RNA Binding Properties of Bunyamwera Virus Nucleocapsid Protein and Selective Binding to an Element in the 5′ Terminus of the Negative-Sense S Segment (original) (raw)
Abstract
The genome of Bunyamwera virus (BUN) (family Bunyaviridae, genus Bunyavirus) comprises three negative-sense RNA segments which act as transcriptional templates for the viral polymerase only when encapsidated by the nucleocapsid protein (N). Previous studies have suggested that the encapsidation signal may reside within the 5′ terminus of each segment. The BUN N protein was expressed as a 6-histidine-tagged fusion protein in Escherichia coli and purified by metal chelate chromatography. An RNA probe containing the 5′-terminal 32 and 3′-terminal 33 bases of the BUN S (small) genome segment was used to investigate binding by the N protein in vitro using gel mobility shift and filter binding assays. On acrylamide gels a number of discrete RNA-N complexes were resolved, and analysis of filter binding data indicated a degree of cooperativity in N protein binding. RNA-N complexes were resistant to digestion with up to 1 μg of RNase A per ml. Competition assays with a variety of viral and nonviral RNAs identified a region within the 5′ terminus of the BUN S segment for which N had a high preference for binding. This site may constitute the signal for initiation of encapsidation by N.
Bunyamwera virus (BUN) is the prototype of the genus Bunyavirus and the family Bunyaviridae and possesses a single-stranded negative-sense tripartite RNA genome. The three RNA segments, termed L (large), M (medium), and S (small), encode six proteins. The L segment codes for the L protein, the viral RNA-dependent RNA polymerase, which is responsible for both transcribing and replicating the genome RNAs. The M segment encodes the two virion glycoproteins, G1 and G2, and a nonstructural protein, NSm, as a polyprotein which is probably cotranslationally cleaved by host proteases. The S segment encodes the nucleocapsid (N) protein and, in an overlapping reading frame, a second nonstructural protein called NSs (reviewed in reference 8).
In common with other negative-sense RNA viruses, the bunyavirus genome RNA segments are replicated via full-length cRNAs termed antigenomes. Both the negative-sense genome and positive-sense antigenome RNAs are encapsidated by the N protein and are associated with the viral polymerase in ribonucleoprotein complexes called nucleocapsids. It is only within the nucleocapsid that the RNA is transcriptionally active. Bunyavirus genome and antigenome RNAs contain highly conserved, complementary terminal sequences that may form panhandle structures in vivo and are probably responsible for the circular appearance of isolated nucleocapsids (19, 20, 22, 26, 28).
Full-length genome and antigenome segments are usually the only RNAs that are encapsidated in the infected cell. Viral mRNAs, which are not encapsidated, are truncated at the 3′ end and contain a nontemplated capped primer on the 5′ terminus (2, 4, 9, 14, 21). It is therefore likely that the terminal sequences of the genome and antigenome RNAs are involved in the encapsidation process. This theory is supported by the observation that an antisense chloramphenicol acetyltransferase gene flanked by BUN terminal sequences was successfully encapsidated by N and transcribed by L in an in vivo system (7). Raju and Kolakofsky (25) reported that in infected cells a minority of encapsidated bunyavirus transcripts either had the 3′-terminal truncation similar to mRNA but not the 5′ primer sequence or were full-length transcripts containing a capped primer on the 5′ terminus (26). Taken together, these data suggest that the encapsidation signal is probably within the 5′ terminal sequence of genome and antigenome RNAs.
Investigations concerning the N proteins of viruses in the Hantavirus (11, 29) and Tospovirus (27) genera of the Bunyaviridae have shown that binding of the N proteins to RNA in in vitro assays is essentially sequence nonspecific. However, Severson et al. (29) reported hantavirus N protein to have a preference for full-length hantavirus S segment RNA over RNA comprising only internal sequences, and Gött et al. (11) reported hantavirus N to have a preference for double-stranded RNA.
The BUN N protein is a 26.7-kDa basic protein of 233 amino acids that does not show any sequence similarity to the hantavirus (ca. 50-kDa) or tospovirus (ca. 29-kDa) N proteins. Like the analogous nucleocapsid proteins of all members of the Bunyaviridae family, it contains no RNA binding consensus sequence (8). In this paper we describe investigations into the binding of bacterially expressed BUN N protein to negative-sense BUN S segment RNA sequences in vitro. We also provide an analysis of the relative selectivity of the binding of N to such sequences with the intent to construct a model for the selective encapsidation of full-length viral genome and antigenome RNA.
MATERIALS AND METHODS
Expression and purification of BUN N protein.
The BUN N open reading frame (ORF), from codon 2 to stop codon 234, was amplified by PCR using primers 5′GCCGCGGATCCATCGAGGGAAGGATTGAGTTGGAATTT and 5′GCCGCGTCGACTTACATGTTGATTCCGAA, which also contain, respectively, _Bam_HI and _Sal_I restriction enzyme sites (underlined). The DNA amplicon was digested with _Bam_HI and _Sal_I and cloned between the _Bam_HI and _Sal_I sites of pQE30 (Qiagen). Recombinant N was expressed as an N-terminally 6-histidine-tagged protein in Escherichia coli strain M15 by induction with 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG). The bacteria were lysed by freeze-thawing and sonication, and N protein was purified under native conditions (23) by Ni-nitrilotriacetic acid (NTA) column chromatography (Bio-Rad Econosystem). Protein immobilized on the Ni-NTA column was washed with 50 mM sodium phosphate buffer (pH 6.0) containing 300 mM NaCl and 10% glycerol, and N was eluted with a linear gradient of 0 to 500 mM imidazole in wash buffer. Fractions containing large amounts of N were eluted at around 250 mM imidazole, pooled, and dialyzed at 4°C against 10 mM NaCl in 10 mM Tris-HCl (pH 8.0). The dialyzed protein was concentrated using a Vivaspin concentrator (molecular weight cutoff, 10,000) (Vivascience) and stored at 4°C.
RNA transcription plasmids.
pT7BUNS5′(32)/3′(33) contains cDNA to the precise 32 5′-terminal bases and 33 3′-terminal bases of the negative-sense S segment, linked by a 5-base sequence which creates a _Sma_I site, under control of a T7 promoter in pUC119. BUNS5′(32)/3′(33) RNA was transcribed following linearization with _Bbs_I, and BUNS-5′(32) RNA was transcribed following linearization with _Sma_I.
pTZBUNS3′(33/22) contains cDNA to the precise 33 3′ terminal bases of the negative-sense BUN S segment under control of a T7 promoter and was constructed by inserting a 63-bp _Sma_I-_Xba_I fragment from pT7BUNS5′(32)/3′(33) into pTZ18. pT7BUNS3′(33/22) RNA was transcribed following linearization of the plasmid with _Bbs_I.
pT7BUNSCAT contains a negative-sense chloramphenicol acetyltransferase gene flanked by the entire untranslated regions of the BUN S segment in pUC119 (7). BUNS5′(65) RNA was transcribed following linearization of this plasmid with _Fau_I, and BUNS5′(135) RNA was transcribed following linearization with _Tsp_509I.
The template for transcription of BUNS5′SL2 RNA was generated by annealing two oligonucleotides, 5′CTAATACGACTCACTATA (modified T7 promoter) and 5′CTAAATCAACATTATATTGTTAATGGTATTTTAATATAGTGAGTCGTATTAG, representing bases 23 to 56 of the BUN S 5′ terminus.
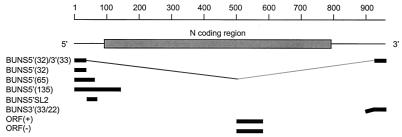
ORF(−) RNA was generated from a PCR product produced by amplification of the N ORF from nucleotides 577 to 86 of the cDNA to which a T7 promoter was incorporated, using primers 5′CTAATACGACTCACTATAGATCCCGATTGCTAAGGG and 5′CTGCGAATTCATGATTGAGTTGGAATTTCACG. The product was linearized with _Mnl_I and made blunt ended using T4 DNA polymerase before being added to a transcription reaction mixture to generate an 87-base RNA. ORF(+) RNA, also 87 bases in length, was generated from a PCR product by amplification of the N ORF from nucleotides 491 to 784 of the cDNA to which a T7 promoter was incorporated, using primers 5′CTAATACGACTCACTATAGATGGAGAGGAAG and 5′CTGCGGATCCTTACATGTTGATTCCGAATTTAGC. The DNA was linearized with _Bst_YI before being added to a transcription reaction mixture. To generate double-stranded ORF (dsORF) RNA, ORF(−) and ORF(+) RNAs were heated together at 95°C for 5 min and 65°C for 10 min, and then annealed by cooling to 25°C over 40 min. Figure 1 shows the regions of the BUN S segment represented by the in vitro-transcribed RNAs.
FIG. 1.
Location of RNA probes used in binding assays relative to the BUN S genome segment. All of the transcripts were initiated with a truncated T7 promoter and did not contain additional, nonviral nucleotides at their 5′ ends, except for BUNS3′(33/22) which had 22 nucleotides of vector sequence (bent line).
In vitro transcription reactions.
RNA transcripts were generated using the Megashortscript T7 in vitro transcription kit (Ambion). BUNS5′(32) RNA was made under the standard reaction conditions recommended by the manufacturer. All other unlabeled transcripts were synthesized in 20-μl reaction volumes containing 3.75 mM each nucleoside triphosphate, 5 μg of plasmid template DNA or 8 μg of oligonucleotide template DNA, 1× transcription buffer, and 1 μl of enzyme mix (both Ambion) and incubated at 37°C for 4 to 6 h. 32P-labeled riboprobes were synthesized in similar reaction mixtures containing 3.75 mM each ATP, GTP, and UTP, 3.75 μM CTP, and 17 pmol of [α-32P]CTP (3,000 Ci/mmol). All reaction mixtures were then treated with 1 μl of DNase I (Ambion) for 15 min at 37°C, and unincorporated NTPs removed with RNA Mini Quick spin columns (Roche Molecular Diagnostics). The transcripts were purified by acid phenol (pH 4.5)-chloroform extraction and ethanol precipitation in the presence of ammonium acetate on dry ice.
RNA-protein binding reactions.
32P-labeled riboprobes were heated at 90°C for 2 min and cooled on ice for 5 min before being incubated with purified N protein in a 10-μl volume binding reaction based on that of Gött et al. (11) containing 1× binding buffer (10 mM HEPES [pH 7.3], 150 mM NaCl, 20 mM KCl, 5 mM MgCl2, 1 mM EDTA, 2 mM dithiothreitol [DTT], 10 U of rRNasin [Promega]) for 20 min at 30°C; N protein was the last component to be added. Control reaction mixtures lacking N contained an equivalent volume of 1× dialysis buffer. In competitive binding reactions the competitor RNA was mixed with the riboprobe in the reaction prior to the addition of N. For gel mobility shift analysis, glycerol was added to the binding-reaction mixtures to 12.5% and the reaction products were separated by electrophoresis on a 6% polyacrylamide gel plus 5% glycerol and 0.5× Tris-borate-EDTA (TBE) at 200 V for 2 h at 4°C or on a 1% agarose gel plus 0.5× TBE at 200 V for 1 h at 4°C. After drying, the gels were analyzed by autoradiography or by using a PhosphorImager (Bio-Rad). For filter-binding assays, 90 μl of 1× binding buffer was added and the reaction products were passed under vacuum through a BA85 nitrocellulose membrane filter (Schleicher & Schuell) that had been presoaked with 1× binding buffer. The filter was washed with 500 μl of 1× binding buffer under vacuum and dried, and radioactivity was determined by Cerenkov counting.
Western blotting.
Purified N was subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on a 15% polyacrylamide gel and blotted onto a nitrocellulose membrane (Amersham Life Science) by using a semidry electrophoretic transfer apparatus. The membrane was blocked for 16 h with blocking buffer (phosphate-buffered saline [PBS] containing 10% nonfat milk and 0.1% Tween 20) and then incubated in blocking buffer for 1 h with rabbit serum raised against purified BUN (1:500 dilution). The membrane was washed four times with PBS–0.1% Tween 20, incubated with protein A-horseradish peroxidase conjugate (1:1,000 dilution) in blocking buffer for 1 h, washed four times as before, and incubated twice in 50 mM Tris-HCl (pH 7.5) for 15 min each. Visualization was performed using enhanced chemiluminescence (Amersham Pharmacia Biotech).
Northwestern blotting.
Purified N was subjected to SDS-PAGE and blotting as described above. The membrane was washed twice with PBS and blocked with PBS plus 5% nonfat milk and 1 mM DTT for 16 h, four times with 1× HBB (25 mM HEPES [pH 7.5], 25 mM NaCl, 5 mM MgCl2, 10 mM DTT) for 15 min each, and once with hybridization buffer (RNA-protein binding buffer plus 0.5% Nonidet P-40) for 15 min. The membrane was then probed with 5 ml of hybridization buffer plus 5 × 105 cpm of 32P-labeled riboprobe and 40 U of rRNasin (Promega) at room temperature for 2 h, washed three times with hybridization buffer, and analyzed by autoradiography.
RNase treatment.
32P-labeled BUNS5′(32)/3′(33) riboprobe was incubated in the presence or absence of 800 ng of N protein in a standard binding reaction. RNase A was added to the concentrations specified, and the reaction mixtures were incubated at 37°C for 10 min. RNA was extracted with acid phenol (pH 4.5)-chloroform and precipitated with ethanol in the presence of ammonium acetate on dry ice. The RNA pellets were washed with 70% ethanol, resuspended in 5 μl of water, and boiled in 50% formamide, and the products were separated by PAGE on a 6% polyacrylamide sequencing-type gel at 300 V. After being dried, the gel was analyzed by autoradiography.
RESULTS
Expression and purification of recombinant BUN N protein.
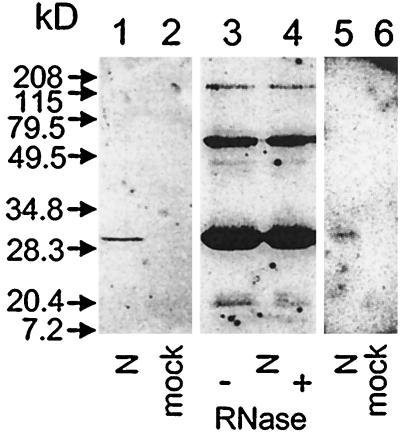
The BUN N protein was expressed as an N-terminally 6-histidine-tagged protein in E. coli and purified under native conditions by nickel affinity chromatography. The bulk of N was eluted by approximately 250 mM imidazole. Eluted fractions were dialyzed, concentrated, and stored at 4°C, at which temperature N was typically stable for approximately 3 weeks. Analysis of the preparation by SDS-PAGE with subsequent staining with Coomassie blue showed a single band at the expected size for His-tagged N protein (data not shown). Further analysis by Western blotting using a rabbit polyclonal antiserum raised against purified BUN virus confirmed the identity of the protein as N (Fig. 2, lane 1). Analysis of the highly concentrated eluate from bacteria containing empty parent plasmid and purified under similar conditions did not yield any bands (lane 2). Western blot analysis of 10-fold-more N than that in lane 1 yielded two additional bands of higher molecular mass (lane 3). The intensity of these bands was unaffected following treatment of the protein with 5 mg of RNase A per ml prior to electrophoresis (lane 4). This concentration of RNase A is more than sufficient to digest RNA in bunyavirus nucleocapsids. Thus, we suggest that these bands represent homodimers and homotrimers of N and were not the result of two or three N molecules binding to the same RNA molecule.
FIG. 2.
Characterization of expressed, histidine-tagged BUN N protein. A 220-ng (lanes 1 and 5) or 2.2-mg (lanes 3 and 4) sample of purified N protein was blotted onto a nitrocellulose membrane following SDS-PAGE; extracts of bacteria containing parent plasmid and subjected to the same purification protocol were run in lanes 2 and 6. The protein preparation in lane 4 was incubated with 5 mg of RNase A per ml before being loaded on the gel. Filters of lanes 1 to 4 were reacted with a polyclonal rabbit antiserum prepared against purified BUN and detected monomeric N (lane 1) and presumed oligomeric forms of N (lanes 3 and 4). No reaction with control lysate was observed (lane 2), and prior treatment with RNase A had no effect (lane 4). The filter of lanes 5 and 6 was reacted with 32P-labeled BUNS5′(32)/3′(33) RNA, and the N band was revealed following autoradiography (lane 5); no bands were detected on the blot of the extract from control bacteria (lane 6).
RNA binding by N protein.
The terminal sequences of each of the three bunyavirus genome segments have been implicated in encapsidation and are proposed to contain the site for nucleocapsid assembly (25). A 69-base riboprobe designated BUNS5′(32)/3′(33) was generated to investigate whether the recombinant N protein would bind the S-segment termini. This RNA corresponds to the exact terminal 32 bases of the 5′ end and 33 bases of the 3′ end of the genome-sense S segment (Fig. 1). Samples (100 pg) of radiolabeled BUNS5′(32)/3′(33) riboprobe were incubated with increasing concentrations of N under reaction conditions based on those described by Gött et al. (11) and then analyzed by polyacrylamide and agarose gel electrophoretic mobility shift assays (GEMSA), filter binding assays, and Northwestern blotting.
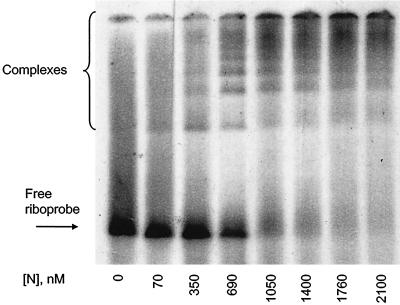
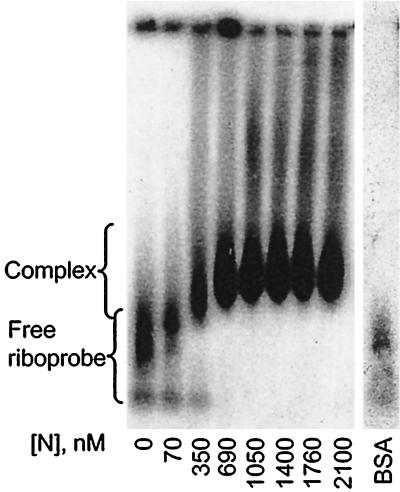
When GEMSA was performed using an acrylamide gel (Fig. 3), at low concentrations of N a small proportion of riboprobe was shifted into a single band of higher molecular mass. At higher concentrations of N the riboprobe was shifted into multiple higher complexes, forming a ladder-like profile, which suggests sequential filling of binding sites on the RNA (3). The multiple bands are interpreted as the result of riboprobe being bound by discrete numbers of N molecules. Further addition of protein resulted in saturation of the riboprobe, which was shifted toward the top of the gel. To investigate the saturated complexes further, identical reactions were analyzed by agarose GEMSA (Fig. 4). In this assay the saturated complexes ran into the gel and could be seen to reach a finite maximum size. To confirm that the mobility shifts of the riboprobe were due to interaction with N, the protein was shown to bind BUNS5′(32)/3′(33) RNA directly by Northwestern blot analysis (Fig. 2, lane 5). No binding of the riboprobe was observed in a mock expression lane in which bacteria containing the empty parent vector had been subjected to an identical induction and purification regime (lane 6), suggesting that the shifts observed in the GEMSAs were not attributable to interaction with a native bacterial protein. When N was replaced by bovine serum albumen in binding reactions, no shift of the riboprobe was observed, indicating that the mobility shift was specific for the N protein (Fig. 4).
FIG. 3.
Analysis of N-RNA binding by polyacrylamide GEMSA. A 100-pg sample of 32P-labeled BUNS5′(32)/3′(33) RNA was incubated with different concentrations of N protein, as indicated, before being subjected to analysis on a polyacrylamide gel. In the presence of increasing concentrations of N, the riboprobe was shifted into multiple higher complexes in a ladder-like profile. At high concentrations of N, the riboprobe was shifted toward the top of the gel.
FIG. 4.
Analysis of N-RNA binding by agarose GEMSA. A 100-pg sample of 32P-labeled BUNS5′(32)/3′(33) RNA was incubated with different concentrations of N protein, as indicated, before being subjected to analysis on an agarose gel. In the presence of N, the riboprobe was shifted into a higher band of finite maximum size. The two bands observed for the free riboprobe are thought to be due to secondary structure in the RNA. Replacement of N by bovine serum albumen (BSA) in the binding-reaction mixture did not result in a mobility shift of the probe.
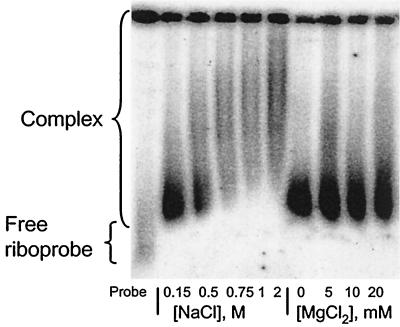
Effect of ionic concentration on binding.
Binding reaction mixtures were assembled in the presence of different concentrations of NaCl (0.15 to 2 M) and of MgCl2 (0 to 20 mM), and the products were analyzed by agarose GEMSA (Fig. 5). Complex formation was not impaired by up to 0.5 M NaCl; at higher salt concentrations there was no evidence of dissociation of the complex, indicated by the lack of free riboprobe, but radioactivity was smeared further up the gel. We assume that this is an electrophoresis artifact caused by the high salt concentration in the sample. Binding of N to the riboprobe was unaffected at the MgCl2 concentrations used (Fig. 5). These results suggest that electrostatic interactions do not play a major role in the binding event. The fact that the N-RNA complexes were stable at high salt concentrations also suggests that the binding is strong.
FIG. 5.
Effect of ionic strength on complex formation. Binding-reaction mixtures contained 100 pg of 32P-labeled BUNS5′(32)/3′(33) RNA, 2,100 nM purified N protein, and different concentrations of NaCl or MgCl2, as indicated, before being subjected to analysis by agarose GEMSA. Binding of N was evident, and no free riboprobe was observed for the range of NaCl and MgCl2 concentrations tested. The increase in retardation coinciding with the increased NaCl concentrations is probably an effect of high salt concentrations on the movement of the complex through the gel.
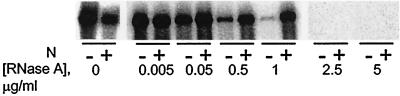
Complexes are resistant to RNase.
The RNA in bunyavirus nucleocapsids is relatively resistant to digestion by “reasonable concentrations” (12, 16) of RNase A but is digested by 100 μg of RNase A per ml (19). To investigate whether in vitro-assembled N-RNA complexes were resistant to RNase A, BUNS5′(32)/3′(33) riboprobe was incubated in binding reactions either with or without N and then different amounts of RNase A were added and incubation was continued for 10 min at 37°C. All reaction mixtures contained 20 U of RNasin RNase inhibitor (Promega), which is needed to inhibit any RNases present in the N protein stock (and thus would otherwise digest the RNA before N was able to bind); this amount of RNasin was considered to exert a nominal effect on the amount of RNase A added (1 U of RNasin inhibits 5 ng of RNase A by 50%; Promega). After phenol extraction the RNAs were analyzed by electrophoresis on a denaturing, sequencing-type polyacrylamide gel. As shown in Fig. 6, RNA complexed with N was resistant to 1 μg of RNase A per ml whereas the naked riboprobe was almost fully digested at this concentration. Neither complexed nor naked riboprobe was resistant to 2.5 μg of RNase A per ml or higher concentrations.
FIG. 6.
Effect of RNase on N-RNA complexes. Binding reactions were performed in mixtures containing 100 pg of 32P-labeled BUNS5′(32)/3′(33) RNA with or without 800 ng of N, and then different concentrations of RNase A were added and incubation was continued for 10 min at 37°C. Following phenol extraction and ethanol precipitation, the RNAs were resolved on a denaturing acrylamide gel. Complexes were resistant to 1 μg of RNase A per ml but not to 2.5 μg/ml, whereas most of the free riboprobe was digested by 1 μg of RNase A per ml.
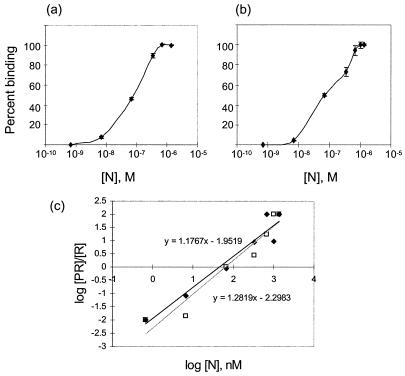
Kinetics of N binding.
The kinetics of binding of N to BUNS5′(32)/3′(33) RNA was measured by filter binding assays with a wider range of concentrations of N than that used in GEMSA. Binding-reaction mixtures containing 100 pg of radiolabeled riboprobe were passed through nitrocellulose membranes under vacuum. Whereas free riboprobe passed through the membrane, riboprobe complexed with N was immobilized on it. The degree of binding could thus be measured as the proportion of radiolabeled RNA retained on the membrane by its association with N (Fig. 7a). Maximal binding was obtained with 180 ng of N in the reaction, equivalent to a molar ratio of 1:1,500 (RNA to protein). The dissociation constant (Kd), which was calculated as half-maximum binding, was approximately 7 × 10−8 M. Analysis of binding kinetics using a Hill plot provides a mathematical calculation of the degree of cooperativity in the binding event (3). The gradient of the resulting line serves as a measure of the number of sites that are bound cooperatively. Analysis of the binding data in Fig. 7a by this technique gave the results shown in Fig. 7c and yielded a gradient of approximately 1.2, indicating that the binding event showed a degree of cooperativity (3).
FIG. 7.
Binding kinetics of purified N protein to RNA. (a and b) A 100-pg 32P-labeled BUNS5′(32)/3′(33) (a) or BUNS5′(65) (b) RNA was incubated with increasing concentrations of N protein before being passed through a nitrocellulose filter. The retained labeled RNA was determined by scintillation counting and corrected by subtracting background counts of riboprobe in the absence of N. The two probes showed similar kinetics and yielded a Kd of approximately 7 × 10−8 M, calculated as equal to 1/2 _V_max. Error bars indicate the standard deviation from three replicates. (c) Hill plot of binding of N to BUNS5′(32)/3′(33) RNA (solid diamonds) and BUNS-5′(65) RNA (open squares). Linear regression was performed on the data points, yielding gradients of 1.2 for BUNS5′(32)/3′(33) RNA and 1.3 for BUNS-5′(65) RNA, indicating a low degree of cooperativity in both cases.
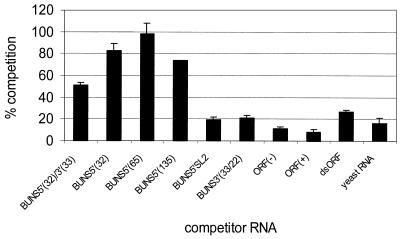
Competitive binding assays.
The encapsidation signal for bunyavirus genome and antigenome segments is proposed to reside within the 5′-terminal sequences (12, 16, 25). We used a panel of RNAs in competitive filter binding assays to investigate the presence of such a signal. Binding-reaction mixtures were assembled containing radiolabeled BUNS5′(32)/3′(33) riboprobe in the presence of a 1,000-fold mass excess of unlabeled competitor RNAs, which were mixed prior to the addition of N (Fig. 8). These experiments would therefore provide a measure of the degree of selectivity of N for the competitor RNA over BUNS5′(32)/3′(33) RNA. The results were expressed as the percentage of competition shown by the competitor RNA for binding to N; a low value indicates that most of N is binding the riboprobe, and a high value indicates that there is competition for binding by the unlabeled RNA. To ensure that any competition observed was not due to loss of labeled riboprobe through RNase degradation, the competitor RNAs were tested for RNase contamination by incubation with the riboprobe, under binding conditions, followed by electrophoresis on a denaturing polyacrylamide gel and autoradiography. No evidence of RNase degradation of the riboprobe was observed (data not shown).
FIG. 8.
Competitive binding assays using a panel of RNAs. A 100-pg sample of 32P-labeled BUNS5′(32)/3′(33) RNA and 100 ng of unlabeled competitor RNA were mixed, prior to the addition of 350 nM purified N, in a standard binding reaction. After incubation, the mixture was passed through a nitrocellulose filter and the retained labeled RNA was determined by scintillation counting and corrected by subtracting background counts of riboprobe in the absence of N. Results are expressed as percent competition. Thus, 100% competition would indicate that N bound the competitor RNA exclusively and 0% competition would indicate that N bound the riboprobe exclusively. The competitor RNAs are described in Materials and Methods.
A 1,000-fold molar excess of the homologous RNA, BUNS5′(32)/3′(33), gave about 50% competition, whereas yeast RNA competed to less than 20% (Fig. 8). Higher levels of competition were observed with competitor RNAs containing the 5′ end of the BUN S segment. BUNS5′(32) and BUNS5′(135) RNAs, comprising the terminal 32 and 135 bases, respectively, reduced retention of riboprobe by about 75 to 80%. BUNS5′(65) RNA consists of the 5′ terminal 65 bases of the BUN negative-sense S segment and is thus a similar length to BUNS5′(32)/3′(33). In the presence of a 1,000-fold excess of this competitor, the proportion of riboprobe retained dropped by approximately 97%, indicating a high degree of competition. However, an RNA comprising an internal region of the 5′ end, BUNS5′SL2 (bases 23 to 56), showed only 20% competition.
The binding kinetics of N to BUNS5′(65) RNA were measured by a filter binding assay (Fig. 7b) and shown to be similar to those of BUNS5′(32)/3′(33). Analysis of the binding data with BUNS5′(65) RNA yielded a similar Hill plot to that of BUNS5′(32)/3′(33) RNA (Fig. 7c).
BUNS3′(33/22) consists of the 33 terminal bases at the 3′ end of the S segment and some vector sequences, and competed to a low level similar to that of yeast RNA. ORF(−) and ORF(+) RNAs comprise 87-base transcripts, genome and antigenome sense, respectively, representing a region of the N ORF which encodes a highly conserved domain in the N protein. Neither of these RNAs competed more than yeast RNA. dsORF RNA was generated by annealing the two single-stranded ORF transcripts and was used to compare the preference of N for dsRNA and ssRNA. dsORF RNA competed only slightly more than the ssRNAs and did not reach the high levels shown by competitors containing 5′ terminal sequences.
DISCUSSION
We have described experiments to study the RNA binding properties of BUN nucleocapsid protein in vitro. Recombinant BUN N protein was expressed as a His-tagged protein in bacteria and purified by nickel affinity chromatography. N was reactive to antiserum raised against purified BUN and demonstrated binding activity for RNA containing the terminal sequences of the negative-sense S RNA segment, with a dissociation constant of 7 × 10−8 M, similar to the value reported for hantavirus N protein (29). RNA binding reactions in the presence of increasing concentrations of N, followed by polyacrylamide GEMSA, indicated the presence of discrete complexes until the RNA was fully encapsidated. Although the observed pattern might represent binding of RNA to misfolded N protein, we think this unlikely since multiple bands of appropriate sizes are seen on Western blots (Fig. 2), suggesting that N is capable of homo-oligomerizing. Analysis of the filter binding assay data by a Hill plot (Fig. 7) indicated a low (nonsimultaneous) degree of cooperativity in the binding of N to the BUNS5′(32)/3′(33) RNA. Richmond et al. (27) reported RNA-binding by recombinant tomato spotted wilt virus N to be a cooperative event, as did Gött et al. (11) for analysis of bacterially expressed hantavirus N protein binding, although neither group presented corroboratory data, for example in the form of a Hill plot.
RNA-N protein complexes showed a finite maximum size when analyzed on an agarose gel (Fig. 4). We interpret this as indicating that N binds along the RNA as opposed to the RNA associating with preformed multimers of N, whose size would be proportional to the concentration of N in the reaction mixture. The RNA-N complexes formed in the binding reactions were shown to be resistant to a level of RNase A digestion similar to that of authentic nucleocapsids, indicating that they possess similar properties.
We observed a discrepancy between the Kd calculated from filter binding data and the equivalent degree of binding in GEMSA: the concentration of N required for 50% maximum binding in the filter binding assay was lower than that required in the gel shift assay. Since this was a consistent observation, we assume that it is because the N-RNA complexes were less stable during electrophoresis, particularly through a polyacrylamide gel. Filter binding was therefore the preferred method for competitive binding assays to investigate the selectivity of binding by N. Interpretation of the results was complicated by the finding that even in the presence of a 1,000-fold excess of homologous unlabeled competitor, the retention of BUNS5′(32)/3′(33) riboprobe was decreased by only 50%. The unexpected lower level of competition observed with the homologous RNA may be due to interactions between the 5′ and 3′ termini of BUNS5′(32)/3′(33) such that the labeled RNA is able to base-pair with unlabeled homologous RNA to form multimers, thereby reducing the sensitivity of the assay. This phenomenon would occur when the RNA possesses both termini on the same strand.
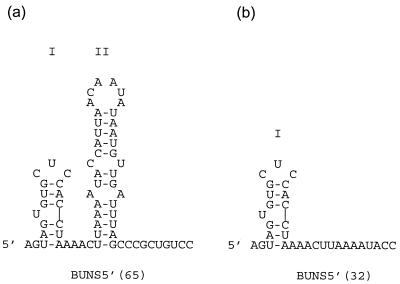
We were able to identify the first 32 bases of the 5′ negative-sense S segment as a region for which N possesses a preference over RNAs lacking this region. Analyses of the RNAs containing 5′-terminal sequences by the Mfold program (18, 35) predict the presence of a stem-loop structure, designated I, near the 5′ terminus (Fig. 9). It is possible that the observed preference for binding is provided by this putative stem-loop, which might act as a signal for N to begin binding the segment RNA. BUNS5′(65) is predicted to form a second stem-loop, II (Fig. 9a), and stem-loops similar to stem-loop II are predicted in the first 65 bases of the 5′ negative-sense termini of the other BUN segments (data not shown). However, RNA comprising just stem-loop II (BUNS5′SL2 RNA) competed similarly to yeast RNA and is therefore not the sole contributor of the high degree of competition seen with BUNS5′(65). In addition, Mfold does not predict that BUNS5′(135) contains stem-loop II, although stem loop I is still predicted (data not shown). BUNS3′(33/22), an RNA possessing the 3′ terminus but lacking the 5′ terminus, did not demonstrate a greater degree of competition than yeast RNA, which suggests that the 3′ terminus does not contribute significantly to the selectivity observed for BUNS5′(32)/3′(33). The preference of N for the 5′ terminus is unlikely to be due to N binding dsRNA nonspecifically, since N did not possess a high preference for ORF dsRNA. Stem-loop I in the 5′ terminus might also have implications for the structure of the putative panhandle involving the ends of the RNA segment, particularly if this structure were retained after N binds, because loop I resembles the hook structures that have been proposed in the 5′ termini of influenza virus and Thogoto virus RNAs (10, 17, 31).
FIG. 9.
Predicted secondary structure of the 5′ terminus of BUN S segment RNA. Secondary structures of BUNS5′(65) (a) and BUNS5′(32) RNA (b) were predicted using Mfold (18, 35). The most energetically favorable structures are shown, and stem-loops I and II are indicated.
Normally only full-length genome and antigenome segments are encapsidated in bunyavirus-infected cells, and hence a mechanism must exist to distinguish between these and viral mRNAs (or indeed cellular RNAs); it has been proposed that the presence of the additional primer-derived bases at the 5′ termini of viral mRNAs plays a role (25). We suggest that such additional bases may affect the secondary structure in the 5′ terminus, and experiments to test this hypothesis are under way. La Crosse bunyavirus N protein is reported to bind S segment-derived mRNA when N is present at high concentrations in infected cells, providing a feedback mechanism to regulate its own concentration (12), and this suggests that although authentic N is capable of binding RNAs other than genomes and antigenomes, it can only do so when present at high concentration. Competitive-binding experiments reported above suggest that BUN N is also capable of binding nonviral RNAs since yeast RNA could compete to a low but reproducible degree (Fig. 8), which is presumably not sequence specific.
Taken together, our observations may indicate two types of binding by N protein to RNA, selective or preferential binding to the 5′-terminal region and nonspecific binding. Other proteins have exhibited characteristics similar to those observed with BUN N. Heterogeneous nuclear ribonucleoproteins are capable of both preferential binding to certain sequences and less sequence-specific binding at high concentrations, probably serving to hinder the formation of secondary structure in RNA (5, 6). Many positive-sense RNA virus core or coat proteins often possess the ability to selectively bind specific sequences (13, 15, 32, 34), sometimes binding hairpins in the 3′ terminus, as well as binding RNA nonspecifically. Preferential binding to an encapsidation signal has also been observed for rabies virus (33) and human immunodeficiency virus (1).
The prediction of a binding site near the 5′ terminus for which N is highly selective is in agreement with the suggestion of Kolakofsky and colleagues (12, 25), who envisaged a scenario in which N binds a site in the 5′ terminus of the segment RNA as it is being transcribed. Addition of the next N monomer could occur through its combined affinity for the RNA and the bound protein, and in this regard, homotypic interactions between tomato spotted wilt virus N proteins have already been reported (30). Encapsidation of the RNA as it is being transcribed removes the necessity for highly cooperative binding unless the transcription elongation event is progressing more quickly than the binding of N to the nascent chain.
ACKNOWLEDGMENTS
We thank Carol Noonan for assisting with protein purification, Ewan Dunn for supplying some of the transcription plasmids, and Paul Yeo for helpful discussion.
J.C.O. was supported by a CASE studentship from BBSRC and Roche Products Ltd., and work in the laboratory of R.M.E. is supported by grants from the Wellcome Trust and the MRC.
REFERENCES
- 1.Allen P, Collins B, Brown D, Hostomsky Z, Gold L. A specific RNA structural motif mediates high affinity binding by the HIV-1 nucleocapsid protein (NCp7) Virology. 1996;225:306–315. doi: 10.1006/viro.1996.0605. [DOI] [PubMed] [Google Scholar]
- 2.Bishop D H L, Gay M E, Matsuoko Y. Non-viral heterogeneous sequences are present at the 5′ ends of one species of snowshoe hare bunyavirus S complementary RNA. Nucleic Acids Res. 1983;11:6409–6418. doi: 10.1093/nar/11.18.6409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Black D L, Chan R, Min H, Wang J, Bell L. The electrophoretic mobility shift assay for RNA binding proteins. In: Smith C W J, editor. RNA:protein interactions. Oxford, United Kingdom: Oxford University Press; 1998. pp. 109–136. [Google Scholar]
- 4.Bouloy M, Pardigon N, Vialet P, Gerbaud S, Girad M. Characterisation of the 5′ and 3′ ends of viral messenger RNAs isolated from BHK21 cells infected with Germiston virus (Bunyavirus) Virology. 1990;75:50–58. doi: 10.1016/0042-6822(90)90185-t. [DOI] [PubMed] [Google Scholar]
- 5.Burd C G, Dreyfuss G. Conserved structures and diversity of functions of RNA-binding proteins. Science. 1994;265:615–621. doi: 10.1126/science.8036511. [DOI] [PubMed] [Google Scholar]
- 6.Burd C G, Dreyfuss G. RNA binding specificity of hnRNP A1: significance of hnRNP A1 high-affinity binding sites in pre-mRNA splicing. EMBO J. 1994;13:1197–1204. doi: 10.1002/j.1460-2075.1994.tb06369.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dunn E F, Pritlove D C, Jin H, Elliott R M. Transcription of a recombinant Bunyavirus RNA template by transiently expressed Bunyavirus proteins. Virology. 1995;211:133–143. doi: 10.1006/viro.1995.1386. [DOI] [PubMed] [Google Scholar]
- 8.Elliott R M, editor. The Bunyaviridae. New York, N.Y: Plenum Press; 1996. [Google Scholar]
- 9.Eshita Y, Ericson B, Romanowski V, Bishop D H L. Analyses of the mRNA transcription processes of snowshoe hare bunyavirus S and M RNA species. J Virol. 1985;55:681–689. doi: 10.1128/jvi.55.3.681-689.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flick R, Neumann G, Hoffmann E, Neumeier E, Hobom G. Promoter elements in the influenza vRNA terminal structure. RNA. 1996;2:1046–1057. [PMC free article] [PubMed] [Google Scholar]
- 11.Gött P, Stohwasser R, Schnitzler P, Darai G, Bautz E K F. RNA binding of recombinant nucleocapsid proteins of hantaviruses. Virology. 1993;194:332–337. doi: 10.1006/viro.1993.1263. [DOI] [PubMed] [Google Scholar]
- 12.Hacker D, Raju R, Kolakofsky D. La Crosse virus nucleocapsid protein controls its own synthesis in mosquito cells by encapsidating its mRNA. J Virol. 1989;63:5166–5174. doi: 10.1128/jvi.63.12.5166-5174.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hacker D L. Identification of a coat protein binding site on southern bean mosaic virus RNA. Virology. 1995;207:562–565. doi: 10.1006/viro.1995.1117. [DOI] [PubMed] [Google Scholar]
- 14.Jin H, Elliott R M. Characterization of Bunyamwera virus S RNA that is transcribed and replicated by the L protein expressed from recombinant vaccinia virus. J Virol. 1993;67:1396–1404. doi: 10.1128/jvi.67.3.1396-1404.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khromykh A A, Westaway E G. RNA binding properties of core protein of the flavivirus Kunjin. Arch Virol. 1996;141:685–699. doi: 10.1007/BF01718326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kolakofsky D, Hacker D. Bunyavirus RNA synthesis: genome transcription and replication. Curr Top Microbiol Immunol. 1991;169:143–159. doi: 10.1007/978-3-642-76018-1_5. [DOI] [PubMed] [Google Scholar]
- 17.Leahy M B, Dessens J T, Nuttall P A. Striking conformational similarities between the transcription promoters of Thogoto and influenza A viruses: evidence for intrastrand base pairing in the 5′ promoter arm. J Virol. 1997;71:8352–8356. doi: 10.1128/jvi.71.11.8352-8356.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mathews D H, Sabina J, Zuker M, Turner D H. Expanded sequence dependence of thermodynamic parameters provides robust prediction of RNA secondary structure. J Mol Biol. 1999;288:911–940. doi: 10.1006/jmbi.1999.2700. [DOI] [PubMed] [Google Scholar]
- 19.Obijeski J F, Bishop D H L, Palmer E L, Murphy F A. Segmented genome and nucleocapsid of La Crosse virus. J Virol. 1976;20:664–675. doi: 10.1128/jvi.20.3.664-675.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pardigon N, Vialat P, Girard M, Bouloy M. Panhandles and hairpin structures at the termini of Germiston virus (Bunyavirus) Virology. 1982;122:191–197. doi: 10.1016/0042-6822(82)90388-9. [DOI] [PubMed] [Google Scholar]
- 21.Patterson J L, Kolakofsky D. Characterization of La Crosse virus small genome transcripts. J Virol. 1984;49:680–685. doi: 10.1128/jvi.49.3.680-685.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pettersson R F, von Bonsdorf C H. Ribonucleoproteins of Uukuniemi virus are circular. J Virol. 1975;15:386–392. doi: 10.1128/jvi.15.2.386-392.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qiagen. The QIAexpressionist. 3rd ed. Hilden, Germany: Qiagen; 1997. [Google Scholar]
- 24.Raju R, Kolakofsky D. Translational requirement of La Crosse virus S-mRNA synthesis: in vivo studies. J Virol. 1986;61:96–103. doi: 10.1128/jvi.61.1.96-103.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raju R, Kolakofsky D. Unusual transcripts in La Crosse virus-infected cells and the site for nucleocapsid assembly. J Virol. 1987;61:667–672. doi: 10.1128/jvi.61.3.667-672.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raju R, Kolakofsky D. The ends of La Crosse virus genome and antigenome RNAs within nucleocapsids are base paired. J Virol. 1989;63:122–128. doi: 10.1128/jvi.63.1.122-128.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Richmond K E, Chenault K, Sherwood J L, German T L. Characterization of the nucleic acid binding properties of tomato spotted wilt virus nucleocapsid protein. Virology. 1998;248:6–11. doi: 10.1006/viro.1998.9223. [DOI] [PubMed] [Google Scholar]
- 28.Samso A, Bouloy M, Hannoun C. Presence de ribonucleoproteins circulaires dans le virus Lumbo (Bunyavirus) C R Acad Sci Ser D. 1975;280:779–782. [PubMed] [Google Scholar]
- 29.Severson W, Partin L, Schmaljohn C S, Jonsson C B. Characterization of the Hantaan nucleocapsid protein-ribonucleic acid interaction. J Biol Chem. 1999;274:33732–33739. doi: 10.1074/jbc.274.47.33732. [DOI] [PubMed] [Google Scholar]
- 30.Uhrig J F, Soellick T-R, Minke C J, Philipp C, Kellmann J-W, Schreier P H. Homotypic interaction and multimerization of nucleocapsid protein of tomato spotted wilt tospovirus: identification and characterization of two interacting domains. Proc Natl Acad Sci USA. 1999;96:55–60. doi: 10.1073/pnas.96.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weber F, Haller O, Kochs G. Conserved vRNA end sequences of Thogoto-orthomyxovirus suggest a new panhandle structure. Arch Virol. 1997;142:1029–1033. doi: 10.1007/s007050050138. [DOI] [PubMed] [Google Scholar]
- 32.Wei N, Morris T J. Interactions between viral coat protein and a specific binding region on turnip crinkle virus RNA. J Mol Biol. 1991;222:437–443. doi: 10.1016/0022-2836(91)90483-m. [DOI] [PubMed] [Google Scholar]
- 33.Yang J, Hooper D C, Wunner W H, Koprowski H, Dietzschold B, Fu Z F. The specificity of rabies virus RNA encapsidation by nucleoprotein. Virology. 1998;242:107–117. doi: 10.1006/viro.1997.9022. [DOI] [PubMed] [Google Scholar]
- 34.Zhou M, Williams A K, Chung S-I, Wang L, Collisson E W. The infectious bronchitis virus nucleocapsid protein binds RNA sequences in the 3′ terminus of the genome. Virology. 1996;217:191–199. [Google Scholar]
- 35.Zuker M, Mathews D H, Turner D H. Algorithms and thermodynamics for RNA secondary structure prediction: a practical guide. NATO ASI Ser. 1999;70:11–43. [Google Scholar]