Sevelamer (original) (raw)
From Wikipedia, the free encyclopedia
Chemical compound
Pharmaceutical compound
Sevelamer
 |
|
|---|---|
| Clinical data | |
| Pronunciation | ( or ) |
| Trade names | Renagel, Renvela |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a601248 |
| License data | US DailyMed: Sevelamer |
| Pregnancy category | AU: B3 |
| Routes of administration | By mouth |
| ATC code | V03AE02 (WHO) |
| Legal status | |
| Legal status | AU: S4 (Prescription only)[1] CA: ℞-only[2] US: ℞-only EU: Rx only[3][4][5][6][7] |
| Pharmacokinetic data | |
| Bioavailability | 0% |
| Excretion | Feces 100% |
| Identifiers | |
| IUPAC name poly(allylamine-co-N,_N'_-diallyl-1,3-diamino-2-hydroxypropane) | |
| CAS Number | 52757-95-6  Y Y |
| PubChem CID | 3085017 |
| DrugBank | DB00658  N N |
| ChemSpider | 2341997  N N |
| UNII | 941N5DUU5C |
| KEGG | D08512  Yas HCl: D01983 Yas HCl: D01983 |
| ChEMBL | ChEMBL1201492  N N |
| CompTox Dashboard (EPA) | DTXSID80872282 |
| Chemical and physical data | |
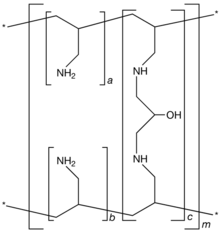
| Formula | [(C3H7N)a+b.(C9H17N2O)c]mwhere a+b:c = 9:1 |
| Molar mass | variable |
 N N Y (what is this?) (verify) Y (what is this?) (verify) |
Sevelamer (rINN) is a phosphate binding medication used to treat hyperphosphatemia in patients with chronic kidney disease. When taken with meals, it binds to dietary phosphate and prevents its absorption. Sevelamer was invented and developed by GelTex Pharmaceuticals. Sevelamer is marketed by Sanofi under the brand names Renagel (sevelamer hydrochloride) and Renvela (sevelamer carbonate).
Chemistry and pharmacology
[edit]
Sevelamer consists of polyallylamine that is crosslinked with epichlorohydrin.[8] The marketed form sevelamer hydrochloride is a partial hydrochloride salt being present as approximately 40% amine hydrochloride and 60% sevelamer base. The amine groups of sevelamer become partially protonated in the intestine and interact with phosphate ions through ionic and hydrogen bonding.[_citation needed_]
Sevelamer is used in the management of hyperphosphatemia in adult patients with stage 4 and 5 chronic kidney disease (CKD) on hemodialysis. Its efficacy at lowering phosphate levels is similar to that of calcium acetate, but without the accompanying risk of hypercalcemia and arterial calcification.[9][10] In patients with CKD, it has also been shown to reduce LDL and cholesterol.[11]
Sevelamer therapy is contraindicated in hypophosphatemia or bowel obstruction.[12] In hypophosphatemia, sevelamer could exacerbate the condition by further lowering phosphate levels in the blood, which could be fatal.[13]
Common adverse drug reactions (ADRs) associated with the use of sevelamer include: nausea and vomiting, constipation, diarrhea, stomach pain, heartburn, gas.[14]
Sevelamer can significantly reduce serum uric acid.[15] This reduction has no known detrimental effect and may be helpful in patients with gout.
Sevelamer is able to sequester advanced glycation end products (AGEs) in the gut, preventing their absorption into the blood. AGEs contribute to oxidative stress, which can damage cells (like beta cells, which produce insulin in the pancreas). As Vlassara and Uribarri explain in a 2014 review on AGEs, this may explain why sevelamer, but not calcium carbonate (a phosphate binder that does not sequester AGEs), has been shown to lower AGEs in the blood, as well as oxidative stress and inflammatory markers.[16]
- ^ "Prescription medicines: registration of new chemical entities in Australia, 2015". Therapeutic Goods Administration (TGA). 21 June 2022. Retrieved 10 April 2023.
- ^ "Kidney disease". Health Canada. 9 May 2018. Retrieved 13 April 2024.
- ^ "Renvela EPAR". European Medicines Agency. 10 June 2009. Retrieved 29 June 2024.
- ^ "Renvela PI". Union Register of medicinal products. 12 June 2009. Retrieved 29 June 2024.
- ^ "Sevelamer carbonate Winthrop (previously Sevelamer carbonate Zentiva) EPAR". European Medicines Agency. 15 January 2015. Retrieved 29 June 2024.
- ^ "Sevelamer carbonate PI". Union Register of medicinal products. 19 January 2015. Retrieved 29 June 2024.
- ^ "Renagel EPAR". European Medicines Agency (EMA). 28 January 2000. Retrieved 5 September 2024.
- ^ Ramsdell R (June 1999). "Renagel: a new and different phosphate binder". review. ANNA Journal. 26 (3): 346–7. PMID 10633608.
- ^ Burke SK (September 2000). "Renagel: reducing serum phosphorus in haemodialysis patients". review. Hospital Medicine. 61 (9): 622–7. doi:10.12968/hosp.2000.61.9.1419. PMID 11048603.
- ^ Habbous S, Przech S, Acedillo R, Sarma S, Garg AX, Martin J (January 2017). "The efficacy and safety of sevelamer and lanthanum versus calcium-containing and iron-based binders in treating hyperphosphatemia in patients with chronic kidney disease: a systematic review and meta-analysis". review. Nephrology, Dialysis, Transplantation. 32 (1): 111–125. doi:10.1093/ndt/gfw312. PMID 27651467.
- ^ Patel L, Bernard LM, Elder GJ (February 2016). "Sevelamer Versus Calcium-Based Binders for Treatment of Hyperphosphatemia in CKD: A Meta-Analysis of Randomized Controlled Trials". review. Clinical Journal of the American Society of Nephrology. 11 (2): 232–44. doi:10.2215/CJN.06800615. PMC 4741042. PMID 26668024.
- ^ Yuste C (2017). "Gastrointestinal complications induced by sevelamer crystals". Clinical Kidney Journal. 10 (4): 539–544. doi:10.1093/ckj/sfx013. PMC 5570024. PMID 28852493.
- ^ Emmett M (September 2004). "A comparison of clinically useful phosphorus binders for patients with chronic kidney failure". review. Kidney International Supplements. 66 (90): S25–32. doi:10.1111/j.1523-1755.2004.09005.x. PMID 15296504.
- ^ "Sevelamer". MedlinePlus.
- ^ Locatelli F, Del Vecchio L (May 2015). "Cardiovascular mortality in chronic kidney disease patients: potential mechanisms and possibilities of inhibition by resin-based phosphate binders". review. Expert Review of Cardiovascular Therapy. 13 (5): 489–99. doi:10.1586/14779072.2015.1029456. PMID 25804298. S2CID 32586527.
- ^ Vlassara H, Uribarri J (January 2014). "Advanced glycation end products (AGE) and diabetes: cause, effect, or both?". review. Current Diabetes Reports. 14 (1): 453. doi:10.1007/s11892-013-0453-1. PMC 3903318. PMID 24292971.