in North America. Pediatrics 1999;103:e28. Available at <http://www.pediatrics.org/cgi/content/full/103/3/e28>. Accessed August 4, 2000.
- Henneberger PK, Galaid EI, Marr JS. Descriptive epidemiology of pneumococcal meningitis in New York City. Am J Epidemiol 1983;117:484--91.
- Filice GA, Van Etta LL, Darby CP, Fraser DW. Bacteremia in Charleston County, South Carolina. Am J Epidemiol 1986;123:128--36.
- Chen FM, Breiman RF, Farley M, Plikaytis B, Deaver KA, Cetron MS. Geocoding and linking data from population-based surveillance and the US census to evaluate the impact of median household income on the epidemiology of invasive Streptococcus pneumoniae infections. Am J Epidemiol 1998;148:1212--8.
- Zangwill KM, Vadheim CM, Vannier AM, Hemenway LS, Greenberg DP, Ward JI. Epidemiology of invasive pneumococcal disease in Southern California: implications for the design and conduct of a pneumococcal conjugate vaccine efficacy trial. J Infect Dis 1996;174:752--9.
- Pastor P, Medley F, Murphy TV. Invasive pneumococcal disease in Dallas County, Texas: results from population-based surveillance in 1995. Clin Infect Dis 1998;26:590--5.
- Overturf GD. Chapter 9: infections and immunizations of children with sickle cell disease. In: Aronoff SC, ed. Advances in pediatric infectious diseases, vol. 14. St. Louis, MO: Mosby, Inc., 1999:191--218.
- Topley JM, Cupidore L, Vaidya S, Hayes RJ, and Serjeant GR. Pneumococcal and other infections in children with sickle cell-hemoglobin C (SC) disease. J Pediatr 1982;101(2):176--9.
- Lane PA, Rogers ZR, Woods GM, et al. Fatal pneumococcal septicemia in hemoglobin SC disease. J Pediatr 1994;124:859--62.
- Pearson HA. Prevention of pneumococcal disease in sickle cell anemia [Editorial]. J Pediatr 1996;129:788--9.
- Winkelstein JA, Drachman RH. Deficiency of pneumococcal serum opsonizing activity in sickle cell disease. N Engl J Med 1968;279:459--66.
- Ammann AJ, Addiego J, Wara DW, Lubin B, Smith WB, Mentzer WC. Polyvalent pneumococcal polysaccharide immunization of patients with sickle-cell anemia and patients with splenectomy. N Engl J Med 1977;297:897--900.
- John AB, Ramlal A, Jackson H, Maude GH, Sharma AW, Serjeant GR. Prevention of pneumococcal infection in children with homozygous sickle cell disease. BM J 1984;288:1567--70.
- Wong WY, Overturf GD, Powars DR. Infection caused by Streptococcus pneumoniae in children with sickle cell disease: epidemiology, immunologic mechanisms, prophylaxis, and vaccination. Clin Infect Dis 1992;14:1124--36.
- Bjornson AB, Falletta JM, Verter JI, et al: Serotype-specific immunoglobulin G antibody responses to pneumococcal polysaccharide vaccine in children with sickle cell anemia: effects of continued penicillin prophylaxis. J Pediatr 1996;129:828--35.
- Fiore AE, Levine OS, Elliott JA, Facklam RR, Butler JC for the Pneumococcal Sentinel Surveillance Working Group. Effectiveness of pneumococcal polysaccharide vaccine for preschool-age children with chronic disease. Emerg Infect Dis 1999;5:828--31.
- Frankel RE, Virata M, Hardalo C, Altice FL, Friedland G. Invasive pneumococcal disease: clinical features, serotypes, and antimicrobial resistance patterns in cases involving patients with and without human immunodeficiency virus infection. Clin Infect Dis 1996;23:577--84.
- Moore D, Nelson M, Henderson D. Pneumococcal vaccination and HIV infection [Review]. Intl J STD AIDS 1998;9:1--7.
- Farley JJ, King JC, Nair P, Hines SE, Tressler RL, Vink PE. Invasive pneumococcal disease among infected and uninfected children of mothers with human immunodeficiency virus infection. J Pediatr 1994;124:853--8.
- Andiman WA, Mezger J, Shapiro E. Invasive bacterial infections in children born to women infected with human immunodeficiency virus type 1. J Pediatr 1994;124:846--52.
- Mao C, Harper M, McIntosh K, et al. Invasive pneumococcal infections in human immunodeficiency virus-infected children. J Infect Dis 1996;173:870--6.
- Gessner BD, Ussery XT, Parkinson AJ, Breiman RF. Risk factors for invasive disease caused by Streptococcus pneumoniae among Alaska native children younger than two years of age. Pediatr Infect Dis J 1995;14:123--8.
- Takala AK, Jussi J, Kela E, Rönnberg P-R, Koskenniemi E, Eskola J. Risk factors for primary invasive pneumococcal disease among children in Finland. JAMA 1995;273:859--64.
- Nuorti JP, Butler JC, Farley MM, et al. Cigarette smoking and invasive pneumococcal disease. N Engl J Med 2000;342:681--9.
- Uhari M, Mantysaari K, Niemelä M. Meta-analytic review of the risk factors for acute otitis media. Clin Infect Dis 1996;22:1079--83.
- Nafstad P, Hagen JA, Oie L, Magnus P, Jaakkola JJK. Day care centers and respiratory health. Pediatrics 1999;103:753--8.
- Paradise JL, Rockette HE, Colborn DK, et al. Otitis media in 2253 Pittsburgh-area infants: prevalence and risk factors during the first two years of life. Pediatrics 1997;99:318--33.
- Fleming DW, Cochi SL, Hightower AW, Broome CV. Childhood upper respiratory tract infections: to what degree is incidence affected by day-care attendance? Pediatrics 1987;79:55--60.
- Breiman RF, Butler JC, Tenover FC, Elliott JA, Facklam RR. Emergence of drug-resistant pneumococcal infections in the United States. JAMA 1994;271:1831--5.
- CDC. Geographic variation in penicillin resistance in _Streptococcus pneumoniae_---selected sites, United States, 1997. MMWR 1999;48:656--61.
- Duchin JS, Breiman RF, Diamond A, et al. High prevalence of multidrug-resistant Streptococcus pneumoniae among children in a rural Kentucky community. Pediatr Infect Dis J 1995;14:745--50.
- Arnold KE, Leggiadro RJ, Breiman RF, et al. Risk factors for carriage of drug-resistant Streptococcus pneumoniae among children in Memphis, Tennessee. J Pediatr 1996;128:757--64.
- Reichler MR, Allphin AA, Breiman RF, et al. Spread of multiply resistant Streptococcus pneumoniae at a day care center in Ohio. J Infect Dis 1992;166:1346--53.
- Dagan R, Abramson O, Leibovitz E, et al. Impaired bacteriologic response to oral cephalosporins in acute otitis media caused by pneumococci with intermediate resistance to penicillin. Pediatr Infect Dis J 1996;15:980--5.
- Catalan MJ, Fernandez JM, Vazquez A, Varela de Seijas E, Suárez A, de Quirós JCL. Failure of cefotaxime in the treatment of meningitis due to relatively resistant Streptococcus pneumoniae. Clin Infect Dis 1994;18:766--9.
- Sloas MM, Barrett FF, Chesney PJ, et al. Cephalosporin treatment failure in penicillin- and cephalosporin-resistant Streptococcus pneumoniae meningitis. Pediatr Infect Dis J 1992;11:662--6.
- Bonafede M, Rice LB. Emerging antibiotic resistance. J Lab Clin Med 1997;130:558--66.
- Tan TQ, Mason EO, Barson WJ, et al. Clinical characteristics and outcome of children with pneumonia attributable to penicillin-susceptible and penicillin-nonsusceptible Streptococcus pneumoniae. Pediatrics 1998;102:1369--75.
- Friedland IR. Comparison of the response to antimicrobial therapy of penicillin-resistant and penicillin-susceptible pneumococcal disease. Pediatr Infect Dis J 1995;14:885--90.
- Feikin DR, Schuchat A, Kolczak M, et al. Mortality from invasive pneumococcal pneumonia in the era of antibiotic resistance, 1995--1997. Am J Public Health 2000;90:223--9.
- Tuomanen EI, Austrian R, Masure HR. Mechanisms of disease: pathogenesis of pneumococcal infection. N Engl J Med 1995;332:1280--4.
- Musher DM, Johnson B, Watson DA. Quantitative relationship between anticapsular antibody measured by enzyme-linked immunosorbent assay or radioimmunoassay and protection of mice against challenge with Streptococcus pneumoniae serotype 4. Infect Immun 1990;58:3871--6.
- Henrichsen J. Six newly recognized types of Streptococcus pneumoniae. J Clin Microbiol 1995;33:2759--62.
- Kalin M. Pneumococcal serotypes and their clinical relevance. Thorax 1998;53:159--62.
- Butler JC. Epidemiology of pneumococcal serotypes and conjugate vaccine formulations. Microb Drug Resist 1997;3(2):125--9.
- Butler JC, Breiman RF, Lipman HB, Hofmann J, Facklam RR. Serotype distribution of Streptococcus pneumoniae infections among preschool children in the United States, 1978--1994: implications for development of a conjugate vaccine. J Infect Dis 1995;171:885--9.
- Sniadack DH, Schwartz B, Lipman HB, et al. Potential interventions for the prevention of childhood pneumoniae: geographic and temporal differences in serotype and serogroup distribution of sterile site pneumococcal isolates from children---implications for vaccine strategies. Pediatr Infect Dis J 1995;14:503--10.
- Hausdorff WP, Bryant J, Paradiso PR, and Siber GR. Which pneumococcal serogroups cause the most invasive disease: implications for conjugate vaccine formulation and use, part I. Clin Infect Dis 2000;30:100--21.
- Block SL, Hedrick JA, Harrison CJ. Pneumococcal serotypes from acute otitis media in rural Kentucky [Abstract 1185]. 39th Interscience Conference on Antimicrobial Agents and Chemotherapy, San Francisco, CA, 1999;677.
- Eskola J, Kilpi T. Efficacy of a heptavalent pneumococcal conjugate vaccine (PcnCRM) against serotype-specific, culture-confirmed pneumococcal acute otitis media (AOM) in infants and children [Abstract LB-13]. 39th Interscience Conference on Antimicrobial Agents and Chemotherapy, San Francisco, CA, 1999;16.
- Klein DL, Ellis RW. Conjugate vaccines against Streptococcus pneumoniae. In: Levine MM, Woodrow GC, Kaper JB, Cobon, GS, eds. New generation vaccines. 2nd ed., rev. New York, NY: Marcel Dekker, Inc., 1997:503--25.
- Stein KE. Thymus-independent and thymus-dependent responses to polysaccharide antigens. J Infect Dis 1992;165 (suppl):S49--52.
- Douglas RM, Paton JC, Duncan SJ, Hansman DJ. Antibody response to pneumococcal vaccination in children younger than five years of age. J Infect Dis 1983;148:131--7.
- Adams WG, Deaver KA, Cochi SL, et al. Decline of childhood Haemophilus influenzae Type b (Hib) disease in the Hib vaccine era. JAMA 1993;269:221--6.
- Romero-Steiner S, Libutti D, Pais LB, et al. Standardization of an opsonophagocytic assay for the measurement of functional antibody activity against Streptococcus pneumoniae using differentiated HL-60 cells. Clin Diagn Lab Immunol 1997;4:415--22.
- Scheifele DW, Halperin SA, Guasparini R, Meekison W, Pim C, Barreto L. Extended follow-up of antibody levels and antigen responsiveness after 2 Haemophilus influenzae type b conjugate vaccines. J Pediatr 1999;135:240--5.
- Käyhty H, Peltola H, Karanko V, and Mäkelä PH. Protective levels of serum antibodies to the capsular polysaccharide of Haemophilus influenzae type b. J Infect Dis 1983;147:1100.
- Anderson P. Protective level of serum antibodies to the capsular polysaccharide of Haemophilus influenzae type b [Letter]. J Infect Dis 1984;149:1034--5.
- Obaro SK, Huo Z, Banya WAS, et al. Glycoprotein pneumococcal conjugate vaccine primes for antibody responses to a pneumococcal polysaccharide vaccine in Gambian children. Pediatr Infect Dis J 1997;16:1135--40.
- Vernacchio L, Neufeld EJ, MacDonald K, et al. Combined schedule of 7-valent pneumococcal conjugate vaccine followed by 23-valent pneumococcal vaccine in children and young adults with sickle cell disease. J Pediatr 1998;133:275--8.
- King JC, Vink PE, Farley JJ, et al. Comparison of the safety and immunogenicity of a pneumococcal conjugate with a licensed polysaccharide vaccine in human immunodeficiency virus and non-human immunodeficiency virus-infected children. Pediatr Infect Dis J 1996;15:192--6.
- Rennels MB, Edwards KM, Keyserling HL, et al. Safety and immunogenicity of heptavalent pneumococcal vaccine conjugated to CRM197 in United States infants. Pediatrics 1998;101:604--11.
- O'Brien KL, Steinhoff MC, Edwards K, Keyserling H, Thoms ML, Madore D. Immunologic priming of young children by pneumococcal glycoprotein conjugate, but not polysaccharide, vaccines. Pediatr Infect Dis J 1996;15:425--30.
- O'Brien KL, Winkelstein JA, Santosham M, et al. Immunogenicity of a pneumococcal protein conjugate vaccine in infants with sickle cell disease. Pediatr Res 1996;39(4):160.
- Dagan R, Muallem M, Melamed R, Leroy O, Yagupsky P. Reduction of pneumococcal nasopharyngeal carriage in early infancy after immunization with tetravalent pneumococcal vaccines conjugated to either tetanus toxoid or diphtheria toxoid. Pediatr Infect Dis J 1997;16:1060--4.
- Nowak-Wegrzyn AH, Winkelstein JA, Stover BM, Swift AJ, Lederman HM. Serum opsonic activity for Streptococcus pneumoniae types 6B and 14 in infants with sickle cell disease after immunization with pneumococcal protein conjugate vaccine [Abstract 57]. Pediatric Academic Societies 1999 Annual Meeting, San Francisco, CA. Pediatr Res 1999;45(4[part 2]):11A.
- King JC, Vink PE, Farley JJ, Smilie M, Parks M, Lichenstein R. Safety and immunogenicity of three doses of a five-valent pneumococcal conjugate vaccine in children younger than two years with and without human immunodeficiency virus infection. Pediatrics 1997;99:575--80.
- Miernyk KM, Parkinson AJ, Rudolph KM, et al. Immunogenicity of a heptavalent pneumococcal conjugate vaccine in Apache and Navajo Indian, Alaska Native, and non-native American infants aged <2 years. Clin Infect Dis 2000;31:34--41.
- Black S, Shinefield H, Ray P, et al. Efficacy of heptavalent conjugate pneumococcal vaccine (Wyeth Lederle) in 37,000 infants and children: impact on pneumonia, otitis media, and an update on invasive disease---results of the Northern California Kaiser Permanente Efficacy Trial [Abstract 1398]. 39th Interscience Conference on Antimicrobial Agents and Chemotherapy, San Francisco, CA 1999;379.
- Kilpi T, Jokinen J, Herra E, et al. Effect of heptavalent pneumococcal conjugate vaccine (PNCCRM) on pneumococcal acute otitis media (AOM) by serotype [Abstract O20]. 2nd International Symposium on Pneumococci and Pneumococcal Diseases, Sun City, South Africa, 2000.
- Black S. Implementation of pneumococcal and meningococcal conjugate vaccines [Presentation]. Pediatric Academic Societies and the American Academy of Pediatrics Joint Meeting, Boston, MA, 2000.
- Mohle-Boetani JC, Ajello G, and Breneman E. Carriage of Haemophilus influenzae type b in children after widespread vaccination with conjugate Haemophilus influenzae type b vaccines. Pediatr Infect Dis J 1993;12:589--93.
- Dagan R. Effect of vaccine on antibiotic-resistant Streptococcus pneumoniae carriage and spread [Abstract 072]. Second International Symposium of Pneumococci and Pneumococcal Diseases, Sun City, South Africa, 2000. Presentation available at <http://216.247.185.154/isppd00/is0d1050/maid1050.htm>. Accessed August 9, 2000.
- Lieu TA, Ray GT, Black SB, et al. Projected cost-effectiveness of pneumococcal conjugate vaccination of healthy infants and young children. JAMA 2000;283:1460--8.
- Lieu TA. Re-analysis of the projected cost-effectiveness of pneumococcal conjugate vaccination of healthy U.S. children [Presentation]. Advisory Committee on Immunization Practices Meeting, Atlanta, GA, 2000.
- Food and Drug Administration. Product approval information---licensing action. Hyattsville, MD: US Department of Health and Human Services, Food and Drug Administration, Center for Biologics Evaluation and Research, 2000. Available at <http://www.fda.gov/cber/products/pneuled021700.htm>. Accessed August 9, 2000.
- American Academy of Pediatrics. Pertussis. In: Pickering LK, ed. 2000 red book: report of the Committee on Infectious Diseases. 25th ed. Elk Grove Village, IL: American Academy of Pediatrics; 2000:444.
- Shinefield H, Black S, Ray P, et al. Safety and immunogenicity of heptavalent pneumococcal CRM197 conjugate vaccine in infants and toddlers. Pediatr Infect Dis J 1999;18:757--63.
- Gaston MH, Verter JI, Woods G, et al. Prophylaxis with oral penicillin in children with sickle cell anemia. N Engl J Med 1986;314:1593--9.
- Norris CF, Mahannah SR, Smith-Whitley K, Ohene-Fremong K, McGowan KL. Pneumococcal colonization in children with sickle cell disease. J Pediatr 1996;129:821--7.
- Adamkiewicz TV, Farley MM, Baughman W, Schrag S, Elliot J. Are children with sickle cell disease at higher risk of developing penicillin-resistant Streptococcus pneumoniae (Pnc) infections than other children [Abstract 831]. Pediatr Res 1999;45:143A.
- O'Brien KL. Streptococcus pneumoniae among Native Americans [Presentation]. International Conference on Emerging Infectious Diseases, Atlanta, GA, 2000. Presentation available at <http://www.cdc.gov/iceid/webcast/pps/obrian.pps>. Accessed August 9, 2000.
- Rudolph KM, Parkinson AJ, Reasonover AL, Bulkow LR, Parks DJ, Butler JC. Serotype distribution and antimicrobial resistance patterns of invasive isolates of Streptococcus pneumoniae: Alaska, 1991--1998. J Infect Dis 2000; (in press).
- Sorensen RU, Leiva LE, Giangrosso PA, et al. Response to a heptavalent conjugate Streptococcus pneumoniae vaccine in children with recurrent infections who are unresponsive to the polysaccharide vaccine. Pediatr Infect Dis J 1998;17:685--91.
- Powers DC, Anderson EL, Lottenbach K, Mink CM. Reactogenicity and immunogenicity of a protein-conjugated pneumococcal oligosaccharide vaccine in older adults. J Infect Dis 1996;173:1014--8.
- Ahmed F, Steinhoff MC, Rodriquez-Barradas MC, Hamilton RG, Musher DM, Nelson KE. Effect of human immunodeficiency virus type 1 infection on the antibody response to a glycoprotein conjugate pneumococcal vaccine: results from a randomized trial. J Infect Dis 1996;173:83--90.
- American Academy of Pediatrics. Pneumococcal infections. In: Pickering LK, ed. 2000 red book: report of the committee on Infectious Diseases. 25th ed. Elk Grove Village, IL: American Academy of Pediatrics; 2000:459.
- Paton JC. Novel pneumococcal surface proteins: role in virulence and vaccine potential. Trends Microbiol 1998;6:85--7.
- Flanagan MP, Michael JG. Oral immunization with a Streptococcal pneumoniae polysaccharide conjugate vaccine in enterocoated microparticles induces serum antibodies against type specific polysaccharides. Vaccine 1999;17:72--81.
- K�nen-Waisman S, Cohen A, Fridkin M, Cohen IR: Self heat-shock protein (hsp60) peptide serves in a conjugate vaccine against a lethal pneumococcal infection. J Infect Dis 1999;179:403--13.
* Additional information regarding ABCs/EIP Network is available at <http://www.cdc.gov/ncidod/dbmd/abcs> (accessed August 8, 2000).
** Abbreviations used in this report are those used by ACIP during development of vaccination recommendations; they are similar to those already published by the American Academy of Pediatrics (Source: American Academy of Pediatrics. Policy statement: recommendations for the prevention of pneumococcal infections, including the use of pneumococcal conjugate vaccine [Prevnar], pneumococcal polysaccharide vaccine, and antibiotic prophylaxis. Pediatrics 2000:106:3626). A new standardized nomenclature system is in development as part of CDC's Vaccine Identification Standards Initiative (available at <http://www.cdc.gov/nip/visi/prototypes/vaxabbrev.htm>; accessed September 15, 2000).
*** Defined in that study as any setting outside the home where a child regularly spends >4 hours/week with >2 unrelated children under adult supervision.
**** Routine vaccinations included diphtheria toxoid-tetanus toxoid-pertussis vaccine; Haemophilus influenzae type b conjugate; hepatitis B; oral and inactivated poliovirus; measles-mumps-rubella; and varicella.
***** Initially all children in the study received the whole-cell pertussis vaccine and oral poliovirus vaccine; however, after changes in vaccine recommendations midway through the study, participants began receiving the diphtheria toxoid-tetanus toxoid-acellular pertussis vaccine and the inactivated poliovirus vaccine.
****** VAERS forms can be obtained by calling (800) 822-7967, and additional information is available at <http://www.vaers.org> (accessed July 31, 2000).
| ******* | A | Strong evidence, including results of efficacy studies, supports vaccine use. |
|---|---|---|
| B | Moderate evidence, including immunogenicity data but not efficacy data, supports vaccine use. | |
| C | No efficacy or immunogenicity studies are available regarding this population, but protection is anticipated on the basis of such studies among other groups; vaccination is supported by respected authorities. |
******** This recommendation excludes children who have received a bone marrow transplant (BMT). Children who undergo BMT have impaired humoral immune system responses for months or years after the procedure and are at increased risk for serious pneumococcal infection. Because studies among this population are not complete, ACIP is currently unable to make recommendations regarding use of PCV7 among BMT patients. PCV7 might produce superior antibody responses compared with PPV23 among BMT patients. However, pending results of studies of PCV7 among BMT patients, health-care providers should vaccinate this population with PPV23 vaccine at 12 and 24 months after BMT, as recommended by an expert panel (Source: CDC. Guidelines for preventing opportunistic infections among hematopoietic stem cell transplant recipients: recommendations of CDC, the Infectious Disease Society of America, and the American Society of Blood and Marrow Transplantation. MMWR 2000;in press).
********* Defined as any setting outside the home where a child regularly spends >4 hours/week with >2 unrelated children under adult supervision.
Table 1
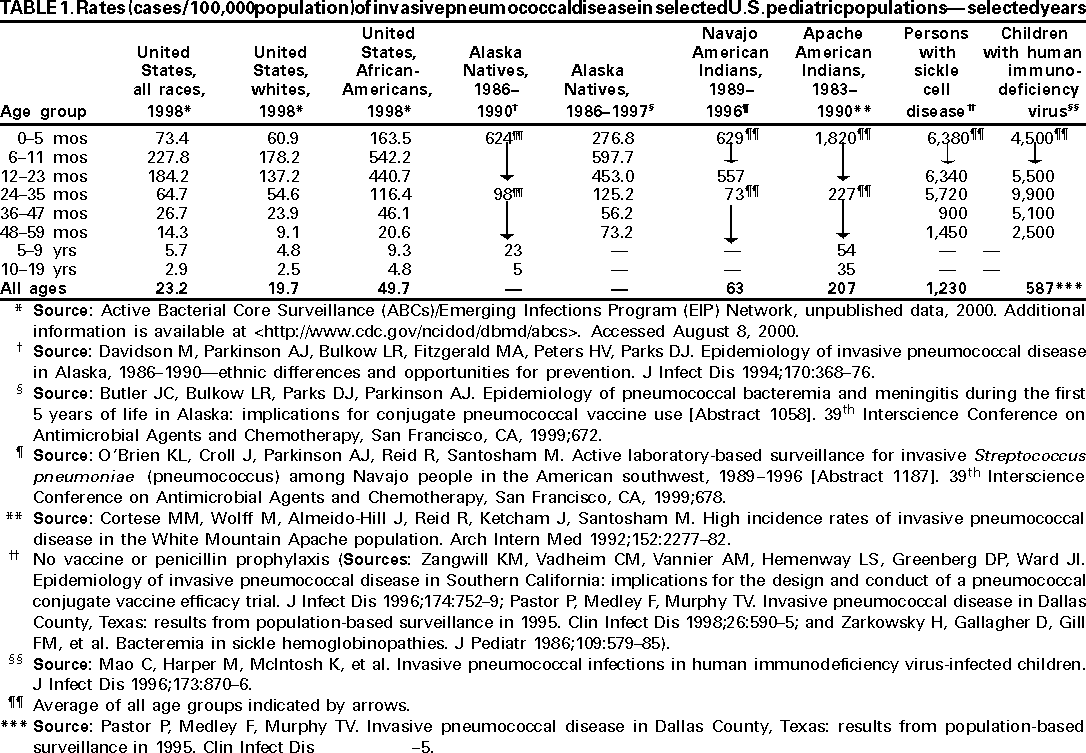
Return to top.
Figure 1
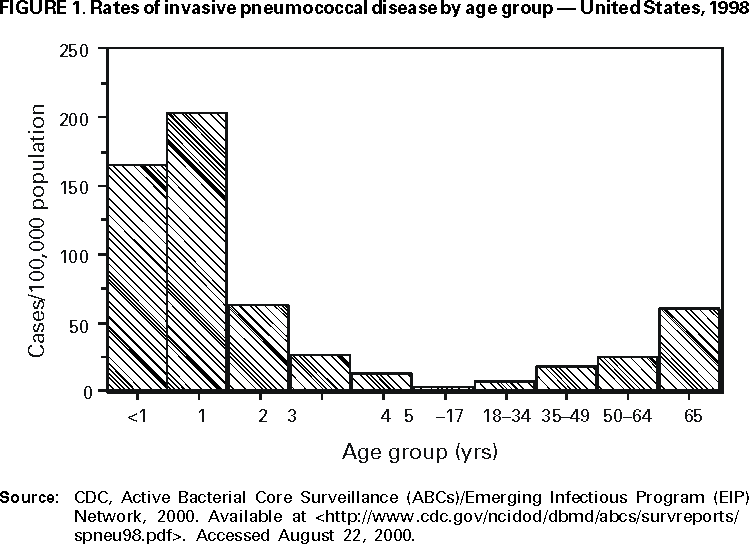
Return to top.
Table 2
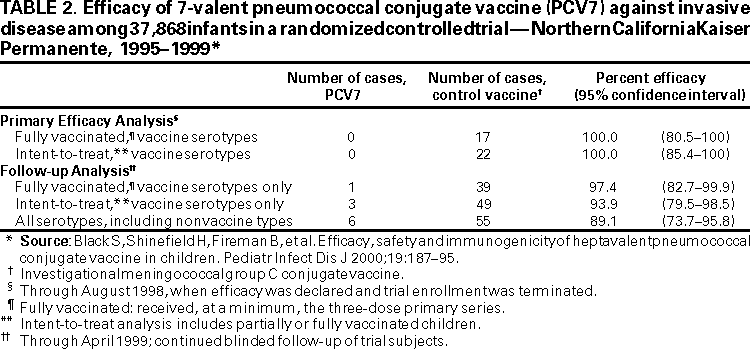
Return to top.
Figure 2
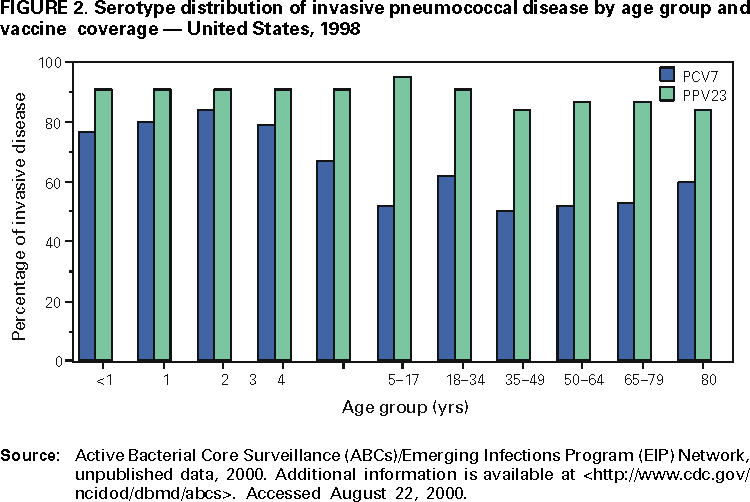
Return to top.
Table 3
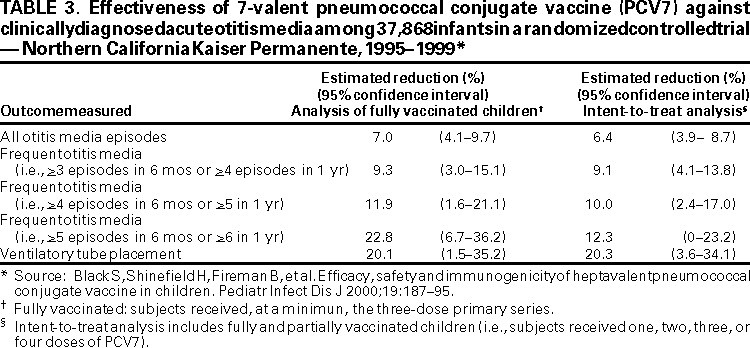
Return to top.
Table 4
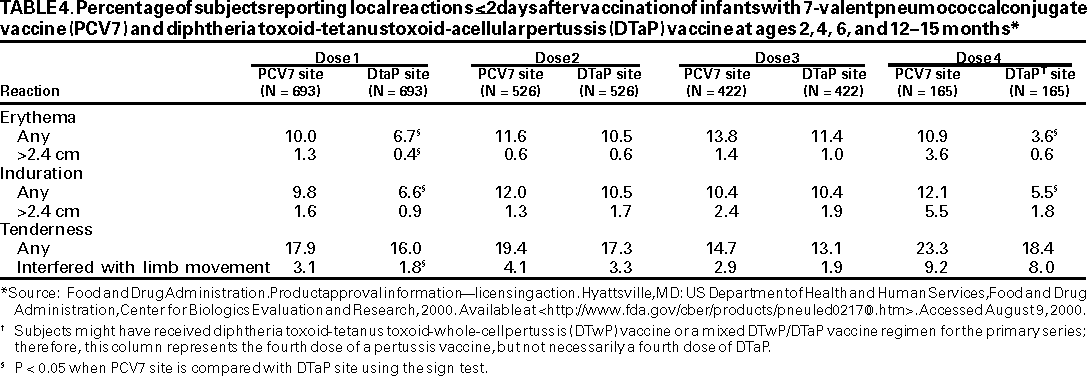
Return to top.
Table 5
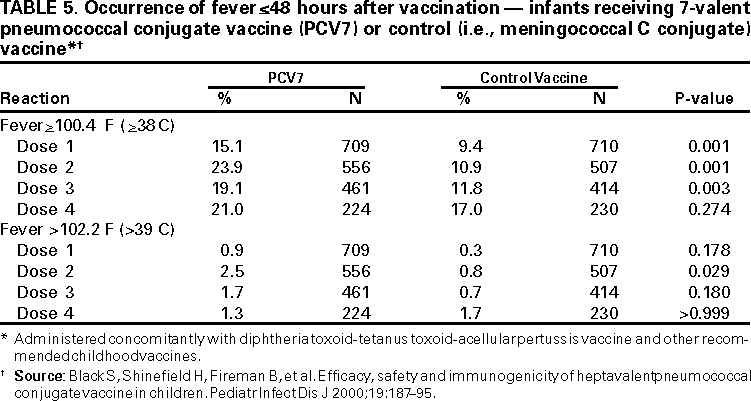
Return to top.
Table 6
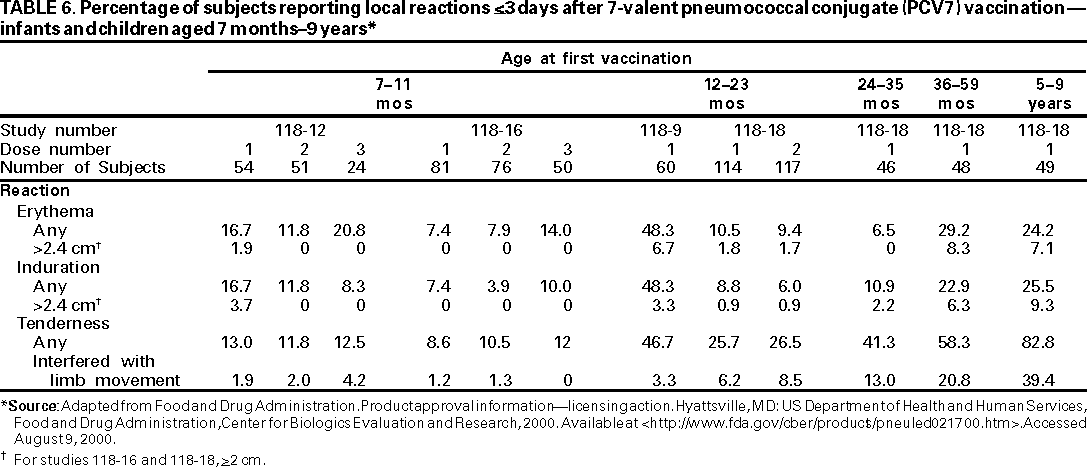
Return to top.
Table 7
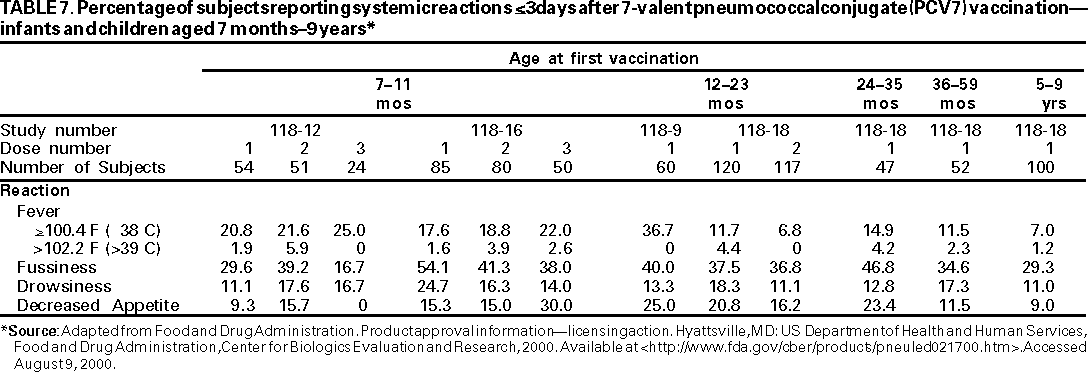
Return to top.
Table 8
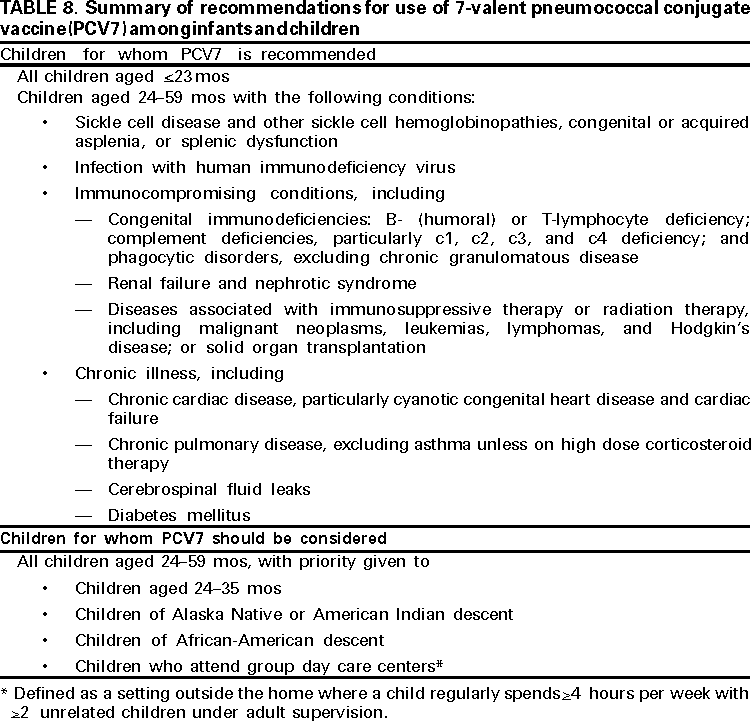
Return to top.
Table 9
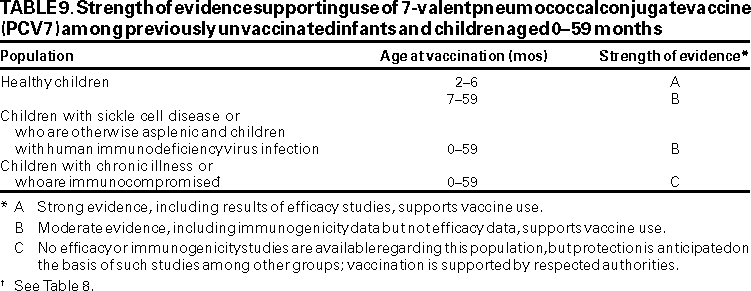
Return to top.
Table 10
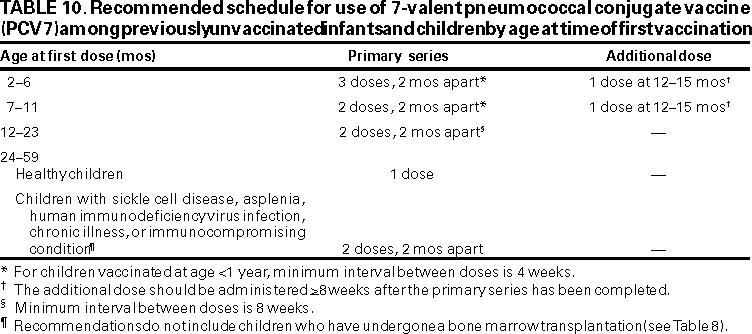
Return to top.
Table 11
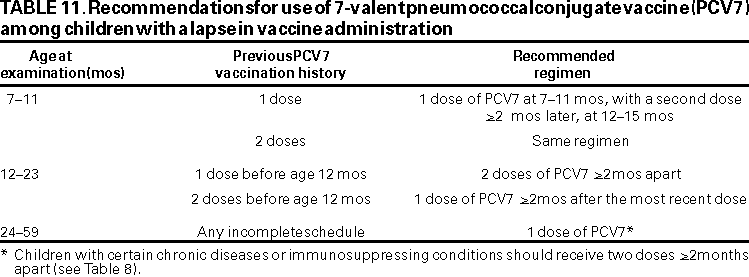
Return to top.
Table 12
Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices.
**Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.
Page converted: 9/29/2000