Common Properties of Nuclear Body Protein SP100 and TIF1α Chromatin Factor: Role of SUMO Modification (original) (raw)
Abstract
The SP100 protein, together with PML, represents a major constituent of the PML-SP100 nuclear bodies (NBs). The function of these ubiquitous subnuclear structures, whose integrity is compromised in pathological situations such as acute promyelocytic leukemia (APL) or DNA virus infection, remains poorly understood. There is little evidence for the occurrence of actual physiological processes within NBs. The two NB proteins PML and SP100 are covalently modified by the ubiquitin-related SUMO-1 modifier, and recent work indicates that this modification is critical for the regulation of NB dynamics. In exploring the functional relationships between NBs and chromatin, we have shown previously that SP100 interacts with members of the HP1 family of nonhistone chromosomal proteins and that a variant SP100 cDNA encodes a high-mobility group (HMG1/2) protein. Here we report the isolation of a further cDNA, encoding the SP100C protein, that contains the PHD-bromodomain motif characteristic of chromatin proteins. We further show that TIF1α, a chromatin-associated factor with homology to both PML and SP100C, is also modified by SUMO-1. Finally, in vitro experiments indicate that SUMO modification of SP100 enhances the stability of SP100-HP1 complexes. Taken together, our results suggest an association of SP100 and its variants with the chromatin compartment and, further, indicate that SUMO modification may play a regulatory role in the functional interplay between the nuclear bodies and chromatin.
The PML-SP100 nuclear bodies (NBs), also referred to as ND10 or PODs (PML oncogenic domains), represent an important model system for the study of the interplay between global nuclear architecture and events surrounding gene regulation, the control of cell growth and differentiation, and apoptosis (reviewed in reference 47). Immunologically they are defined as containing two major protein constituents: SP100 and PML. The SP100 protein was first characterized as an antigen reactive with antibodies from patients with autoimmune disorders (52). The more recent interest in NBs, however, is due to their alteration in pathological situations. The PML protein was identified as part of the oncogenic PML-RARα fusion derived from the t(15; 17) chromosomal translocation characteristic of acute promyelocytic leukemia (APL) (for a review, see reference 33). This leukemia is treatable to complete clinical remission with retinoic acid, the physiological ligand of the retinoic acid receptor α (RARα). In APL cells, expression of PML-RARα leads to the disruption of the NBs and retinoic acid treatment induces their reorganization. The integrity of NBs is also compromised in some neurodegenerative disorders (reviewed in reference 47), as well as during infection by DNA viruses such as adenovirus, cytomegalovirus and herpes simplex virus (reviewed in reference 36). In this regard, it is also of interest that interferons (IFNs), key inducers in cellular antiproliferative and anti-viral responses, cause the specific transcriptional up-regulation of the two NB components, PML and SP100 (17, 27, 49). Finally, NBs disaggregate nearly completely during cell division (2).
The NBs are intimately associated with a novel pathway of posttranslational protein modification by members of the SUMO family of ubiquitin-like proteins. This pathway, enzymatically analogous to ubiquitination, gives rise to covalent adducts consisting of a SUMO moiety linked to its target protein via a glycine-lysine isopeptide link (reviewed in reference 22). Among a growing list of substrates, both PML and SP100 are targets for this type of modification (40, 51). Indeed, conjugation to SUMO may play a crucial role in the establishment or maintenance of proper NB structure. First, SUMO-modified PML is preferentially targeted to the NBs, whereas the unmodified form remains in the nucleoplasmic diffuse fraction (40, 51). Second, in PML-/- cells, in which the absence of PML leads to the disaggregation of the NBs, only exogenously added PML subject to SUMO modification leads to NB restoration whereas addition of a nonmodifiable PML mutant does not (20, 60). Finally, the direct interaction of PML with another NB component, Daxx, depends on PML being modified by SUMO (20). For SP100, the role of SUMO modification remains unclear since it appears not to be requisite for NB targeting (50).
Thus, while much has been learned about the dynamics of the NBs as subnuclear structures, evidence implicating them as actual sites of physiological processes remains controversial (6, 26). In this context, DNA viral replication appears to take place not within, but in close proximity to the NBs (36). Therefore, a model in which NB components play important roles outside these structures appears plausible: indeed, treatment of cells with trivalent arsenicals, also shown to have a therapeutic effect in APL (8), leads to the rapid targeting of the diffuse nucleoplasmic form of the NB proteins back to the NBs (40, 61), thereby providing a striking demonstration that a significant fraction of these proteins exists outside the NBs and hence may perform critical functions there.
We and others have previously demonstrated that the NB protein SP100 interacts and colocalizes with members of the heterochromatin protein 1 (HP1) family of nonhistone chromosomal proteins (31, 48). Interestingly, a similar link between the NBs and the chromatin compartment was also recently demonstrated for the Daxx protein (20). Besides being found in the NBs as a direct partner of PML, Daxx also localizes to centromeric heterochromatin, possibly by binding CENP-C (12, 44). Further, like SP100, Daxx exhibits transcriptional corepressor activity (19, 55) that may be subject to regulation by PML (20, 32).
In this report we describe a novel splice variant, SP100C, that contains a PHD finger-bromodomain motif and displays distinct subnuclear localization behavior. Further, we show that covalent modification of SP100 proteins by SUMO is a common property with TIF1α, a chromatin-associated protein with homology to both PML and SP100. Finally, we provide evidence that SUMO modification affects the binding of SP100 to its partner HP1. Our findings thus suggest additional links between NB components and chromatin.
MATERIALS AND METHODS
Isolation of cDNA clones.
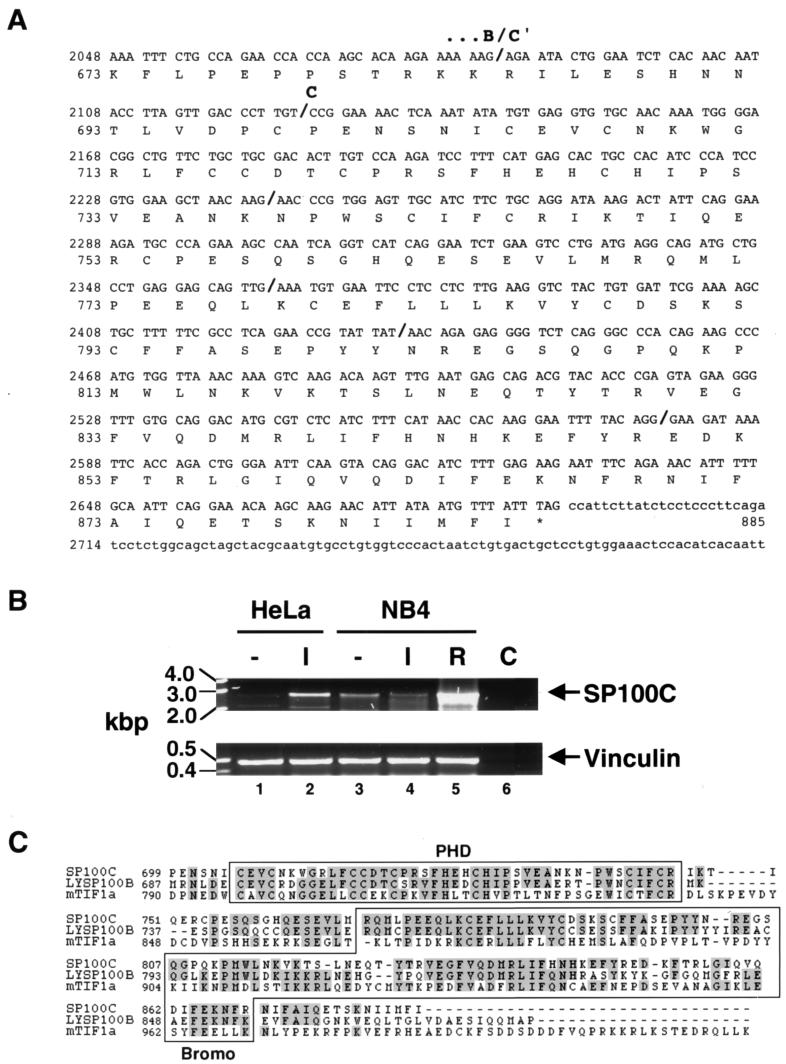
cDNA screens and plasmid constructions were carried out using standard methods. Three overlapping phage λgt10 clones encoding SP100B and SP100C sequences were isolated from a liver cDNA library (11) using multimerized oligonucleotide probes derived from the 3′ end of an open-framed cDNA isolated by Xie et al. (58). The 3′ end of the SP100C clone was obtained by reverse transcriptase PCR (RT-PCR) using a specific 5′ primer (nucleotides 1974 to 1998)-poly(dT) primer pair combination. For consistency, we have retained the nucleotide numbering of the first SP100 clone (here called SP100A [52]). Semiquantitative RT-PCR verification of a near-full-length SP100C cDNA was performed using a primer pair directing the synthesis of an amplified product spanning nucleotides 69 to 2879 (5′-TGAATGAATGTATTTCACCAGTAG-3′ and 5′-TATTGAGAATTTTTAACATGAAGG-3′) on total cDNA prepared from HeLa cells left untreated or treated with 200 U of IFN-α per ml for 16 h or from NB4 cells left untreated or treated with either IFN-α or 1 μM retinoic acid for 48 h. PCR products from 30 amplification cycles were subcloned into pGEM T-Easy (Promega) and sequenced. Equal loading of PCR products was verified by control amplifications using primers directing the synthesis of human vinculin.
Plasmid constructions.
Plasmids for transient overexpression of SP100 and its variants in mammalian cells and for T7 in vitro translation-transcription were constructed in the simian virus 40 enhancer-promoter-driven pSG5 vector (Stratagene) using convenient restriction sites and/or PCR-mediated mutagenesis. HA and His6 tag fusions were first made in the pACT (Clontech) and pQE-30 (Qiagen) vectors, respectively, prior to insertion into the pSG5 expression vector. The SP100AΔN construction was prepared by in-frame insertion of a _Hin_cII-_Bgl_II SP100A fragment downstream of an _Nco_I site providing an ATG start codon. Glutathione _S_-transferase (GST) fusions were constructed in the pGEX2T or pGEX3X vectors (Pharmacia). Site-directed mutagenesis was carried out using components and protocols of the QuikChange kit (Stratagene). The SUMO-1 cDNA used in transfection and bacterial expression experiments encodes amino acids (aa) 1 to 97. The murine TIF1α expression vector has been described previously (29). All constructions were verified by sequencing, and detailed maps and primer sequences are available on request.
Immunofluorescent labeling.
Indirect immunofluorescent labeling was carried out as described previously (7). The nuclear bodies were labeled with an anti-PML polyclonal rabbit serum (at a 1:200 dilution) raised against a GST-PML fusion protein (7). Overexpressed HA-tagged proteins were revealed with the 12CA5 monoclonal antibody (Boehringer). Confocal images were acquired with a Leica scanning laser confocal microscope equipped with PlanApo optics and processed using Adobe Photoshop software.
Nickel affinity pull-down assays and Western blots.
HeLa cells at 30 to 50% confluency in 10-cm dishes were transfected with 2 μg each of expression constructs encoding SP100 (or its mutant derivatives) and His6-SUMO1 (or empty control vector) cloned in the pSG5 (Stratagene) vector background, using Lipofectamine Plus reagent (Gibco-BRL). At 30 h posttransfection, the cells were washed in ice-cold phosphate-buffered saline, harvested directly in 1 ml of Gua8 buffer (6 M guanidine-HCl, 100 mM NaCl, 10 mM Tris, 50 mM NaH2PO4 [pH 8.0]), briefly sonicated, and centrifuged. Clarified extracts were incubated for 1 to 2 h with 15 μl (packed volume) of Ni-agarose affinity beads (Quiagen). Bound proteins were washed twice in Gua8 buffer, three times in Urea6.5 buffer (8 M urea, 100 mM NaCl, 50mM NaH2PO4 [pH 6.5]), and once in cold phosphate-buffered saline before being eluted by boiling in Laemmli loading buffer and electrophoresed on sodium dodecyl sulfate–8% acrylamide gels. Western blots for detecting SP100 proteins were prepared with anti-SP100 monoclonal antibody C1 (J. Seeler and A. Dejean, unpublished data), using protocols and reagents of the Western-Star kit (Cetus/Perkin-Elmer). Murine TIF1α was detected with monoclonal antibodies 5T and 6T (29) as indicated (see Fig. 5A and C).
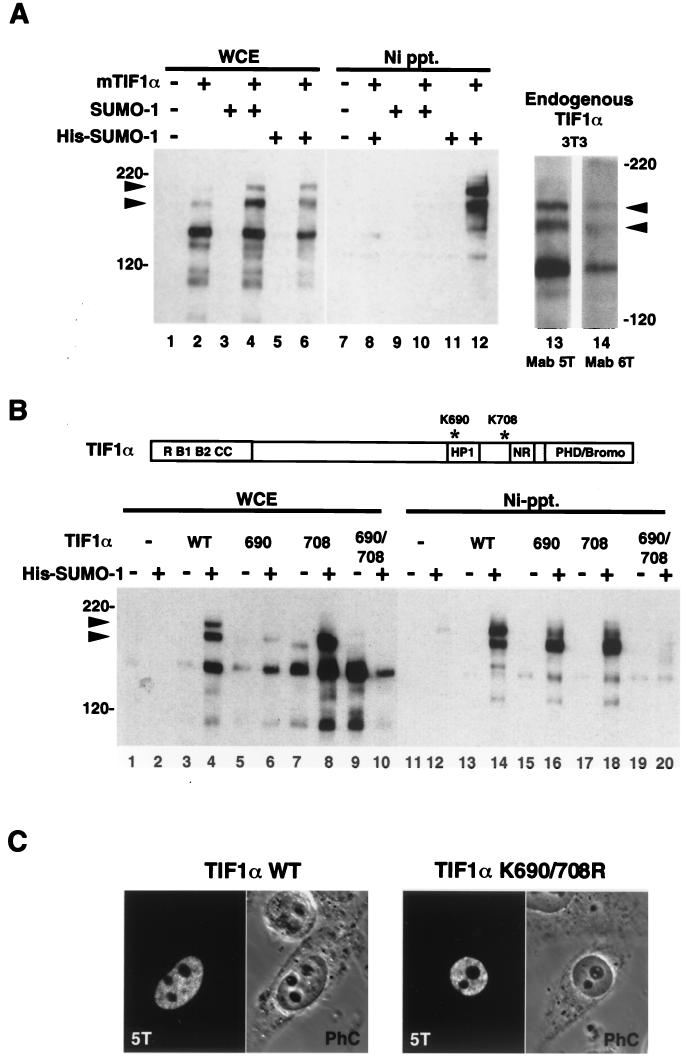
FIG. 5.
(A) TIF1α is covalently modified by SUMO-1 in vivo. HeLa cells were transfected with vectors expressing TIF1α, SUMO-1, or His–SUMO-1, as indicated (lanes 1 to 12). Whole-cell extracts (WCE) prepared under denaturing conditions were either trichloroacetic acid (TCA) precipitated (lanes 1 to 6) or subjected to Ni-agarose chromatography (Ni Ppt.) (lanes 7 to 12) prior to electrophoresis and Western blotting with the anti-TIF1α monoclonal antibody 5T. Arrowheads indicate the positions of the TIF1α–SUMO-1 conjugates. TCA precipitates of extracts from NIH 3T3 cells (lanes 13 and 14) were Western blotted with two different mouse TIF1α-specific monoclonal antibodies, 5T (lane 13) and 6T (lanes 14). Arrowheads indicate the positions of the two TIF1α-SUMO conjugates. (B) Lysines at positions 690 and 708 of TIF1α are the SUMO-1 conjugation sites. HeLa cells were transfected with vectors expressing wild-type (WT) TIF1α or Lys-to-Arg point mutations at position 690, 708, or both, either in the presence (+) or in the absence (−) of a His–SUMO-1 expression vector, as indicated. Whole-cell extracts were either TCA precipitated (lanes 1 to 10) or purified on nickel-agarose (lanes 11 to 20) before being subjected to Western blotting with anti-TIF1α monoclonal antibody 5T. The positions of the TIF1α–SUMO-1 conjugates are indicated by arrows. (C) Mutation of SUMO target lysines does not affect nuclear localization of TIF1α. HeLa cells were transfected with plasmids for wild-type (left panel) or mutant (K690/708R; right panel) TIF1α and treated for immunofluorescence analysis with anti-TIF1α monoclonal antibody 5T and fluorescein isothiocyanate-conjugated anti-mouse secondary antibodies. Phase-contrast (PhC) images confirm the nucleoplasmic (extranucleolar) distribution of both proteins.
Binding assays and in vitro SUMO modification.
GST pull-down assays were carried out as described previously (48), using 2 to 10 μg of GST fusion protein immobilized on 10 μl of glutathione-S-Sepharose beads (Pharmacia) and incubating with 5 μl of a standard [35S]methionine-labeled rabbit reticulocyte lysate reaction (TNT T7 kit; Promega), or the SUMO-modified equivalent (see below). His6-tagged recombinant SUMO-1 was prepared by bacterial expression and purification from a pQE-30 vector (Qiagen). Bacterially expressed Ubc9 was prepared as a GST fusion from a construction in vector pGEX2T (Pharmacia), cleaved from GST using thrombin (Sigma), and purified by passage over glutathione-S-agarose (Sigma) using protocols of the manufacturer. In vitro SUMO modification was carried out using 4 μl of rabbit reticulocyte lysate in a reaction volume of 20 μl, as described previously (10), and scaled up as necessary for use in GST pull-down assays. Band intensity on autoradiographs was estimated by PhosphorImager analysis using the area integration function of the ImageQuant software.
RESULTS
SP100C, a novel SP100 variant with homology to chromatin-associated factors.
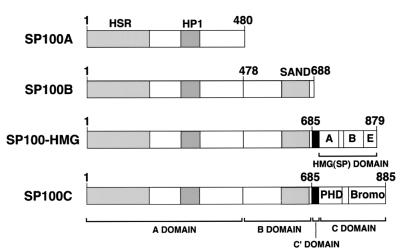
Using a specific DNA probe from the SP100 B domain (Fig. 1) to screen a human liver-derived cDNA library, we isolated a partial cDNA encoding a variant SP100 protein which we have named SP100C. Rapid amplification of cDNA ends-PCR was performed to obtain the remaining 3′ end cDNA (Fig. 2A). RT-PCR analysis of mRNA from HeLa and NB4 cell lines confirmed the existence of mRNAs extending from 5′ SP100A-specific to 3′ SP100C-specific sequences that direct the expression of the SP100C protein. Figure 2B shows PCR products obtained with a primer pair spanning DNA sequences from the second to the last exon. Treatment with interferon (HeLa cells) or with retinoic acid (NB4 cells) led to a marked increase in the amount of amplified product, consistent with previous results for SP100 and its homologs LYSP100/SP140 and SP110 (5, 9, 17) (see Discussion). The full open reading frame of the assembled cDNA therefore encodes a protein of 885 aa with a predicted molecular mass of 101 kDa. Like SP100-HMG, the SP100C protein contains the 477 amino-terminal residues of SP100A (including the HSR homodimerization and HP1-binding motifs) and 207 aa of SP100B (the B domain), which contains the SAND domain, a recently described motif whose function is still unclear (15). Linking the B and the carboxy-terminal SP100C-specific domain (C; aa 700 to 885) is a 14 aa stretch (denoted C′), whose coding sequence is frequently spliced out, as was also found previously for cDNAs encoding SP100-HMG (data not shown).
FIG. 1.
Major protein domains of the SP100 variant proteins generated by alternative splicing. Schematic representation of the SP100A, SP100B, SP100-HMG, and SP100C protein variants derived from alternatively spliced mRNAs (the corresponding cDNA sequences are deposited in the GenEMBL database under accession numbers M60618 [52], U36501 [9], AF056322 [18, 48], and AF255565 [this study], respectively). Brackets indicate the extent of the major domains: A, B, C′, HMG(SP), and C. Note that domains A, B, and C′ are shared by SP100-HMG and SP100C. The positions of the HSR dimerization, HP1-binding, and SAND domains, as well as of the PHD and bromodomains, are indicated.
FIG. 2.
(A) Nucleotide and deduced amino acid sequence of the SP100C variant. The nucleotide numbering of the original SP100A sequence (GenEMBL accession number M60618) has been retained, but only the C termini of the SP100B-specific domain (B domain), the C′ domain and the C domain are shown here, as indicated above the sequence. Bold slashes in the cDNA sequence indicate exon boundaries deduced from analysis of HTGS sequences (GenEMBL accession numbers AC010149 and AC022295). (B) Expression of SP100C mRNA. RT-PCR was performed on total RNA extracted from HeLa and NB4 cells untreated (−) or treated with 200 U of IFN-α per ml for 16 h (I) or 1 μM all-_trans_-retinoic acid for 48 hrs (R) as indicated, using a primer pair combination spanning nucleotides 69 to 2879 of the SP100C cDNA (upper panel). Subcloning and sequencing of reaction products confirmed the specific amplification of SP100C cDNA and furthermore, the existence of minor splice variants (data not shown), as found previously by Guldner et al. (18). Control amplification (C) was performed on cDNA reaction products in which RT had been omitted. The amount of template cDNA was normalized by PCR with primers directing the synthesis of vinculin (lower panel). Interestingly, IFN-α treatment of NB4 cells does not lead to a marked increase in SP100C expression. (C) Amino acid alignment of the SP100C domain with homologous domains from LYSP100B (GenEMBL accession number U36500) and mouse TIF1α (GenEMBL accession number S78219). Amino acid identities are shaded grey. The PHD finger and bromodomains as defined by Aasland et al. (1) and Jeanmougin et al. (21), respectively, are boxed.
Inspection of genomic sequences deposited in the HTGS (High-Throughput Genomic Sequence) database confirmed that SP100A, SP100B, SP100-HMG, and SP100G-encoding sequences are contained in two overlapping BAC clones that have been mapped to chromosome 2q37. Analysis of draft sequence from these two clones showed that the C′ domain corresponds to a single exon and that the C domain is divided into five exons (Fig. 2A).
Homology searches of the GenEMBL data bank revealed two proteins, LYSP100B and TIF1α, to be the closest homologues of SP100C. Figure 2C shows the amino acid alignment of the C domain of SP100C with homologous domains of LYSP100B and TIF1α. In this domain, SP100C is 59 and 34% identical to LYSP100B and TIF1α, respectively. This region contains two protein motifs that are found in chromatin-associated proteins: a cysteine- and histidine-rich PHD finger (1) and a Bromodomain (21).
The SP100A, SP100-HMG and SP100C proteins display distinct subnuclear localization patterns.
The association of SP100 with NBs prompted us to investigate whether the variants SP100-HMG and SP100C are similarly targeted to these structures or whether the additional carboxy-terminal sequences [i.e., HMG(SP) and C domains] led to an altered subcellular localization compared to the primarily NB-associated SP100A protein. To address this question, we performed confocal immunofluorescence analysis of HeLa cells transfected with constructs expressing HA epitope-tagged SP100A, SP100-HMG, and SP100C proteins with monoclonal anti-HA and polyclonal anti-PML antibodies. Transfections were carried out using a range of DNA quantities (1 to 10 μg of expression plasmid) to achieve a broader range of expression levels.
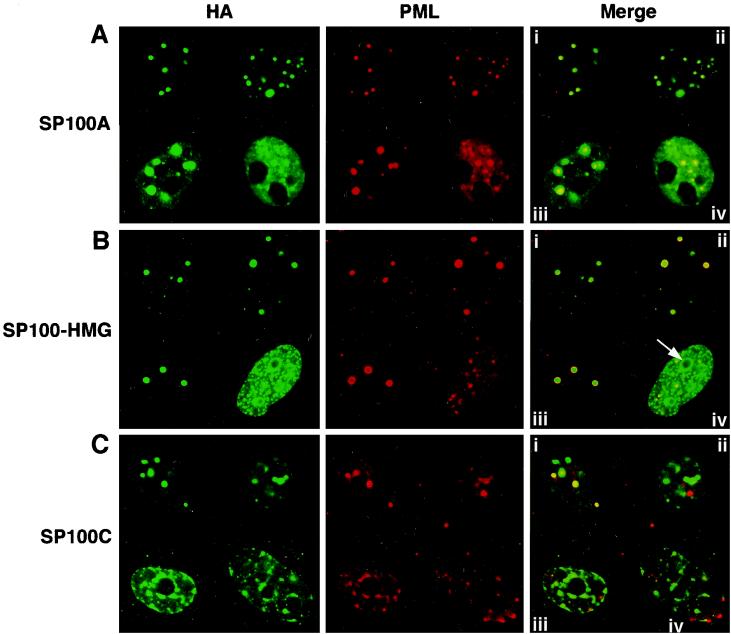
Representative examples of cells overexpressing these SP100 variants are shown in Fig. 3. The labeling of the SP100A protein is coincident with that of the NB-associated PML protein, at both low and high expression levels (Fig. 3A). It appears that higher expression induces the formation of large subnuclear aggregates, in addition to increasing the intensity of the nuclear diffuse labeling (Fig. 3A, iii and iv). Mild overexpression SP100-HMG similarly results in faithful targeting to the PML-positive NBs (Fig. 3B), whereas higher expression produces numerous, densely packed aggregates, the largest of which frequently decorate the outside of the nucleoli (Fig. 3B, iv). These results differ somewhat from those previously obtained by Guldner et al. (18). These authors found that SP100-HMG targets non-NB sites on low-level expression in HEp-2 cells, indicating that the localization pattern of SP100-HMG may be cell type specific.
FIG. 3.
The SP100 variants display distinct subnuclear localization behavior. HeLa cells grown on coverslips were transfected with expression plasmids encoding HA-tagged SP100A (A), SP100-HMG (B), or SP100C (C), immunolabeled with anti-HA monoclonal antibody 12CA5 (left panels) or rabbit polyclonal antibodies against PML (middle panels), and revealed with anti-mouse immunoglobulin G–fluorescein isothiocyanate (green) or anti-rabbit immunoglobulin G–Texas red (red) secondary antibodies, respectively, for confocal immunofluorescence microscopy. Colocalization appears yellow in the merged images (right panels). Four representative nuclei (i to iv) are shown in each panel, arranged in order of increasing apparent expression level. Arrow, densely packed aggregate at high expression levels.
Strikingly, and in contrast to SP100A and SP100-HMG, even at low expression levels SP100C appeared to target only a subset of the PML-containing NBs (Fig. 3C, i and ii). In about 20% of the transfected cells, a different pattern was observed (Fig. 3C, iii and iv). SP100C formed a reticulate or track-like nuclear pattern with denser concentrations at the nuclear lamina and surrounding the nucleoli, a pattern reminiscent of heterochromatin-rich regions. Further, it appears that SP100C partially delocalized PML staining, either into this reticulate pattern or into aggregates at the nuclear periphery (Fig. 3C, iii and iv). These results suggest that differences in the subnuclear targeting behavior of SP100 proteins exist and that they may be of functional significance.
SP100-HMG and SP100C are covalently modified by SUMO-1.
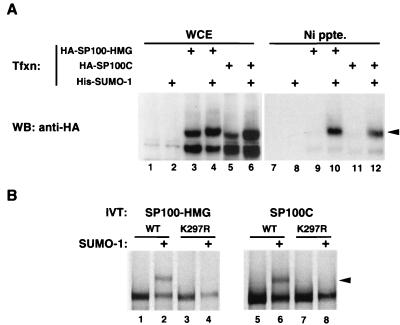
The SP100A protein was reported to be modified covalently by SUMO-1 (51). The interaction between SP100 and SUMO was further indicated by the finding that three members of the SUMO family (SUMO-1, SUMO-2, and SUMO-3) associate with SP100A in yeast two-hybrid assays, as does the human Ubc9 protein, the SUMO-specific E2 conjugating enzyme (Seeler and Dejean, unpublished). We therefore wished to confirm that the longer SP100 variants, SP100-HMG and SP100C, are similarly SUMO modified. As shown in Fig. 4A, transient overexpression of either protein in HeLa cells, either in the presence or in the absence of exogeous (His-tagged) SUMO-1, yielded two immunoreactive bands in a Western blot prepared from whole-cell extracts (lanes 3 to 6). On nickel affinity purification of His-tagged protein complexes from these same extracts, only the slower-migrating bands corresponding to His–SUMO-1 conjugates of SP100-HMG (lane 10) or SP100C (lane 12) were detected. These bands were not seen in the absence of exogenously expressed His–SUMO-1 (lanes 9 and 11), since conjugates of the untagged endogenous SUMO are not retained on the Ni affinity matrix. These results demonstrate that both SP100-HMG and SP100C are efficiently modified by SUMO-1 in vivo, an expected finding, given that the target lysine (Lys-297) for modification of SP100 falls within the amino-terminal A domain common to all SP100 isoforms (reference 50 and our unpublished results). To determine, however, whether the C-terminal extensions of SP100-HMG and SP100C contain additional SUMO-1 targets, we employed an in vitro modification assay. In this assay, radioactively labeled, in vitro-translated substrate proteins were incubated with a HeLa cell fraction providing the E1 activity, together with bacterially produced SUMO-1 and Ubc9 proteins. As seen in Fig. 4B, both SP100-HMG (lane 2) and SP100C (lane 6) were efficiently modified using this system. However, proteins in which lysine 297 was mutated to arginine remained unmodified (lanes 4 and 8), showing that the carboxy-terminal domains of these proteins contain no additional modification sites. For SP100C this is particularly noteworthy, because lysine 778, found at the amino terminus of the Bromodomain, falls within a (LVIA)KXE consensus sequence (LKCE) previously defined for numerous SUMO-1 targets (50) yet appears to be untargeted here.
FIG. 4.
(A) SP100-HMG and SP100C are covalently modified by SUMO-1 in vivo. Whole-cell extracts (WCE) of HeLa cells transfected with vectors expressing HA-tagged SP100-HMG or SP100C or His-tagged SUMO-1, as indicated, were either immunoblotted with anti-HA antibody directly (lanes 1 to 6) or subjected to Ni affinity chromatography prior to blotting (Ni ppte.) (lanes 7 to 12). The arrowhead marks the position of the SUMO-1-conjugated species. Note the presence of conjugates modified by the endogenous SUMO protein(s) (lanes 3 and 5) which are not precipitated by Ni affinity beads. (B) SUMO modification in vitro. [35S]methionine-labeled, in vitro-translated SP100-HMG and SP100C and their K297R point mutant derivatives, as indicated, were subjected to the in vitro modification reaction in the presence (even lanes) or absence (odd lanes) of recombinant SUMO-1 protein. Arrowheads mark the position of SUMO-1 conjugates.
TIF1α is covalently modified by SUMO-1.
The novel SP100C variant has significant homology to the TIF1 proteins in sharing the carboxy-terminal PHD finger-bromodomain region. In addition, TIF1 proteins belong to the RING finger–B-box–coiled-coil (RBCC) family of proteins and thus have homology to PML (29). Furthermore, two of the three known TIF1 isoforms, TIF1α, and TIF1β, have been shown, like SP100, to interact with HP1 proteins (28). Given these common structural and functional properties of TIF1 and the NB proteins PML and SP100, we hypothesized that TIF1 could be, like PML and SP100, a target for SUMO modification.
To test this hypothesis, we transfected HeLa cells with plasmids directing the expression of mouse TIF1α, SUMO-1, or His–SUMO-1 and used the in vivo conjugation assay as described above for SP100. Whole-cell extracts prepared under denaturing conditions were either electrophoresed directly or subjected to Ni-agarose affinity purification before being subjected to Western blotting with an anti-TIF1α monoclonal antibody. As seen in Fig. 5A, in crude extracts a major 140-kDa band corresponding to the unmodified TIF1α protein was detected (lanes 2, 4, and 6). In addition, two slower-migrating bands were visible, both of which were retained on nickel-agarose beads on coexpression of His–SUMO-1 (lane 12). These bands were not seen when TIF1α was coexpressed with untagged SUMO-1 (lane 10), indicating that they correspond to SUMO-1 conjugates of TIF1α. The electrophoretic mobility of the TIF1α forms is consistent with the attachment of two SUMO-1 molecules per molecule of TIF1α. A similar pattern of bands was obtained on Western blotting of untransfected NIH 3T3 cell extracts with two different monoclonal antibodies against TIF1α protein (lanes 13 and 14), indicating that a nonnegligible fraction of endogenous TIF1α is readily conjugated to SUMO-1.
To map the target lysine residues for SUMO modification TIF1α, we constructed a series of deletion and point mutations for testing in the in vivo SUMO conjugation assay (data not shown). Two single-point mutations, K690R and K708R, led to the disappearance or diminution of the upper slower-migrating band presumably corresponding to the doubly modified TIF1α protein (Fig. 5B, lanes 6, 8, 16, and 18). As expected, mutation of both lysines (K690R and K708R) entirely abrogated the modification (lanes 10 and 20), demonstrating that the lysines at positions 690 and 708 are critical for SUMO-1 modification of TIF1α. It is noteworthy that the sequences at both sites conform to the (LVIA)KXE consensus. Finally, to rule out the possibility that the absence of SUMO-1 modification in the TIF1α mutant derivatives is the consequence of aberrant subcellular localization, HeLa cells transfected with plasmids encoding wild-type and mutant TIF1α proteins were analyzed by immunofluorescence microscopy. As shown in Fig. 5C, the K690 K708R double mutant displayed the same nuclear diffuse-granular distribution as did its wild-type counterpart, arguing that these lysine residues indeed correspond to the direct targets of SUMO-1.
SUMO-1 modification of SP100 stabilizes the SP100-HP1 interaction in vitro.
A striking finding in the above concerns the location of the SUMO modification sites in SP100 (K297) (50) and TIF1α (K690), which in both cases fall within the HP1-binding domain of these proteins. Moreover, the second modification site in TIF1α (K708) is located close to the so-called NR box, a short conserved sequence involved in binding nuclear receptors (28). This prompted us to analyze whether conjugation to SUMO-1 may affect the binding of SP100 and TIF1α to their partner proteins.
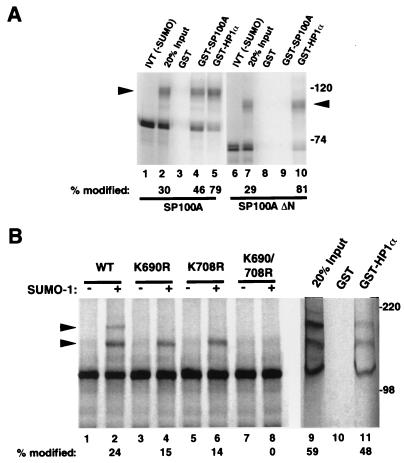
To test whether SUMO modification of SP100 could affect binding to HP1, we performed a pull-down experiment with glutathione-Sepharose-immobilized GST, GST-SP100A (used here as a positive control) or GST-HP1α as target proteins for incubations with radiolabeled in vitro-translated then SUMO-1-modifed SP100A protein produced by the in vitro modification system. As shown in Fig. 6A, 30% of the total SP100 was modified in the in vitro reaction (lane 2). Neither the unmodified nor the modified proteins bound the GST control (lane 3), but both were bound by beads charged with GST-SP100A and GST-HP1α (lanes 4 and 5). Interestingly, binding to GST-HP1α was accompanied by a significant enrichment of the modified form (79%; lane 5) while homomeric binding to GST-SP100A led to only a modest increase in the proportion of modified protein in the eluates (46%; lane 4). The preferential binding of SUMO-1-modified SP100A to HP1α was confirmed using a homodimerization-deficient, N-terminally truncated form of SP100A (SP100AΔN; lanes 6 to 10). As expected, this protein failed to bind GST-SP100A (lane 9), but, again, the SUMO-1-modified form displayed increased binding to GST-HP1α (81%; lane 10). These findings suggest that SUMO-1 modification of SP100 plays a role in stabilizing SP100-HP1 complexes and, to a lesser extent, SP100 dimers or multimers.
FIG. 6.
(A) SUMO-1 modification of SP100A enhances binding to HP1α in vitro. [35S]methionine-labeled, in vitro-translated SP100A protein was incubated with the SUMO-1 conjugation reaction mixture in the absence (lane 1) or presence (lane 2) of recombinant SUMO-1 protein. This mixture of unmodified and SUMO-1-modified (arrowhead) SP100A protein was then used in a pull-down assay with GST (negative control [lane 3]), GST-SP100A (positive control [lane 4]), or GST-HP1α (lane 5) immobilized on glutathione-Sepharose beads. Quantitative PhosphorImager analysis was used to determine the percentage of SUMO-1-modified SP100A protein in the input (lane 2) as well as the proteins eluted from the beads (lanes 4 and 5). To rule out the possibility that the enhancement of HP1α binding on SUMO modification requires dimerization of SP100A, a similar experiment (lanes 6 to 10) was carried out with the mutant SP100AΔN protein in which the N-terminal 129 aa (the HSR dimerization domain) are deleted. (B) SUMO-1 modification of TIF1α in vitro does not significantly affect HP1α -binding. In vitro-translated wild-type TIF1α (lanes 1 and 2) and mutants with the Lys-to-Arg point mutations at position 690, 708, or both, as indicated, were subjected to the modification reaction in the presence (+) or absence (−) of recombinant SUMO-1 protein. In vitro-modified, wild-type TIF1α was used in a pull-down assay with GST (lane 10, negative control) and GST-HP1α (lane 11) as in panel A; the modification led to the appearance of two slower-migrating bands (arrowheads).
To explore whether SUMO-1 modification may similarly modulate the binding of TIF1α to HP1α, we carried out similar experiments using in vitro-modified TIF1α. As shown in Fig. 6B, in vitro modification of TIF1α led to the appearance of two slower-migrating bands (lane 2). The uppermost band was lost on mutation of either the K690 or K708 site (lanes 4 and 6), and both bands were lost in the K690R K708R double mutant (lane 8), confirming the requirement of these two lysine residues for SUMO-1 modification in vitro. By contrast to what was observed for SP100A, the binding to GST-HP1α of TIF1α subjected to SUMO-1 modification in vitro resulted in almost identical, perhaps even slightly decreased binding of modified TIF1α protein (59% modified before and 48% modified after binding GST-HP1α [lanes 9 and 11, respectively]), suggesting that the attachment of SUMO-1 to TIF1α has little effect on the binding properties to HP1α.
DISCUSSION
The present work contributes to a growing body of evidence suggesting a link between the PML-SP100 NB components and processes taking place at the chromatin level. This link was first established by the demonstration of an interaction between SP100 and members of the HP1 family of nonhistone chromosomal proteins, as well as by the molecular cloning of an SP100 variant, SP100-HMG, that contains an HMG1/2 protein domain at the carboxy- terminus (31, 48). Here we extend these findings by the cloning of an additional variant, SP100C, characterized by the presence of two domains frequently found in proteins affecting chromatin structure: a PHD finger and a bromodomain. Furthermore, we show that the posttranslational modification by SUMO of the PML and SP100 NB proteins applies also to the chromatin-associated transcriptional intermediary factor TIF1α, a homolog of both PML and SP100. Finally, we provide evidence that the SUMO-1 modification serves to enhance the stability of the interaction of SP100 with its HP1α partner protein.
SP100: an adapter with multiple functionalities.
Four major SP100 variants resulting from alternative splicing are now known. All four share the HSR homodimerization interface as well as the HP1 interaction region at the N terminus, while the SP100B, SP100C, and SP100-HMG variants possess a novel sequence motif of unknown function, the SAND domain (15). Perhaps the most telling motifs of the SP100 proteins are the HMG domain and PHD-bromodomain at the C termini of the longest variants. The HMG domain of SP100-HMG exhibits DNA-binding capacity in vitro (data not shown), but the strength and specificity of this binding in the context of the full-length protein remains to be established.
The SP100C variant belongs to a group of proteins possessing both PHD finger and bromodomain motifs. All of these proteins are believed to play important roles in mediating events at the chromatin level. For example, the p300-CBP coactivators are integral to histone acetyltransferase complexes with P/CAF and, in fact, display acetyltransferase activity themselves. The recent demonstration that the P/CAF bromodomain displays increased affinity for acetylated lysines in these modified histones has opened up the interesting possibility that this motif recognizes specific acetylated protein targets (for a review, see reference 57). It is tempting to speculate that SP100C, through its bromodomain, interacts with acetylated histones or other as yet undiscovered acetylated protein partners. Interesting in this context is the recent demonstration (43) of a potential role of the NBs in the acetylation of p53 following cellular stimulation by oncogenic ras.
The existence of four variant SP100 proteins with a common amino-terminal domain but differing in their carboxy termini suggests that these proteins may be bifunctional. The N-terminal moiety that contains the dimerization domain may thus be primarily implicated in aggregating a given SP100 protein at the NBs, whereas the C-terminal domains may perform as yet undetermined functions in the nucleoplasm or at the chromatin level. This idea is supported by localization studies showing that the HSR dimerization domain alone suffices for NB targeting (references 41 and 50 and our unpublished results) while the C-terminal HMG and PHD-bromodomains extend the number of targeted subnuclear sites beyond the NBs (Fig. 3).
Parallels between the TIF1 and SP100 protein families.
TIF1α was first isolated as a cofactor to ligand-bound nuclear receptors (29, 54) and later shown to exhibit kinase activity (13). TIF1β (KAP-1 or KRIP-1) was found as an interacting partner to KRAB (Krüppel-associated box)-containing repressor proteins (14, 23, 38). A third protein, TIF1γ, isolated by low-stringency screening, has also been reported recently (56). The TIF1 family of transcriptional intermediary factors offers some interesting parallels to the two NB-associated PML and SP100 proteins. In addition to the structural similarity of TIF1 proteins and NB components in sharing the RBCC motif with PML and the PHD-bromodomain motif with SP100C, TIF1α and TIFβ interact with members of the HP1 family of proteins (28). Like HP1 and SP100, all three TIF1 proteins behave as potent transcriptional repressors when artificially tethered to DNA (28, 31, 42, 46, 48). While the significance of the observed repressing effects remains unclear, it nevertheless suggests that TIF1 proteins interact with additional chromatin-associated factors that remain to be identified. TIF1 proteins may thus perform both activating (as initially described for the TIF1α-nuclear receptor interaction [28]) and repressing (as in the TIF1β-KRAB-ZFP interaction [46]) functions.
Recent evidence has implicated PML as a cofactor in TIF1α-dependent enhancement of RARα-mediated transcriptional activation (59), thus providing a first functional link between TIF1 proteins and NB components in transcriptional regulation. Furthermore, like PML, two TIF1 proteins form part of oncogenic fusion proteins. TIF1α, when fused with the B-Raf kinase, gives rise to the T18 oncogenic protein in mice (37), and TIF1α and TIF1γ (also called rfg) have both been isolated as part of fusions with the RET receptor tyrosine kinase in human papillary thyroid carcinomas (25).
While this paper was in preparation, Bloch et al. (5) reported the molecular cloning of SP110, which, after LYSP100/SP140, represents a further SP100-homologous protein. Like SP100C (this study) and LYSP100B/SP140 (4, 9), this protein contains a carboxy-terminal PHD-bromodomain motif and associates with a subset of PML-SP100 NBs. Most interesting, however, is the finding that this protein, like TIF1α and PML, may function as a coactivator for nuclear hormone receptors, an activity possibly mediated by a LXXLL nuclear receptor-binding motif present in SP110, but absent in LYSP100B/SP140 and SP100C. Taken together, these findings define the SP100 and TIF1 family of proteins as containing members possessing both shared and specific functional properties. Apart from the structural similarities common to all, these include transcriptional repression (SP100, TIF1α, TIF1β, and TIF1γ) or activation (LYSP100/SP140, SP110), interaction with HP1 proteins (SP100, TIF1α, TIF1β), and SUMO modification (SP100, TIF1α, TIF1β [this study and our unpublished results]). Although as yet poorly defined mechanistically, the concept of the transcriptional intermediary factor may therefore be used to unite the TIF1 with the SP100 family of NB-associated proteins.
SUMO modification and chromatin.
The possible importance of SUMO modification in the regulation of processes at the chromatin level is supported by the findings that a number of SUMO-modified substrate proteins are transcriptional regulators. Examples include the transcription factors p53, c-jun (16, 39, 45), the Drosophila NF-κB homolog Dorsal (3), the Drosophila transcriptional repressor Tramtrack-69 (ttk69) (30), and HIPK2 (homeodomain interacting protein kinase [24]). In yeast, mutations now known to affect genes encoding components of the ySMT3 (yeast SUMO) conjugation pathway result in defects in chromosome segregation and centromere function, again suggesting that SUMO modification affects the activity of chromatin-associated multiprotein complexes (reference 53 and references therein). A transient association of SUMO-1 with human centromeres has also been reported recently (12).
The biological roles of SUMO modification remain poorly understood. For IκBα, the evidence supports a model by which SUMO modification antagonizes ubiquitination, since both SUMO and ubiquitin target the same lysines (10). However, the majority of cases support a model by which SUMO modification regulates the subcellular localization of its substrates, as has been shown for PML (40, 60), RanGAP1 (34, 35), and Dorsal (3). Thus, it appears that SUMO modification alters the protein binding specificity of its targets, such that, for example, SUMO-modified PML acquires the capacity to bind Daxx (20) and SUMO-RanGAP acquires the ablity to bind RanBP2 (34, 35) with consequent changes in subcellular localization. The stabilization of protein complexes by SUMO modification, as shown here with SUMO-SP100 and HP1, may therefore similarly affect the mobility and localization of the SUMO target protein or of its partner(s). The role played by SUMO modification of TIF1α remains to be established, and further work is required to determine its possible effects on the subnuclear localization, kinase activity, and transcriptional properties of this protein.
ACKNOWLEDGMENTS
We are grateful to M. Cerviño for technical assistance.
This work was supported by grants from the European Community, the Association for International Cancer Research, and the Association pour la Recherche sur le Cancer.
REFERENCES
- 1.Aasland R, Gibson T, Stewart A. The PHD finger: implications for chromatin-mediated transcriptional regulation. Trends Biochem Sci. 1995;20:56–59. doi: 10.1016/s0968-0004(00)88957-4. [DOI] [PubMed] [Google Scholar]
- 2.Ascoli C A, Maul G G. Identification of a novel nuclear domain. J Cell Biol. 1991;112:785–795. doi: 10.1083/jcb.112.5.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhaskar V, Valentine S A, Courey A J. A functional interaction between dorsal and components of the smt3 conjugation machinery. J Biol Chem. 2000;275:4033–4040. doi: 10.1074/jbc.275.6.4033. [DOI] [PubMed] [Google Scholar]
- 4.Bloch D B, de la Monte S M, Guigaouri P, Filippov A, Bloch K D. Identification and characterization of a leukocyte-specific component of the nuclear body. J Biol Chem. 1996;271:29198–29204. doi: 10.1074/jbc.271.46.29198. [DOI] [PubMed] [Google Scholar]
- 5.Bloch D B, Nakajima A, Gulick T, Chiche J D, Orth D, de La Monte S M, Bloch K D. Sp110 localizes to the PML-Sp100 nuclear body and may function as a nuclear hormone receptor transcriptional coactivator. Mol Cell Biol. 2000;20:6138–6146. doi: 10.1128/mcb.20.16.6138-6146.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boisvert F, Hendzel M, Bazett-Jones D. Promyelocytic leukemia (PML) nuclear bodies are protein structures that do not accumulate RNA. J Cell Biol. 2000;148:283–292. doi: 10.1083/jcb.148.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carvalho T, Seeler J S, Ohman K, Jordan P, Pettersson U, Akusjarvi G, Carmo-Fonseca M, Dejean A. Targeting of adenovirus E1A and E4-ORF3 proteins to nuclear matrix-associated PML bodies. J Cell Biol. 1995;131:45–56. doi: 10.1083/jcb.131.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen G Q, Zhu J, Shi X G, Ni J H, Zhong H J, Si G Y, Jin X L, Tang W, Li X S, Xong S M, Shen Z X, Sun G L, Ma J, Zhang P, Zhang T D, Gazin C, Naoe T, Chen S J, Wang Z Y, Chen Z. In vitro studies on cellular and molecular mechanisms of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia: As2O3 induces NB4 cell apoptosis with downregulation of Bcl-2 expression and modulation of PML-RAR alpha/PML proteins. Blood. 1996;88:1052–1061. [PubMed] [Google Scholar]
- 9.Dent A L, Yewdell J, Puvion-Dutilleul F, Koken M H, de The H, Staudt L M. LYSP100-associated nuclear domains (LANDs): description of a new class of subnuclear structures and their relationship to PML nuclear bodies. Blood. 1996;88:1423–1426. [PubMed] [Google Scholar]
- 10.Desterro J M, Rodriguez M S, Hay R T. SUMO-1 modification of IkappaBalpha inhibits NF-kappaB activation. Mol Cell. 1998;2:233–239. doi: 10.1016/s1097-2765(00)80133-1. [DOI] [PubMed] [Google Scholar]
- 11.de The H, Lavau C, Marchio A, Chomienne C, Degos L, Dejean A. The PML-RAR-α fusion mRNA generated by the t(15;17) translocation in acute promyelocytic leukemia encodes a functionally altered RAR. Cell. 1991;66:675–684. doi: 10.1016/0092-8674(91)90113-d. [DOI] [PubMed] [Google Scholar]
- 12.Everett R D, Earnshaw W C, Pluta A F, Sternsdorf T, Ainsztein A M, Carmena M, Ruchaud S, Hsu W L, Orr A. A dynamic connection between centromeres and ND10 proteins. J Cell Sci. 1999;112:3443–3454. doi: 10.1242/jcs.112.20.3443. [DOI] [PubMed] [Google Scholar]
- 13.Fraser R A, Heard D J, Adam S, Lavigne A C, Le Douarin B, Tora L, Losson R, Rochette-Egly C, Chambon P. The putative cofactor TIF1alpha is a protein kinase that is hyperphosphorylated upon interaction with liganded nuclear receptors. J Biol Chem. 1998;273:16199–16204. doi: 10.1074/jbc.273.26.16199. [DOI] [PubMed] [Google Scholar]
- 14.Friedman J R, Fredericks W J, Jensen D E, Speicher D W, Huang X P, Neilson E G, Rauscher F J. KAP-1, a novel corepressor for the highly conserved KRAB repression domain. Genes Dev. 1996;10:2067–2078. doi: 10.1101/gad.10.16.2067. [DOI] [PubMed] [Google Scholar]
- 15.Gibson T, Ramu C, Gemünd C, Aasland R. The APECED polyglandular autoimmune syndrome protein, AIRE-1, contains the SAND domain and is probably a transcription factor. Trends Biochem Sci. 1998;23:242–244. doi: 10.1016/s0968-0004(98)01231-6. [DOI] [PubMed] [Google Scholar]
- 16.Gostissa M, Hengstermann A, Fogal V, Sandy P, Schwarz S E, Scheffner M, Del Sal G. Activation of p53 by conjugation to the ubiquitin-like protein SUMO-1. EMBO J. 1999;18:6462–6471. doi: 10.1093/emboj/18.22.6462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guldner H H, Szostecki C, Grotzinger T, Will H. IFN enhance expression of Sp100, an autoantigen in primary biliary cirrhosis. J Immunol. 1992;149:4067–4073. [PubMed] [Google Scholar]
- 18.Guldner H H, Szostecki C, Schroder P, Matschl U, Jensen K, Luders C, Will H, Sternsdorf T. Splice variants of the nuclear dot-associated Sp100 protein contain homologies to HMG-1 and a human nuclear phosphoprotein-box motif. J Cell Sci. 1999;112:733–747. doi: 10.1242/jcs.112.5.733. [DOI] [PubMed] [Google Scholar]
- 19.Hollenbach A D, Sublett J E, McPherson C J, Grosveld G. The Pax3-FKHR oncoprotein is unresponsive to the Pax3-associated repressor hDaxx. EMBO J. 1999;18:3702–3711. doi: 10.1093/emboj/18.13.3702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ishov A M, Sotnikov A G, Negorev D, Vladimirova O V, Neff N, Kamitani T, Yeh E T, Strauss J F, Maul G G. PML is critical for ND10 formation and recruits the PML-interacting protein daxx to this nuclear structure when modified by SUMO-1. J Cell Biol. 1999;147:221–234. doi: 10.1083/jcb.147.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jeanmougin F, Wurtz J M, Le Douarin B, Chambon P, Losson R. The bromodomain revisited. Trends Biochem Sci. 1997;22:151–153. doi: 10.1016/s0968-0004(97)01042-6. [DOI] [PubMed] [Google Scholar]
- 22.Johnson P R, Hochstrasser M. SUMO-1: ubiquitin gains weight. Trends Cell Biol. 1997;7:408–413. doi: 10.1016/S0962-8924(97)01132-X. [DOI] [PubMed] [Google Scholar]
- 23.Kim S S, Chen Y M, O'Leary E, Witzgall R, Vidal M, Bonventre J V. A novel member of the RING finger family, KRIP-1, associates with the KRAB-A transcriptional repressor domain of zinc finger proteins. Proc Natl Acad Sci USA. 1996;93:15299–15304. doi: 10.1073/pnas.93.26.15299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim Y H, Choi C Y, Kim Y. Covalent modification of the homeodomain-interacting protein kinase 2 (HIPK2) by the ubiquitin-like protein SUMO-1. Proc Natl Acad Sci USA. 1999;96:12350–12355. doi: 10.1073/pnas.96.22.12350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klugbauer S, Rabes H. The transcription coactivator HTIF1 and a related protein are fused to the RET receptor tyrosine kinase in childhood papillary thyroid carcinomas. Oncogene. 1999;18:4388–4393. doi: 10.1038/sj.onc.1202824. [DOI] [PubMed] [Google Scholar]
- 26.LaMorte V J, Dyck J A, Ochs R L, Evans R M. Localization of nascent RNA and CREB binding protein with the PML-containing nuclear body. Proc Natl Acad Sci USA. 1998;95:4991–4996. doi: 10.1073/pnas.95.9.4991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lavau C, Marchio A, Fagioli M, Jansen J, Falini B, Lebon P, Grosveld F, Pandolfi P P, Pelicci P G, Dejean A. The acute promyelocytic leukaemia-associated PML gene is induced by interferon. Oncogene. 1995;11:871–876. [PubMed] [Google Scholar]
- 28.LeDouarin B, Nielsen A L, Garnier J M, Ichinose H, Jeanmougin F, Losson R, Chambon P. A possible involvement of TIF1 alpha and TIF1 beta in the epigenetic control of transcription by nuclear receptors. EMBO J. 1996;15:6701–6715. [PMC free article] [PubMed] [Google Scholar]
- 29.LeDouarin B, Zechel C, Garnier J M, Lutz Y, Tora L, Pierrat P, Heery D, Gronemeyer H, Chambon P, Losson R. The N-terminal part of TIF1, a putative mediator of the ligand- dependent activation function (AF-2) of nuclear receptors, is fused to B-raf in the oncogenic protein T18. EMBO J. 1995;14:2020–2033. doi: 10.1002/j.1460-2075.1995.tb07194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lehembre F, Badenhorst P, Muller S, Travers A, Schweisguth F, Dejean A. Covalent modification of the transcriptional repressor tramtrack by the ubiquitin-related protein Smt3 in Drosophila flies. Mol Cell Biol. 2000;20:1072–1082. doi: 10.1128/mcb.20.3.1072-1082.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lehming N, Le S A, Schüller J, Ptashne M. Chromatin components as part of a putative transcriptional repressing complex. Proc Natl Acad Sci USA. 1998;95:7322–7326. doi: 10.1073/pnas.95.13.7322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li H, Leo C, Zhu J, Wu X, O'Neil J, Park E J, Chen J D. Sequestration and inhibition of Daxx-mediated transcriptional repression by PML. Mol Cell Biol. 2000;20:1784–1796. doi: 10.1128/mcb.20.5.1784-1796.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin R, Egan D, Evans R. Molecular genetics of acute promyelocytic leukemia. Trends Genet. 1999;15:179–184. doi: 10.1016/s0168-9525(99)01710-2. [DOI] [PubMed] [Google Scholar]
- 34.Mahajan R, Delphin C, Guan T, Gerace L, Melchior F. A small ubiquitin-related polypeptide involved in targeting RanGAP1 to nuclear pore complex protein RanBP2. Cell. 1997;88:97–107. doi: 10.1016/s0092-8674(00)81862-0. [DOI] [PubMed] [Google Scholar]
- 35.Matunis M J, Wu J, Blobel G. SUMO-1 modification and its role in targeting the Ran GTPase-activating protein, RanGAP1, to the nuclear pore complex. J Cell Biol. 1998;140:499–509. doi: 10.1083/jcb.140.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maul G G. Nuclear domain 10, the site of DNA virus transcription and replication. Bioessays. 1998;20:660–667. doi: 10.1002/(SICI)1521-1878(199808)20:8<660::AID-BIES9>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 37.Miki T, Fleming T P, Crescenzi M, Molloy C J, Blam S B, Reynolds S H, Aaronson S A. Development of a highly efficient expression cDNA cloning system: application to oncogene isolation. Proc Natl Acad Sci USA. 1991;88:5167–5171. doi: 10.1073/pnas.88.12.5167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moosmann P, Georgiev O, Le Douarin B, Bourquin J P, Schaffner W. Transcriptional repression by RING finger protein TIF1 beta that interacts with the KRAB repressor domain of KOX1. Nucleic Acids Res. 1996;24:4859–4867. doi: 10.1093/nar/24.24.4859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Müller S, Berger M, Lehembre F, Seeler J, Haupt Y, Dejean A. SUMO-1 modification of c-jun and p53 is co-regulated with ubiquitination in a phosphorylation-dependent manner. J Biol Chem. 2000;275:13321–13329. doi: 10.1074/jbc.275.18.13321. [DOI] [PubMed] [Google Scholar]
- 40.Müller S, Matunis M J, Dejean A. Conjugation with the ubiquitin-related modifier SUMO-1 regulates the partitioning of PML within the nucleus. EMBO J. 1998;17:61–70. doi: 10.1093/emboj/17.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Negorev D, Ishov A M, Maul G G. Evidence for separate ND10-binding and homo-oligomerization domains of Sp100. J Cell Sci. 2001;114:59–68. doi: 10.1242/jcs.114.1.59. [DOI] [PubMed] [Google Scholar]
- 42.Nielsen A, Ortiz J A, You J, Oulad-Abdelghani M, Khechumian R, Gansmuller A, Chambon P, Losson R. Interaction with the members of the heterochromatin protein 1 (HP1) family and histone deacetylation are differentially involved in the transcriptional silencing by members of the TIF1 family. EMBO J. 1999;18:6385–6395. doi: 10.1093/emboj/18.22.6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pearson M, Carbone R, Sebastiani C, Cioce M, Fagioli M, Saito S, Higashimoto Y, Appella E, Minucci S, Pandolfi P P, Pelicci P G. PML regulates p53 acetylation and premature senescence induced by oncogenic Ras. Nature. 2000;406:207–210. doi: 10.1038/35018127. [DOI] [PubMed] [Google Scholar]
- 44.Pluta A F, Earnshaw W C, Goldberg I G. Interphase-specific association of intrinsic centromere protein CENP-C with HDaxx, a death domain-binding protein implicated in Fas-mediated cell death. J Cell Sci. 1998;111:2029–2041. doi: 10.1242/jcs.111.14.2029. [DOI] [PubMed] [Google Scholar]
- 45.Rodriguez M S, Desterro J M, Lain S, Midgley C A, Lane D P, Hay R T. SUMO-1 modification activates the transcriptional response of p53. EMBO J. 1999;18:6455–6461. doi: 10.1093/emboj/18.22.6455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ryan R F, Schultz D C, Ayyanathan K, Singh P B, Friedman J R, Fredericks W J, Rauscher F J. KAP-1 corepressor protein interacts and colocalizes with heterochromatic and euchromatic HP1 proteins: a potential role for Kruppel-associated box-zinc finger proteins in heterochromatin-mediated gene silencing. Mol Cell Biol. 1999;19:4366–4378. doi: 10.1128/mcb.19.6.4366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Seeler J-S, Dejean A. The PML nuclear bodies: actors or extras? Curr Opin Genet Dev. 1999;9:362–367. doi: 10.1016/s0959-437x(99)80054-9. [DOI] [PubMed] [Google Scholar]
- 48.Seeler J-S, Marchio A, Sitterlin D, Transy C, Dejean A. Interaction of SP100 with HP1 proteins: a link between the promyelocytic leukemia-associated nuclear bodies and the chromatin compartment. Proc Natl Acad Sci USA. 1998;95:7316–7321. doi: 10.1073/pnas.95.13.7316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stadler M, Chelbi-Alix M K, Koken M H, Venturini L, Lee C, Saib A, Quignon F, Pelicano L, Guillemin M C, Schindler C, de The H. Transcriptional induction of the PML growth suppressor gene by interferons is mediated through an ISRE and a GAS element. Oncogene. 1995;11:2565–2573. [PubMed] [Google Scholar]
- 50.Sternsdorf T, Jensen K, Reich B, Will H. The nuclear dot protein Sp100, characterization of domains necessary for dimerization, subcellular localization, and modification by small ubiquitin-like modifiers. J Biol Chem. 1999;274:12555–12566. doi: 10.1074/jbc.274.18.12555. [DOI] [PubMed] [Google Scholar]
- 51.Sternsdorf T, Jensen K, Will H. Evidence for covalent modification of the nuclear dot-associated proteins PML and Sp100 by PIC1/SUMO-1. J Cell Biol. 1997;139:1621–1634. doi: 10.1083/jcb.139.7.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Szostecki C, Guldner H H, Netter H J, Will H. Isolation and characterization of cDNA encoding a human nuclear antigen predominantly recognized by autoantibodies from patients with primary biliary cirrhosis. J Immunol. 1990;145:4338–4347. [PubMed] [Google Scholar]
- 53.Tanaka K, Nishide J, Okazaki K, Kato H, Niwa O, Nakagawa T, Matsuda H, Kawamukai M, Murakami Y. Characterization of a fission yeast SUMO-1 homologue, pmt3p, required for multiple nuclear events, including the control of telomere length and chromosome segregation. Mol Cell Biol. 1999;19:8660–8672. doi: 10.1128/mcb.19.12.8660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thenot S, Henriquet C, Rochefort H, Cavailles V. Differential interaction of nuclear receptors with the putative human transcriptional coactivator hTIF1. J Biol Chem. 1997;272:12062–12068. doi: 10.1074/jbc.272.18.12062. [DOI] [PubMed] [Google Scholar]
- 55.Torii S, Egan D A, Evans R A, Reed J C. Human Daxx regulates Fas-induced apoptosis from nuclear PML oncogenic domains (PODs) EMBO J. 1999;18:6037–6049. doi: 10.1093/emboj/18.21.6037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Venturini L, You J, Stadler M, Galien R, Lallemand V, Koken M H, Mattei M G, Ganser A, Chambon P, Losson R, de Thé H. TIF1gamma, a novel member of the transcriptional intermediary factor 1 family. Oncogene. 1999;18:1209–1217. doi: 10.1038/sj.onc.1202655. [DOI] [PubMed] [Google Scholar]
- 57.Winston F, Allis C. The bromodomain: a chromatin-targeting module? Nat Struct Biol. 1999;6:601–604. doi: 10.1038/10640. [DOI] [PubMed] [Google Scholar]
- 58.Xie K, Lambie E J, Snyder M. Nuclear dot antigens may specify transcriptional domains in the nucleus. Mol Cell Biol. 1993;13:6170–6179. doi: 10.1128/mcb.13.10.6170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhong S, Delva L, Rachez C, Cenciarelli C, Gandini D, Zhang H, Kalantry S, Freedman L P, Pandolfi P P. A RA-dependent, tumour-growth suppressive transcription complex is the target of the PML-RARalpha and T18 oncoproteins. Nat Genet. 1999;23:287–295. doi: 10.1038/15463. [DOI] [PubMed] [Google Scholar]
- 60.Zhong S, Müller S, Ronchetti S, Freemont P, Dejean A, Pandolfi P. A role of SUMO-1-modified PML in nuclear body formation. Blood. 2000;95:2748–2753. [PubMed] [Google Scholar]
- 61.Zhu J, Koken M H, Quignon F, Chelbi-Alix M K, Degos L, Wang Z Y, Chen Z, de Thé H. Arsenic-induced PML targeting onto nuclear bodies: implications for the treatment of acute promyelocytic leukemia. Proc Natl Acad Sci USA. 1997;94:3978–3983. doi: 10.1073/pnas.94.8.3978. [DOI] [PMC free article] [PubMed] [Google Scholar]