Revolutionizing diabetic retinopathy screening and management: The role of artificial intelligence and machine learning (original) (raw)
Editorial Open Access
Copyright ©The Author(s) 2025. Published by Baishideng Publishing Group Inc. All rights reserved.
World J Clin Cases. Feb 16, 2025; 13(5): 101306
Published online Feb 16, 2025. doi: 10.12998/wjcc.v13.i5.101306
Revolutionizing diabetic retinopathy screening and management: The role of artificial intelligence and machine learning
Mona Mohamed Ibrahim Abdalla, Jaiprakash Mohanraj, Department of Human Biology, School of Medicine, International Medical University, Bukit Jalil 57000, Kuala Lumpur, Malaysia
Co-first authors: Mona Mohamed Ibrahim Abdalla and Jaiprakash Mohanraj.
Author contributions: Abdalla MMI and Mohanraj J contribute equally to this article as co-first authors. Abdalla MMI and Mohanraj J performed the research and wrote the paper.
Conflict-of-interest statement: The authors declare no conflict of interest to this paper.
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Corresponding author: Mona Mohamed Ibrahim Abdalla, MD, MSc, PhD, Doctor, Senior Lec
turer, Department of Human Biology, School of Medicine, International Medical University, No. 126 Jln Jalil Perkasa 19, Bukit Jalil 57000, Kuala Lumpur, Malaysia. monamohamed@imu.edu.my
Received: September 10, 2024
Revised: October 9, 2024
Accepted: November 5, 2024
Published online: February 16, 2025
Processing time: 69 Days and 18.6 Hours
Abstract
Diabetic retinopathy (DR) remains a leading cause of vision impairment and blindness among individuals with diabetes, necessitating innovative approaches to screening and management. This editorial explores the transformative potential of artificial intelligence (AI) and machine learning (ML) in revolutionizing DR care. AI and ML technologies have demonstrated remarkable advancements in enhancing the accuracy, efficiency, and accessibility of DR screening, helping to overcome barriers to early detection. These technologies leverage vast datasets to identify patterns and predict disease progression with unprecedented precision, enabling clinicians to make more informed decisions. Furthermore, AI-driven solutions hold promise in personalizing management strategies for DR, incorporating predictive analytics to tailor interventions and optimize treatment pathways. By automating routine tasks, AI can reduce the burden on healthcare providers, allowing for a more focused allocation of resources towards complex patient care. This review aims to evaluate the current advancements and applications of AI and ML in DR screening, and to discuss the potential of these technologies in developing personalized management strategies, ultimately aiming to improve patient outcomes and reduce the global burden of DR. The integration of AI and ML in DR care represents a paradigm shift, offering a glimpse into the future of ophthalmic healthcare.
Core Tip: Leveraging artificial intelligence (AI) and machine learning in diabetic retinopathy care can significantly enhance early detection and personalized treatment. Clinicians should embrace AI-driven screening tools that analyze retinal images with high precision, reducing the risk of human error and improving diagnostic accuracy. Implementing predictive analytics can help in identifying patients at higher risk, allowing for timely interventions and tailored treatment plans. To maximize the benefits, healthcare systems must invest in training and integrating these technologies seamlessly into clinical workflows. Collaborations between technologists and healthcare providers are crucial for developing robust, ethical, and equitable AI solutions in ophthalmic care.
- Citation: Abdalla MMI, Mohanraj J. Revolutionizing diabetic retinopathy screening and management: The role of artificial intelligence and machine learning. World J Clin Cases 2025; 13(5): 101306
- URL: https://www.wjgnet.com/2307-8960/full/v13/i5/101306.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v13.i5.101306
INTRODUCTION
Diabetic retinopathy (DR) is a serious and common complication of diabetes mellitus, affecting millions globally and representing a leading cause of vision impairment and blindness. The International Diabetes Federation estimates that the global population with diabetes will rise from 463 million in 2019 to 700 million by 2045, further exacerbating the burden of DR[1]. Current global prevalence estimates indicate that 22.27% of individuals with diabetes develop DR, with 6.17% suffering from vision-threatening DR and 4.07% experiencing clinically significant macular edema[2].
The pathogenesis of DR begins with chronic hyperglycemia, which progressively damages the retinal microvasculature, advancing through stages from mild non-proliferative DR (NPDR) to proliferative DR (PDR), potentially leading to diabetic macular edema[3,4].
Mild NPDR is often asymptomatic and reversible with proper glycemic control. Moderate NPDR involves increased retinal vessel leakage and intraretinal hemorrhages, which require early detection to prevent progression. Severe NPDR, with more extensive hemorrhages and microvascular abnormalities, poses a significant risk for progression to PDR, which is marked by neovascularization and can result in irreversible vision loss if not promptly treated[5].
Timely diagnosis is crucial, especially in the early stages of DR (mild to moderate NPDR), when intervention can halt or reverse disease progression. However, in many cases, DR is diagnosed late, particularly in low- and middle-income countries (LMICs), contributing significantly to the global burden of vision loss. Approximately 50% of people with diabetes in LMICs are diagnosed too late to prevent severe vision impairment, underscoring the need for more accessible and efficient screening methods[6]. This delay in diagnosis and treatment results in a substantial increase in the risk of blindness.
Artificial intelligence (AI) and machine learning (ML) present transformative potential in improving DR care. These technologies utilize large datasets and advanced algorithms to enhance the accuracy and efficiency of DR screening. AI-driven systems, such as the EyeArt and IDx-DR, have demonstrated high sensitivity and specificity in detecting DR, offering immediate diagnostic feedback and facilitating early intervention[7,8]. For instance, EyeArt demonstrated a sensitivity of 97% and specificity of 88% in detecting DR, outperforming traditional methods[7]. Furthermore, AI supports personalized management by analyzing individual risk profiles to tailor treatment and reduce the burden on healthcare providers by automating routine tasks[9]. Mobile-based AI solutions, such as the Medios AI algorithm combined with the Remidio NM fundus-on-phone camera, have further enhanced screening accessibility in resource-limited settings[10].
Overall, AI and ML technologies represent a paradigm shift in ophthalmic healthcare, providing accurate, efficient, and accessible DR screening and management solutions. Continued research and development will undoubtedly improve patient outcomes and reduce the global burden of DR.
Despite the advancements in AI and ML technologies, significant gaps remain in their widespread clinical integration and accessibility, particularly in resource-limited settings. Many healthcare systems, especially in LMICs, face barriers such as inadequate infrastructure, limited access to trained personnel, and the high costs of implementing AI-driven screening tools[11]. Additionally, while several AI models have demonstrated high sensitivity and specificity in detecting DR, there is still a need for real-world validation and long-term studies assessing their clinical effectiveness and cost-efficiency across diverse populations[12]. This review aims to address these gaps by providing a comprehensive evaluation of the current advancements in AI and ML for DR detection, focusing on the challenges of their implementation, and offering insights into how these technologies can be scaled to benefit underserved regions.
CURRENT CHALLENGES IN DR SCREENING AND MANAGEMENT
Limitations of traditional screening methods
Traditional DR screening primarily relies on fundus photography, a technique introduced by Carl Zeiss in the mid-20th century in Germany[13]. While fundus photography captures detailed retinal images that are interpreted by ophthalmologists, the method is resource-intensive, requiring specialized personnel, expensive equipment, and substantial infrastructure. These factors limit its scalability, particularly in resource-constrained settings, such as rural and low-income areas[14-16]. The high costs associated with retinal cameras and the necessity for dilated eye exams further constrain the widespread adoption of this method. Additionally, the availability of trained ophthalmologists is often limited, leading to delays in diagnosis and treatment. These delays can result in the progression of DR to more advanced stages, reducing the effectiveness of available treatment options. These limitations highlight the need for more accessible, scalable, and cost-effective screening solutions[9,17].
Barriers to early detection and timely intervention
Early detection and timely intervention are critical for preventing vision loss due to DR. However, access to healthcare services, particularly in rural and low-income regions, is limited, delaying diagnosis and treatment. Socioeconomic factors such as the cost of care and a lack of awareness about the importance of regular screenings further exacerbate these delays. Many patients seek medical attention only after symptoms become severe, by which time the disease has often advanced significantly[9]. Cultural beliefs and misconceptions about diabetes and its complications also contribute to delays in seeking medical advice. Moreover, inadequacies within healthcare infrastructure, such as ineffective referral systems and poor integration between primary care and specialist services, further impede effective DR management[17,18].
AI-powered mobile apps and community health programs can significantly boost DR awareness and screening rates. Mobile apps offer educational resources, screening reminders, and appointment booking, while integrating portable AI screening tools into community health programs increases accessibility. Training community health workers to use these tools allows for screenings during routine visits or events, empowering individuals and enabling earlier DR diagnosis[6].
Challenges in personalized management and treatment adherence
The management of DR is increasingly moving toward personalized treatment strategies, which are crucial for optimizing patient outcomes. However, several challenges complicate the implementation of these personalized approaches. The variability in patient responses to treatment necessitates tailored therapeutic strategies, which are difficult to execute without comprehensive data and advanced predictive tools. Moreover, ensuring adherence to treatment regimens remains a significant challenge[19-21].
Factors such as the complexity of treatment protocols, potential side effects, and the need for regular follow-up appointments often contribute to poor adherence, undermining the efficacy of treatment. Economic barriers, particularly in low-income populations, further complicate adherence, as the cost of medications and follow-up care may be prohibitive. Additionally, psychological factors, including fear of treatment and a lack of perceived benefit, contribute to non-adherence[22]. Addressing these challenges requires a multifaceted approach, integrating patient education, simplified treatment protocols, and robust support systems to enhance adherence and ensure regular monitoring[23].
ADVANCED SCREENING AND DIAGNOSTIC TOOLS AND THEIR CHALLENGES
Advancements in technology have led to the development of several new tools for DR screening and diagnosis, each with its own set of benefits and challenges.
Optical coherence tomography
Developed by Huang _et al_[24] in 1991 in the United States, optical coherence tomography (OCT) provides high-resolution cross-sectional images of the retina, making it particularly effective for detecting diabetic macular edema and subtle retinal changes. While OCT has proven invaluable for precise imaging, its high cost and the specialized training required to operate and interpret results pose limitations, especially in resource-poor settings, reducing its widespread accessibility[24,25].
Fluorescein angiography
Introduced by Novotny and Alvis in 1961 in the United States, fluorescein angiography remains the gold standard for visualizing retinal vasculature through the injection of a fluorescent dye. This technique is highly accurate in detecting vascular leakage and abnormal blood vessels. However, its invasive nature, requirement for specialized equipment, and potential side effects limit its utility, particularly in rural and low-income areas where healthcare infrastructure is limited[26-30].
Ultrawide-field imaging
Emerging in the early 2000s, ultrawide-field imaging (UWFI) was pioneered by companies such as Annidis Corporation in Canada and Optos in the United Kingdom[31]. This technology provides a broad retinal view, capturing up to 200 degrees in a single image, surpassing traditional fundus photography. UWFI enhances the detection of peripheral retinal lesions that may be missed with standard imaging. However, its high cost and the need for specialized training restrict widespread adoption, particularly in resource-constrained environments[32,33].
Confocal scanning laser ophthalmoscopy
Developed in the late 1980s by Heidelberg Engineering in Germany, revolutionized retinal imaging by providing high-resolution images of the retina. Confocal scanning laser ophthalmoscopy (cSLO)'s ability to capture detailed retinal structures with enhanced contrast significantly improves diagnostic accuracy, particularly in detecting subtle retinal abnormalities. However, despite its advanced capabilities, the high cost of cSLO systems and the specialized training required for their operation have limited their broader adoption, particularly in low-resource settings[34,35].
Multispectral imaging
First commercially introduced by Annidis Corporation in Canada in 2012, multispectral imaging (MSI) captures multiple wavelengths of light, enhancing contrast and detail in retinal images. This technology is valuable for early diagnosis and management of retinal diseases, as it detects subtle retinal changes. Despite these advantages, MSI remains costly and is not widely available, especially in low-income regions[36].
Smartphone-based retinal imaging
Smartphone-based retinal imaging, developed by the Peek Vision Foundation in the United Kingdom in the early 2010s, offers a cost-effective and portable solution for retinal imaging. It is particularly beneficial in remote and low-resource settings. This technology is designed to be user-friendly, allowing healthcare workers with minimal training to conduct retinal exams in the field. However, challenges include variability in image quality due to factors such as lighting conditions and operator skill, which can affect diagnostic accuracy[37,38].
Hyperspectral imaging
Pioneered in the early 2010s by Akbari and Kosugi[39] at Ryerson University in Canada, represents a cutting-edge technology capable of capturing detailed biochemical information about the retina. By analyzing a broad range of wavelengths, hyperspectral imaging (HSI) provides high-resolution spectral data that enhances tissue composition analysis, allowing for the identification of subtle retinal changes that may be overlooked by conventional imaging techniques[39,40]. This makes HSI particularly valuable in the early detection and differentiation of retinal diseases[41]. However, HSI is complex and costly to implement, with its adoption in clinical practice still limited due to the high cost of equipment, specialized training needs, and lack of widespread availability[42].
Photoacoustic imaging
Developed by Hu and Wang _et al_[43] at Washington University in St. Louis in the early 2010s, photoacoustic imaging combines laser-induced ultrasound with optical imaging to visualize blood vessels and oxygenation levels in the retina. Though still in the research phase, this technology offers high accuracy in functional retinal assessments. However, its clinical application is limited by cost and complexity, making it more suitable for research purposes rather than widespread clinical use.
Teleophthalmology
Teleophthalmology developed with significant contributions from the American Telemedicine Association (ATA) in the early 2000s, has greatly expanded access to DR screening by enabling remote retinal imaging and evaluation by specialists. Since 2004, the ATA has provided guidelines to ensure consistency and quality in its application. Using digital fundus photography and non-mydriatic cameras, teleophthalmology has been particularly impactful in regions with limited access to ophthalmic care[44-46]. However, it relies on robust internet connectivity, high-quality imaging devices, and trained personnel for operation and interpretation, which presents challenges in resource-poor settings. Moreover, the lack of direct patient interaction can limit comprehensive DR management[47].
AI and ML algorithms
AI and ML algorithms, introduced in the late 2010s, have transformed DR screening by automating the analysis of retinal images. Systems such as IDx-DR [Food and Drug Administration (FDA)-approved in 2018] and EyeArt (FDA-cleared in 2020) have demonstrated high sensitivity and specificity, making them reliable tools for early DR detection. These systems provide immediate diagnostic feedback, facilitating timely intervention to prevent vision loss[7,8]. However, implementing AI in clinical settings requires substantial initial investment, robust data privacy measures, and continuous updates to the algorithms based on new data. Integrating AI into existing healthcare workflows also poses challenges[48].
A summarized overview of these tools, their advantages, disadvantages, and the associated challenges is presented in Table 1.
Table 1 Overview of diabetic retinopathy diagnostic tools.
| Tool | Year introduced | Country of origin | Ref. | Advantages | Disadvantages | AI/ML-based |
|---|---|---|---|---|---|---|
| Fundus photography | Mid-20th century | Germany | Srinivasan __et al_[13], 2023 | Established method for capturing detailed retinal images | Resource-intensive requires specialized personnel, expensive, and not scalable in low-resource settings | No |
| Optical coherence tomography | 1991 | United States | Huang __et al_[24], 1991 | High-resolution cross-sectional images; effective in detecting diabetic macular edema. | High cost, requires specialized training, limited availability in low-resource settings | No |
| Fluorescein angiography | 1961 | United States | Norton and Gutman[27], 1965 | Gold standard for visualizing retinal vasculature; highly precise. | Invasive, requires dye injection, potential side effects, limited use in rural and low-income areas. | No |
| Ultrawide-field imaging | Early 2000s | Canada, United Kingdom | Nagiel __et al_[32], 2016 | Captures up to 200 degrees of the retina; detects peripheral lesions often missed by standard imaging | High cost, requires specialized training, limited adoption in low-resource settings | No |
| Confocal scanning laser ophthalmoscopy | Late 1980s | Germany | Webb __et al_[35], 1987 | Provides high-resolution, high-contrast images; improves diagnostic accuracy for subtle abnormalities | High cost, requires specialized training, limited adoption, particularly in low-resource settings | No |
| Multispectral Imaging | 2012 | Canada | Ma __et al_[36], 2023 | Enhances contrast and detail in retinal images by capturing muliple wavelengths of light | High cost, limited availability, not widely adopted in low-resource settings | No |
| Smartphone-based retinal imaging | Early 2010s | United Kingdom | Kim __et al_[37], 2018 | Cost-effective, portable, accessible; useful in remote and low-resource settings | Variable image quality depending on lighting and operator skill; requires adequate training | No |
| Hyperspectral imaging | Early 2010s | Canada | Akbari and Kosugi[39], 2009 | Captures detailed biochemical information; high accuracy in tissue composition analysis; valuable for early detection | Complex, expensive, not widely available, limited adoption in clinical practice | No |
| Photoacoustic imaging | Early 2010s | United States | Hu and Wang[43], 2010 | Combines laser-induced ultrasound with optical imaging; provides functional assessment of the retina | Still in research phase, high cost, complex, limited clinical application | No |
| Teleophthalmology | Early 2000s | United States | Whited[44], 2006 | Expands access to DR screening, particularly in underserved areas; allows remote retinal imaging and analysis | Dependent on internet connectivity, requires high-quality imaging devices and trained personnel, lack of direct patient interaction | No |
| AI and ML algorithms | 2018, 2020 | United States | Esmaeilzadeh[48], 2024 | High sensitivity and specificity; automates retinal image analysis; provides immediate diagnostic feedback | High initial investment, requires continuous algorithm updates, data privacy concerns, integration challenges in clinical workflows | Yes |
THE ROLE OF AI AND ML IN DR DETECTION
AI and ML are transforming healthcare by handling large datasets and performing sophisticated analyses that can assist in diagnosing and managing various diseases, including[49,50].
AI and ML approaches for DR detection
Two primary AI/ML approaches dominate the automated detection of DR: (1) Deep learning, particularly convolutional neural networks (CNNs); and (2) traditional ML classifiers.
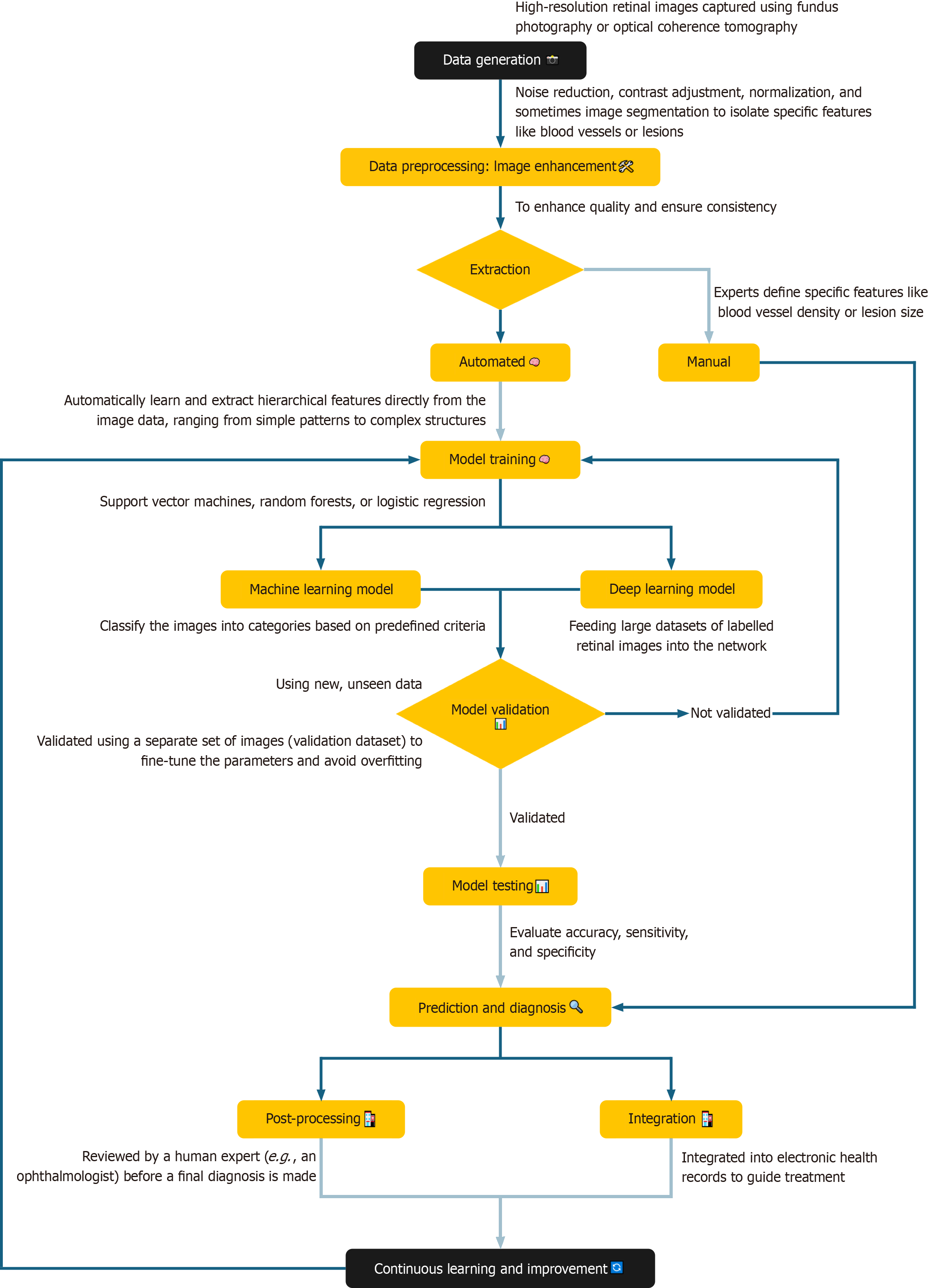
Deep learning and CNNs: Deep learning, particularly using CNNs, has shown remarkable success in automated DR diagnosis. CNNs are designed to process image data, learning hierarchical features from raw pixels to complex patterns[49] from large data sets as shown in Figure 1. Researchers have explored CNN architectures, such as ResNet and VGG, each offering unique advantages in terms of accuracy and efficiency. The effectiveness of CNNs depends on large labelled datasets of retinal images, which train models to distinguish between healthy retinas and those with DR[49].
Figure 1 Process flow showing the integration of artificial intelligence in the prediction and diagnosis of diabetic retinopathy.
Traditional ML classifiers: Traditional ML classifiers, such as support vector machines, random forests, and logistic regression are used in the detection of DR[51]. Unlike deep learning methods that learn directly from raw image data, these classifiers require pre-extracted features, such as texture analysis or vessel segmentation, which serve as input for the algorithms. These extracted features enable the classifiers to differentiate between healthy and diseased retinas. While these methods are effective, they are less scalable and flexible compared to CNNs, which can operate directly on raw images without manual feature extraction[48]. A comparison of traditional ML classifiers with CNN-based models is summarized in Table 2.
Table 2 Summary of artificial intelligence/machine learning techniques in diabetic retinopathy detection.
| Technique | Description | Advantages | Limitations |
|---|---|---|---|
| CNNs | Deep learning model for image analysis; learns hierarchical features | High accuracy, effective for image data | Requires large datasets, computationally intensive |
| Support vector machines | Supervised learning model; used for classifying pre-extracted features | Robust with small datasets, interpretable results | Less effective with large-scale image data |
| Random forests | Ensemble learning method using decision trees; used for feature-based classification | Good performance with noisy data | Requires feature extraction, less flexible than CNNs |
Evaluating model performance: The performance of both deep learning and traditional ML models is evaluated using key metrics like accuracy, sensitivity, and specificity. Accuracy measures the overall correctness of the model's predictions, while sensitivity reflects its ability to correctly identify positive cases (i.e., those with DR). Specificity, on the other hand, measures the model's ability to correctly identify negative cases (i.e., those without the disease). These metrics help researchers and clinicians understand the strengths and limitations of each approach, guiding the development and implementation of more effective screening programs in DR[52,53]. The performance of AI/ML models vs traditional screening methods for DR is shown in Table 3.
Table 3 Comparison of artificial intelligence/machine learning models and traditional screening methods for diabetic retinopathy.
| Screening method | Accuracy | Sensitivity | Specificity | Key points |
|---|---|---|---|---|
| CNNs | High | High | High | Capable of analysing complex retinal images with high accuracy and scalability |
| Support vector machines | Moderate | Moderate | Moderate | Effective in classifying pre-extracted features but less scalable than CNNs |
| Random forests | Moderate | Moderate | Moderate | Good for feature extraction-based classification; robust but less flexible |
| Traditional manual fundus examination | Variable | Low to moderate | Low to moderate | Dependent on the skill of the ophthalmologist; less accessible and scalable |
THE FUTURE OF DR CARE: AI AND ML PAVE THE WAY
AI and ML technologies are not only improving the accuracy of DR screening but are also reshaping management strategies and enabling personalized treatment approaches[49,51,52]. By automating the analysis of retinal images, these technologies increase the efficiency and accessibility of DR screening, allowing for earlier detection and intervention, particularly in underserved areas.
In healthcare, AI and ML are being utilized to diagnose diseases, predict disease progression, discover new drugs, and personalize treatments. In ophthalmology, these tools are instrumental in developing automated screening systems, assisting with diagnosis through image analysis, and customizing treatment plans based on patient-specific data[48].
Recent advancements in AI/ML algorithms
Recent advances in AI/ML algorithms, particularly deep learning models, have led to the creation of highly accurate DR detection systems[54,55]. These models, inspired by the structure of the human brain, are trained on extensive datasets of retinal images, learning to recognize subtle disease markers. For instance, Tan _et al_[56] (2019) and Farahat _et al_[57] (2021) provided examples of AI-driven systems achieving performance levels comparable to or surpassing those of expert ophthalmologists.
Key AI/ML systems in DR screening
Key AI/ML systems in DR screening include: (1) IDx-DR, the first FDA-authorized AI system for autonomous DR detection, achieving high sensitivity and specificity; (2) EyeArt, an FDA-cleared AI system that uses deep learning algorithms to analyze retinal images with high accuracy in detecting referable DR; (3) Google's deep learning algorithm, developed by Google Research, which has achieved high accuracy in detecting DR from retinal images, outperforming ophthalmologists in some studies; and (4) Stanford University's ML system, which combines deep learning with other techniques to detect DR and predict its progression, showing promise in identifying patients at high risk of developing sight-threatening DR[58-61]. While promising, AI/ML in healthcare is still evolving. Ongoing research is vital to enhance these technologies’ accuracy, reliability, and accessibility.
AI for accessibility and scalability
AI-powered systems address the challenges of delivering healthcare to underserved regions by increasing scalability and efficiency. These systems can analyze large volumes of retinal images quickly, reducing the workload on healthcare providers. Portable AI systems, combined with trained technicians, can make high-quality DR screening available in remote areas. Moreover, AI algorithms can seamlessly integrate with teleophthalmology platforms to support remote diagnosis and DR management. Early detection through AI-driven technologies facilitates timely intervention, preventing vision loss and reducing the overall burden on healthcare systems[62].
AI is also revolutionizing DR management by enabling predictive analytics that personalize treatment plans by analyzing patient-specific data, such as disease severity and progression patterns, AI helps tailor treatment regimens for optimal outcomes as summarized in Table 4.
Table 4 Artificial intelligence-driven personalized management strategies in diabetic retinopathy.
| AI application | Ref. | Description | Impact on patient care |
|---|---|---|---|
| Predicting disease progression | Kong and Song[73], 2024 | Analyse vast and diverse datasets, including retinal images, genetic information, blood glucose levels, and other patient-specific variables, to identify subtle patterns and predict the likelihood of disease advancement with higher accuracy | Allows for timely intervention and personalized treatment plans |
| Optimizing treatment regimens | Patibandla __et al_[74], 2024 | Analyse patient data to predict the effectiveness of different treatment options, such as laser therapy or anti-VEGF injections, and recommend the most suitable approach for each individual | Ensures patients receive the most effective treatments based on individual data |
| Personalizing follow-up schedules | Silva __et al_[75], 2024 | Determine the optimal frequency of eye exams and other monitoring measures, ensuring timely detection of any changes in a patient's condition | Helps in timely detection of changes in the patient's condition |
Teleophthalmology combined with portable AI solutions offers a powerful approach to delivering accessible DR diagnostics in remote, underserved areas[63]. By capturing retinal images digitally and transmitting them for remote, AI-powered analysis, teleophthalmology reduces the need for on-site specialists[64]. Portable AI tools, including smartphone apps and handheld devices, further enhance accessibility by enabling healthcare workers in remote locations to conduct screenings and receive immediate diagnostic results.
IMPLEMENTATION AND INTEGRATION CHALLENGES
While AI and ML hold great promise for transforming DR care, their implementation faces significant technological, ethical, and regulatory challenges. Key issues include data standardization, interoperability, ethical concerns such as algorithmic bias, and the need for robust data privacy measures. These issues along with their potential solutions are presented in Table 5.
Table 5 Challenges and solutions for artificial intelligence/machine learning implementation in diabetic retinopathy screening.
| Challenge | Ref. | Description | Potential solution |
|---|---|---|---|
| Data standardization and interoperability | Mandl __et al_[76], 2024 | Difficulty in integrating AI tools with diverse electronic health record systems | Develop universal data standards and use fast healthcare interoperability resources APIs |
| Clinicians need AI tools that seamlessly integrate into their existing workflows without adding complexity or disrupting patient care | |||
| Ensuring scalability, security, and ongoing technical support are critical considerations | |||
| Ethical and regulatory concerns | Goldberg __et al_[77], 2024 | Issues related to data privacy, algorithmic bias, and lack of clear regulatory guidelines | Promote diverse datasets, establish clear regulatory frameworks, and ensure data security |
| Scalability and maintenance | Marvasti __et al_[78], 2024 | Challenges in deploying AI systems across large healthcare networks | Use cloud-based platforms for scalability and provide ongoing technical support |
Despite the advancements in AI-based DR screening, these technologies may have limitations. In particular, AI tools may still need improvement to match human specialists in complex cases[65]. Furthermore, the variety of data used to train these systems may be limited, affecting their reliability across different populations[66]. Future research should focus on enhancing AI's accuracy by leveraging larger and more diverse datasets[67]. Additionally, there is significant potential for AI to aid in monitoring the progression of DR over time, allowing for more personalized and timely interventions[20]. Successful integration of AI into routine clinical practice will also require trust-building among both clinicians and patients through transparent communication of the benefits, risks, and limitations of these technologies[68,69].
A vision for the future: Scalable and sustainable AI for DR care
The future of AI in DR care promises greater accessibility, affordability, and quality of care. By integrating AI with telemedicine platforms, patients can receive timely care regardless of location[6]. AI can automate the analysis of retinal images, enabling healthcare providers to screen a larger volume of patients efficiently. Grzybowski and Kanclerz[50] in 2019 provided an overview of AI-based DR screening technologies. This increased efficiency can help identify individuals at risk of DR earlier, facilitating timely interventions and potentially reducing the incidence of vision loss[50].
However, for AI to reach its full potential, collaboration among stakeholders, AI experts, clinicians, and ethicists is essential. Comprehensive education and training programs are needed to equip healthcare professionals with the skills required to effectively utilize AI technologies. Moreover, transparent communication about the benefits, risks, and limitations of AI is essential for building public trust, encouraging responsible adoption, and promoting ethical use.
Within this dynamic environment, regulatory frameworks play a critical role in ensuring the responsible integration of AI in DR care. Countries like the United States have provided clearance for several AI-powered medical devices, such as IDx-DR, through the FDA, while Europe, under the general data protection regulation, has begun implementing frameworks that govern AI's use in healthcare[70]. Despite these developments, global efforts to standardize AI regulations remain fragmented, necessitating the advocacy for international guidelines that will ensure consistent patient safety, equitable access, and the responsible use of AI-driven healthcare technologies[71]. As the technology continues to evolve, fostering a collaborative ecosystem between regulatory bodies, healthcare providers, and AI developers will be vital in ensuring that AI is used ethically and equitably across all healthcare settings.
Ensuring sustainability and equity
Sustainability, equity, and economic considerations are crucial for implementing AI-driven DR care. While initial investments may be substantial, long-term savings can be achieved by preventing vision loss and reducing the burden on healthcare systems[72]. Addressing algorithmic biases is critical to ensure accurate and equitable diagnosis for all patients[53]. The successful integration of AI in DR care will require investments in training, infrastructure, and regulatory frameworks for data privacy and algorithm accountability.
AI-powered teleophthalmology offers a solution for extending DR screening services to areas lacking specialized eye care infrastructure. Patients in under-served areas can have their retinal images taken locally and transmitted to specialists or AI systems for interpretation, facilitating timely diagnosis and treatment even without access to on-site ophthalmologists.
CONCLUSION
The promise of AI and ML integration into the care of DR is immense in changing the course of screening, as well as management approaches. By enhancing early detection, optimizing treatment pathways, and facilitating personalized care, AI and ML technologies hold the potential to significantly reduce the prevalence of vision impairment and blindness caused by DR. These tools are particularly valuable in improving accessibility in underserved regions and streamlining the workload for healthcare professionals. AI-based screening tools, such as IDx-DR and EyeArt, can be deployed in primary care settings and remote locations, enabling early detection of DR without the need for specialized expertise. By automating the screening process and offering high accuracy, these AI systems can reduce the burden on healthcare professionals and ensure timely diagnosis, particularly in areas where access to ophthalmic care is limited. However, to fully harness the potential of AI-driven solutions, several key challenges must be addressed. Technological limitations, such as ensuring the scalability of AI models and integrating them into existing healthcare infrastructures, must be overcome. Ethical concerns, including algorithmic bias and data privacy, must also be carefully managed to guarantee equitable care for all patients. Additionally, robust regulatory frameworks will be necessary to govern the deployment of these technologies responsibly. Ongoing advancements in AI and ML promise further improvements in vision preservation and the quality of life for patients with diabetes. Moving forward, the successful implementation of scalable and sustainable AI solutions for DR care will require close collaboration among researchers, clinicians, policymakers, and technologists. By working together, we can achieve a future where AI and ML are central to preventing vision loss and reducing the global burden of DR.
Footnotes
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country of origin: Malaysia
Peer-review report’s classification
Scientific Quality: Grade A, Grade B
Novelty: Grade A, Grade C
Creativity or Innovation: Grade B, Grade B
Scientific Significance: Grade B, Grade B
P-Reviewer: Li DH; Tayeb BA S-Editor: Lin C L-Editor: A P-Editor: Wang WB
References
| 1. | Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, Colagiuri S, Guariguata L, Motala AA, Ogurtsova K, Shaw JE, Bright D, Williams R; IDF Diabetes Atlas Committee. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res Clin Pract. 2019;157:107843. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5345] [Cited by in F6Publishing: 5185] [Article Influence: 1037.0] [Reference Citation Analysis (8)] |
|---|
| 2. | Teo ZL, Tham YC, Yu M, Chee ML, Rim TH, Cheung N, Bikbov MM, Wang YX, Tang Y, Lu Y, Wong IY, Ting DSW, Tan GSW, Jonas JB, Sabanayagam C, Wong TY, Cheng CY. Global Prevalence of Diabetic Retinopathy and Projection of Burden through 2045: Systematic Review and Meta-analysis. Ophthalmology. 2021;128:1580-1591. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 127] [Cited by in F6Publishing: 715] [Article Influence: 238.3] [Reference Citation Analysis (1)] |
|---|
| 4. | Kropp M, Golubnitschaja O, Mazurakova A, Koklesova L, Sargheini N, Vo TKS, de Clerck E, Polivka J Jr, Potuznik P, Polivka J, Stetkarova I, Kubatka P, Thumann G. Diabetic retinopathy as the leading cause of blindness and early predictor of cascading complications-risks and mitigation. EPMA J. 2023;14:21-42. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 65] [Article Influence: 65.0] [Reference Citation Analysis (0)] |
|---|
| 12. | Zhang J, Lin S, Cheng T, Xu Y, Lu L, He J, Yu T, Peng Y, Zhang Y, Zou H, Ma Y. RETFound-enhanced community-based fundus disease screening: real-world evidence and decision curve analysis. NPJ Digit Med. 2024;7:108. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
|---|
| 14. | Raj A, Tiwari AK, Martini MG. Fundus image quality assessment: survey, challenges, and future scope. IET Imag Process. 2019;13:1211-1224. [PubMed] [DOI] [Cited in This Article: ] |
|---|
| 18. | Graham-Rowe E, Lorencatto F, Lawrenson JG, Burr JM, Grimshaw JM, Ivers NM, Presseau J, Vale L, Peto T, Bunce C, J Francis J. Barriers to and enablers of diabetic retinopathy screening attendance: a systematic review of published and grey literature. Diabet Med. 2018;35:1308-1319. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 69] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
|---|
| 19. | Sugandh F, Chandio M, Raveena F, Kumar L, Karishma F, Khuwaja S, Memon UA, Bai K, Kashif M, Varrassi G, Khatri M, Kumar S. Advances in the Management of Diabetes Mellitus: A Focus on Personalized Medicine. Cureus. 2023;15:e43697. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 16] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
|---|
| 29. | DuPont M, Arthur E, Shihab Y, Kenny M, Ravichandran S, Parsons-Wingerter P, Vyas R, Murray MC, Predovic M, Lim S, Jacobs N, Ramesh S, Vu A, Sekaran S, Chalam KV, Moorthy RS, Crosson J, Mason J, Grant MB. Use of VESsel GENeration with Optical Coherence Tomography Angiography and Fluorescein Angiography for Detection and Quantification of Vascular Changes in Mild and Moderate Diabetic Retinopathy. Life (Basel). 2024;14. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
|---|
| 34. | Fischer J, Otto T, Delori F, Pace L, Staurenghi G. Scanning Laser Ophthalmoscopy (SLO). In: High Resolution Imaging in Microscopy and Ophthalmology: New Frontiers in Biomedical Optics [Internet]. Cham (CH): Springer, 2019. [PubMed] [DOI] [Cited in This Article: ] |
|---|
| 37. | Kim TN, Myers F, Reber C, Loury PJ, Loumou P, Webster D, Echanique C, Li P, Davila JR, Maamari RN, Switz NA, Keenan J, Woodward MA, Paulus YM, Margolis T, Fletcher DA. A Smartphone-Based Tool for Rapid, Portable, and Automated Wide-Field Retinal Imaging. Transl Vis Sci Technol. 2018;7:21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 57] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
|---|
| 40. | Capobianco G, Pronti L, Gorga E, Romani M, Cestelli-Guidi M, Serranti S, Bonifazi G. Methodological approach for the automatic discrimination of pictorial materials using fused hyperspectral imaging data from the visible to mid-infrared range coupled with machine learning methods. Spectrochim Acta A Mol Biomol Spectrosc. 2024;304:123412. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
|---|
| 48. | Esmaeilzadeh P. Challenges and strategies for wide-scale artificial intelligence (AI) deployment in healthcare practices: A perspective for healthcare organizations. Artif Intell Med. 2024;151:102861. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Reference Citation Analysis (0)] |
|---|
| 49. | Fulkar B, Burle R, Patil P, Gaurkhade S, Gundewar S, Pacharaney U. Machine Learning and Deep Learning Approaches for Automated Diabetic Retinopathy Diagnosis. 2024 International Conference on Intelligent Systems for Cybersecurity (ISCS); 2024; India. [PubMed] [DOI] [Cited in This Article: ] |
|---|
| 53. | Deepa R, Sivasamy A. Advancements in early detection of diabetes and diabetic retinopathy screening using artificial intelligence. AIP Advances. 2023;13:115307. [PubMed] [DOI] [Cited in This Article: ] |
|---|
| 57. | Farahat Z, Souissi N, Belmekki M, Megdiche K, Benamar S, Bennani Y, Bencherif S, Ngote N. Diabetic Retinopathy: New Perspectives with Artificial Intelligence. 2021 Fifth International Conference On Intelligent Computing in Data Sciences (ICDS); 2021; Morocco. [PubMed] [DOI] [Cited in This Article: ] |
|---|
| 59. | Ting DSW, Cheung CY, Lim G, Tan GSW, Quang ND, Gan A, Hamzah H, Garcia-Franco R, San Yeo IY, Lee SY, Wong EYM, Sabanayagam C, Baskaran M, Ibrahim F, Tan NC, Finkelstein EA, Lamoureux EL, Wong IY, Bressler NM, Sivaprasad S, Varma R, Jonas JB, He MG, Cheng CY, Cheung GCM, Aung T, Hsu W, Lee ML, Wong TY. Development and Validation of a Deep Learning System for Diabetic Retinopathy and Related Eye Diseases Using Retinal Images From Multiethnic Populations With Diabetes. JAMA. 2017;318:2211-2223. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1098] [Cited by in F6Publishing: 1158] [Article Influence: 165.4] [Reference Citation Analysis (0)] |
|---|
| 60. | Gulshan V, Peng L, Coram M, Stumpe MC, Wu D, Narayanaswamy A, Venugopalan S, Widner K, Madams T, Cuadros J, Kim R, Raman R, Nelson PC, Mega JL, Webster DR. Development and Validation of a Deep Learning Algorithm for Detection of Diabetic Retinopathy in Retinal Fundus Photographs. JAMA. 2016;316:2402-2410. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3669] [Cited by in F6Publishing: 3139] [Article Influence: 392.4] [Reference Citation Analysis (0)] |
|---|
| 61. | Bora A, Balasubramanian S, Babenko B, Virmani S, Venugopalan S, Mitani A, de Oliveira Marinho G, Cuadros J, Ruamviboonsuk P, Corrado GS, Peng L, Webster DR, Varadarajan AV, Hammel N, Liu Y, Bavishi P. Predicting the risk of developing diabetic retinopathy using deep learning. Lancet Digit Health. 2021;3:e10-e19. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 74] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
|---|
| 64. | Redd TK, Al-Khaled T, Paul Chan RV, Campbell JP; American Academy of Ophthalmology Task Force on Academic Global Ophthalmology; American Academy of Ophthalmology Task Force on Academic Global Ophthalmology. Technology and Innovation in Global Ophthalmology: The Past, the Potential, and a Path Forward. Int Ophthalmol Clin. 2023;63:25-32. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
|---|
| 65. | Jarrahi MH. Artificial intelligence and the future of work: Human-AI symbiosis in organizational decision making. Bus Horiz. 2018;61:577-586. [PubMed] [DOI] [Cited in This Article: ] |
|---|
| 67. | Huang K, Zhou Y, Yu X, Su X. Innovative entrepreneurial market trend prediction model based on deep learning: Case study and performance evaluation. Sci Prog. 2024;107:368504241272722. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
|---|
| 68. | Adler-Milstein J, Aggarwal N, Ahmed M, Castner J, Evans BJ, Gonzalez AA, James CA, Lin S, Mandl KD, Matheny ME, Sendak MP, Shachar C, Williams A. Meeting the Moment: Addressing Barriers and Facilitating Clinical Adoption of Artificial Intelligence in Medical Diagnosis. NAM Perspect. 2022;2022. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 11] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
|---|
| 69. | Tuan DA. Bridging the Gap Between Black Box AI and Clinical Practice: Advancing Explainable AI for Trust, Ethics, and Personalized Healthcare Diagnostics. 2024 Preprint. [PubMed] [DOI] [Cited in This Article: ] |
|---|
| 74. | Patibandla RSML, Rao BT, Murty MR. Revolutionizing Diabetic Retinopathy Diagnostics and Therapy Through Artificial Intelligence. Advances Healthc Inf Syst Adm. 2024;. [PubMed] [DOI] [Cited in This Article: ] |
|---|
| 75. | Silva PS, Zhang D, Jacoba CMP, Fickweiler W, Lewis D, Leitmeyer J, Curran K, Salongcay RP, Doan D, Ashraf M, Cavallerano JD, Sun JK, Peto T, Aiello LP. Automated Machine Learning for Predicting Diabetic Retinopathy Progression From Ultra-Widefield Retinal Images. JAMA Ophthalmol. 2024;142:171-177. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
|---|
| 77. | Goldberg CB, Adams L, Blumenthal D, Brennan PF, Brown N, Butte AJ, Cheatham M, deBronkart D, Dixon J, Drazen J, Evans BJ, Hoffman SM, Holmes C, Lee P, Manrai AK, Omenn GS, Perlin JB, Ramoni R, Sapiro G, Sarkar R, Sood H, Vayena E, Kohane IS; RAISE Consortium. To do no harm - and the most good - with AI in health care. Nat Med. 2024;30:623-627. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
|---|
| 78. | Marvasti TB, Gao Y, Murray KR, Hershman S, McIntosh C, Moayedi Y. Unlocking Tomorrow's Health Care: Expanding the Clinical Scope of Wearables by Applying Artificial Intelligence. Can J Cardiol. 2024;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
|---|