v-Src Generates a p53-Independent Apoptotic Signal (original) (raw)
Abstract
Evasion of apoptosis appears to be a necessary event in tumor progression. Some oncogenes, such as c-myc and E1A, induce apoptosis in the absence of survival factors. However, others, such as bcl-2 and v-src, activate antiapoptotic pathways. For v-Src, these antiapoptotic pathways are dependent on the function of Ras, phosphatidylinositol (PI) 3-kinase, and Stat3. Here we asked whether v-Src can activate a proapoptotic signal when survival signaling is inhibited. We show that when the functions of Ras and PI 3-kinase are inhibited, v-_src_-transformed Rat-2 fibroblasts undergo apoptosis, evidenced by loss of adherence, nuclear fragmentation, and chromosomal DNA degradation. The apoptotic response is dependent on activation of caspase 3. Under similar conditions nontransformed Rat-2 cells undergo considerably lower levels of apoptosis. Apoptosis induced by v-Src is accompanied by a loss of mitochondrial membrane potential and release of cytochrome c and is blocked by overexpression of bcl-2, indicating that it is mediated by the mitochondrial pathway. However apoptosis induced by v-Src is not accompanied by an increase in the level of p53 and is not dependent on p53 function. Thus v-Src generates a p53-independent proapoptotic signal.
The proliferation of metazoan cells requires not only mitogenic signals but also survival signals (29). These survival signals are supplied by cell-matrix contacts and certain growth factors (32) that prevent apoptosis (programmed cell death). The requirement for survival signals is a cellular safeguard against the deregulated proliferation induced by oncogenes (20). Thus, mitogenic signaling without accompanying survival signaling triggers apoptosis and leads to the elimination of potentially malignant cells (21, 47). The oncogenes c-myc and E1A, for example, which generate strong proliferative signals, have been shown to induce apoptosis in the absence of exogenous survival factors (22, 52, 55).
Two distinct but interconnected apoptotic pathways have been implicated in the apoptosis induced by oncogenes, the extrinsic or death receptor pathway and the intrinsic or mitochondrial pathway (11). The death receptor pathway is activated by the binding of ligands, such as tumor necrosis factor (TNF) and Fas, to their respective death receptors, resulting in activation of the upstream initiator caspase, caspase 8, and ultimately in the activation of downstream executioner caspases (4). The mitochondrial pathway is activated by a diverse range of cellular stresses (21). These stresses lead to the loss of the inner mitochondrial membrane potential and release of cytochrome c from the intermembrane space (26). Cytosolic cytochrome c binds to adapter protein Apaf-1, which in turn activates another upstream initiator caspase, caspase 9 (74). These mitochondrial events are inhibited by antiapoptotic members of the Bcl-2 family (Bcl-2, BclXL) and promoted by proapoptotic members (Bax, Bad) (1). A link between these two pathways was demonstrated with the finding that caspase 8 can cleave Bcl-2 family member Bid to generate a cleaved Bid product that induces cytochrome c release (42). Thus, the mitochondrial pathway can serve to amplify the response to ligands such as Fas and TNF (41). Apoptosis induced by expression of oncogenes, such as c-myc and E1A, has been shown to be mediated by the mitochondrial pathway (22, 52, 55). The involvement of the death receptor pathway has also been demonstrated, as Myc-induced apoptosis is inhibited by a dominant-negative FADD mutant (30), indicating that sensitivity to cytochrome c release may be affected by signaling through the death receptor pathway.
Certain oncogenes, such as v-src, activate both mitogenic and survival signaling pathways (3, 70). v-Src is a mutationally activated form of the non-receptor tyrosine kinase Src, and Src family kinases appear to play an important role in cell survival pathways (57, 61). Importantly, v-Src expression does not lead to apoptosis despite its being a potent inducer of proliferation. The expression of v-Src leads to the activation of multiple signaling pathways, although the nature of the pathways activated by v-Src depends to some extent on the cell type (2). The signaling pathways implicated in Src transformation are dependent on the functions of Ras, phosphatidylinositol (PI) 3-kinase, and Stat3 (2, 8, 53, 59, 60, 64). Ras and PI 3-kinase have dual signaling functions, generating both mitogenic and survival signals. Survival signaling by Ras has been shown to occur by multiple mechanisms. The Erk mitogen-activated protein kinases, which are activated by Ras, promote cell survival through activation of the protein kinase Rsk, which phosphorylates and inactivates BAD, a proapoptotic member of the Bcl-2 family (7). Rsk also phosphorylates the transcription factor CREB, thereby upregulating expression of the antiapoptotic gene bcl-2 (7). In addition, the Ras effector Raf-1 can be targeted to the mitochondria by Bcl-2, where it can phosphorylate BAD (68). Ras has also been found to generate survival signaling through activation of the transcription factor NF-κB (36, 50, 56), which promotes the expression of antiapoptotic genes such as bcl-2, bcl-xL, XIAP, TRAF1, TRAF2, c-IAP1, and c-IAP2 (14, 16, 67, 73). PI 3-kinase mediates survival signaling through activation of the serine/threonine kinase Akt (38, 45). Akt promotes cell survival by phosphorylating a number of different proteins involved in the regulation of apoptosis, including caspase 9 (12), BAD (17), the Forkhead family of transcription factors (10, 40), NF-κB (36, 50, 56), and endothelial nitric oxide synthase (19, 24). Stat3 promotes survival by inducing the expression of bcl-xL and possibly other antiapoptotic genes (48).
Since v-Src is a potent inducer of cell proliferation, we hypothesized that it might induce apoptosis when survival signaling is inhibited. We therefore examined the significance of the survival signaling generated by Ras and PI 3-kinase in the transformation of mammalian cells by v-Src. Here we report that v-Src induces apoptosis in Rat-2 fibroblasts when Ras and PI 3-kinase signaling is inhibited. The apoptotic response induced by v-Src is mediated by the mitochondrial pathway but is p53 independent.
MATERIALS AND METHODS
Cell cultures and plasmids.
The Rat-2 fibroblast cell line expressing a dominant-negative version of the ras gene (the N17 H-ras mutant) under the control of the metal-inducible metallothionein promoter has been described previously (2). This cell line was infected with pBABE-Hygro/v-src virus to generate a v-_src_-transformed cell line (referred to here as N17Ras/Src) or with the empty pBABE-Hygro vector to generate a cell line referred to as N17Ras/Vec, as described previously (2). These cells were cultured in Dulbecco's modified Eagle medium (1 part) and Ham's F-10 nutrient mixture (2 parts) supplemented with 10% fetal bovine serum, penicillin (100 U/ml), and streptomycin (100 μg/ml) in a humidified incubator at 37°C with 5% CO2. The human bcl-2 expression plasmid pSFFV.neo/bcl-2 was obtained from Astar Winoto (University of California at Berkeley), wild-type p53 expression plasmid pCMV-p53 was obtained from Gary Firestone (University of California at Berkeley), and dominant-negative p53 construct pCMV-p53-DD was obtained from Moshe Oren (Weizman Institute).
Western blot analysis.
For detection of Ras and p27, cells were lysed in radioimmunoprecipitation assay lysis buffer (10 mM Tris-HCl [pH 7.5], 150 mM NaCl, 1 mM EDTA, 20 mM MgCl2, 1% Nonidet P-40, 1% sodium deoxycholate, 0.05% sodium dodecyl sulfate (SDS), and protease inhibitors [1 mM phenylmethylsulfonyl fluoride, 10 μM benzamidine, 5 μM phenanthroline, and 0.5 μg each of antipain, leupeptin, pepstatin, aprotinin, and chymostatin per ml]). For detection of phospho-Akt and phospho-Erk, cells were lysed in a buffer containing 10 mM Tris-HCl (pH 7.5), 137 mM NaCl, 10% glycerol, 1% Nonidet P-40, 20 mM sodium fluoride, 1 mM sodium pyrophosphate, 1 mM sodium orthovanadate, and protease inhibitors. For detection of caspase 3 and p53, cells were lysed in the lysis buffer provided in the R&D Systems caspase 9 colorimetric assay kit. Similar results were obtained when cells were lysed directly in SDS sample buffer. For detection of poly(ADP-ribose) polymerase (PARP), cells were lysed in 62.5 mM Tris-HCl (pH 7.5)–6 M urea–10% glycerol–2% SDS–5%–β-mercaptoethanol. The protein concentration of the total cell lysates was determined by the bicinchoninic acid protein assay (Pierce). Equal amounts of protein were resolved by SDS-polyacrylamide gel electrophoresis and transferred to an Immobilon-P transfer membrane (Millipore). Proteins were incubated with primary antibodies and then visualized with the appropriate secondary antibodies using Chemiluminescence Reagent Plus (NEN). Primary antibodies were obtained from the following sources: phosphospecific Akt antibodies, New England BioLabs; pan-Ras (Ab-2), PARP (Ab-2), and p53 (Ab-7), Oncogene Research Products; phospho-Erk and p27 (F-8), Santa Cruz Biotechnology.
Quantitation of apoptosis.
To compare the levels of apoptosis in nontransformed N17Ras/Vec and v-_src_-transformed N17Ras/Src Rat-2 cells, equal numbers of cells (1.2 × 106) of all cell types were plated in 10-cm-diameter tissue culture plates. After approximately 8 h, the medium was changed to remove any detached cells. CdCl2 (2 μM) and ZnCl2 (100 μM) were then added to the medium to induce the expression of N17Ras; alternatively, or in addition, LY294002 (20 μM) was added to inhibit PI 3-kinase. Apoptosis was generally monitored following 18 h of further incubation. Apoptosis was quantified by cell detachment assays (46, 69) or by nuclear fragmentation or TUNEL (deoxynucleotidyl transferase-mediated dUTP nick end labeling) assays. All three assays gave similar results. To assay cell detachment, nonadherent cells were collected and counted in a Coulter counter. Greater than 90% of these nonadherent cells were apoptotic, as they contained fragmented nuclei and nicked chromosomal DNA (detected by TUNEL assays). The remaining adherent cells were trypsinized and likewise counted. The percentages of apoptotic cells reported in Fig. 1 represent the ratios of detached cells to total cells. Nuclear fragmentation assays were performed as follows. Detached and adherent cells were combined, fixed with 1% formaldehyde for 10 min at room temperature, permeabilized with 0.2% Triton X-100, and stained with Hoechst 33258 (5 μM). Apoptosis was quantified by scoring cells with nuclear fragmentation by fluorescence microscopy. TUNEL assays were performed using the Apoptosis Detection Kit, Fluorescein (Promega) according to the manufacturer's instructions.
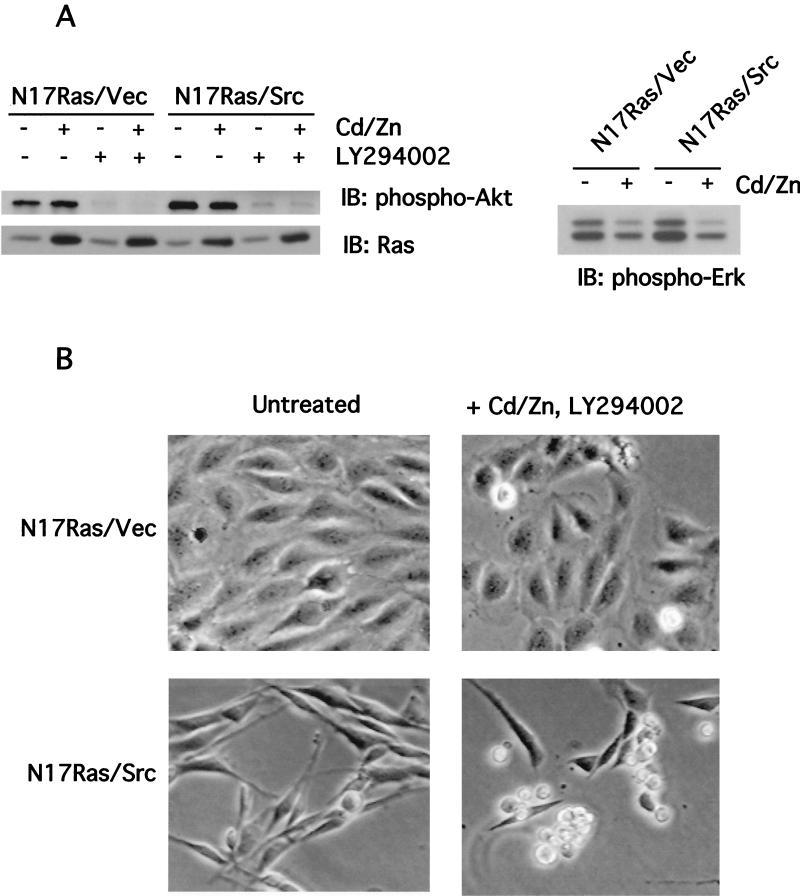
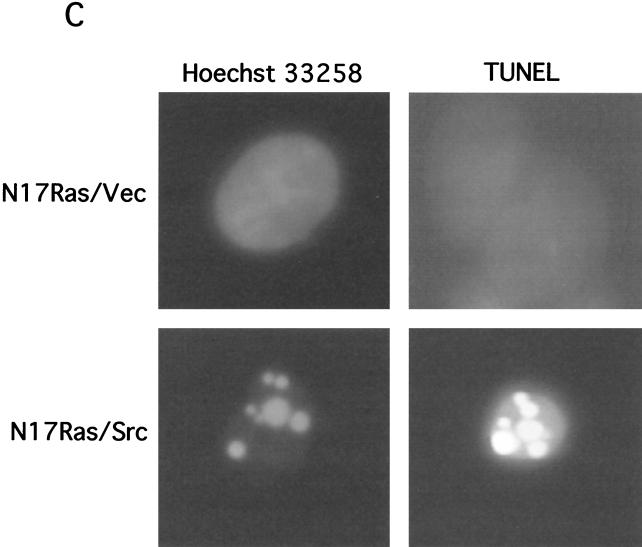
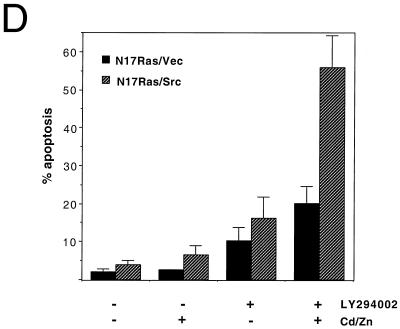
FIG. 1.
Inhibition of Ras and PI 3-kinase induces apoptosis in v-_src_-transformed Rat-2 fibroblasts. (A) Inhibition of Ras and PI 3-kinase signaling by N17Ras and LY294002. Rat-2 fibroblast cell lines expressing N17Ras under the control of the metal-inducible metallothionein promoter and coexpressing v-src (N17Ras/Src) were treated with CdCl2 (2 μM) and ZnCl2 (100 μM) for 18 h to induce the expression of N17Ras or with LY294002 (20 μM) for 1 h. For phospho-Erk analysis, N17Ras was induced in the absence of serum. Lysates were subjected to immunoblotting (IB) with anti-pan-Ras, anti-phospho-Akt, or anti-phospho-Erk antibodies. (B) Morphology of N17Ras/Vec and N17Ras/Src cells following inhibition of Ras and PI 3-kinase. Cells were treated with CdCl2 (2 μM), ZnCl2 (100 μM), and LY294002 (20 μM) for 18 h. The N17Ras/Src cells rounded up and detached from the tissue culture dish. (C) Apoptosis induced by inhibition of Ras and PI 3-kinase in N17Ras/Src cells. Pooled N17Ras/Vec and N17Ras/Src cells were stained with Hoechst 33258 to detect nuclear fragmentation. Cells were also examined by TUNEL assay to detect DNA nicking. Shown are representative nonapoptotic N17Ras/Vec cells and apoptotic N17Ras/Src cells. (D) Quantitation of apoptosis in N17Ras/Vec and N17Ras/Src cells. Cells were treated with CdCl2 (2 μM) and ZnCl2 (100 μM) and/or LY294002 (20 μM) for 18 h. The percentage of apoptotic cells was calculated as the ratio of nonadherent cells to total cells.
For experiments with caspase inhibitor Z-VAD-FMK, N17Ras/Src cells (200,000) were plated into six-well plates. CdCl2 (2 μM) and ZnCl2 (100 μM) were added to the medium for 18 h. Fresh medium containing CdCl2 and ZnCl2 was then added, and the cells were treated with 125 μM Z-VAD-FMK (Calbiochem) for 1 h before LY294002 (20 μM) was added. After 6 h the detached and adherent cells were collected and the percentages of detached apoptotic cells were determined as described above.
Expression of bcl-2.
N17Ras/Src cells were transiently transfected with pSFFV.neo/bcl-2 (4 μg) (or pSFFV.neo vector [4 μg] as a control), together with a vector expressing green fluorescent protein (GFP), pEGFP-C1 (0.4 μg) (Clontech), as a transfection marker by using Lipofectamine Plus reagent (Life Technologies, Inc.) according to the procedure recommended by the manufacturer. The transfection efficiency was approximately 5%. Twenty-four hours posttransfection, CdCl2 (2 μM), ZnCl2 (100 μM), and LY294002 (20 μM) were added to the medium to induce apoptosis. The detached and adherent cells were collected after 9 h, and nuclear staining was performed as described above. Successfully transfected cells were identified by the green autofluorescence of GFP using fluorescence microscopy, and the nuclear morphology of these cells was scored. The percentages of apoptotic transfected cells are reported.
Caspase 3 assays.
Caspase 3 activity in cell lysates was measured using colorimetric caspase 3 substrate DEVD-pNA (Calbiochem). N17Ras/Vec and N17Ras/Src cells were treated with CdCl2 (2 μM), ZnCl2 (100 μM), and LY294002 (20 μM) or with dimethyl sulfoxide (DMSO) carrier for 8 h, after which detached and adherent cells were harvested and pooled. The cells were resuspended in R&D Systems caspase-9 colorimetric assay kit (BF10100) cell lysis buffer and frozen at −20°C. Thawed lysates (50 μg of protein) were incubated at 37°C with 200 μM DEVD-pNA in the supplied reaction buffer in 96-well microtiter plates, and _A_405 was measured at 10-min intervals.
Cytochrome c localization.
N17Ras/Src cells were plated onto glass coverslips within six-well (35-mm-diameter) tissue culture plates (4 × 105 cells/well). CdCl2 (2 μM) and ZnCl2 (100 μM) were added to the medium. After 18 h, fresh medium containing CdCl2 (2 μM), ZnCl2 (100 μM), and LY294002 (20 μM) was added. The cells were then incubated for 3 h prior to fixation; this early time point was chosen because the cells had initiated apoptosis but were not yet detached. Cells were fixed in 4% formaldehyde for 10 min at room temperature and permeabilized for 5 min with 0.2% Triton X-100. The coverslips were then blocked with 10% goat serum-phosphate-buffered saline for 30 min at room temperature, followed by incubation with a monoclonal cytochrome c antibody (Pharmingen; 65981A) and a fluorescein isothiocyanate (FITC)-conjugated antimouse secondary antibody. Nuclei were visualized by DAPI (4′,6′-diamidino-α-phenylindole) staining. Immunofluorescence images were obtained using a Zeiss Axiophot microscope.
Mitochondrial membrane potential.
To assay cells for mitochondrial membrane potential, the fluorogenic mitochondrial dye rhodamine 123 (Molecular Probes, Eugene, Oreg.) was used. Briefly, 1.3 × 106 N17Ras/Src cells were plated onto 10-cm-diameter tissue culture plates. CdCl2 (2 μM) and ZnCl2 (100 μM) were added to the medium for 18 h. Then fresh medium containing CdCl2 (2 μM), ZnCl2 (100 μM), and LY294002 (20 μM) was added. After 0, 2, 4, and 6 h, the extent of apoptosis was determined by calculating the percentages of detached cells as described above. Then, for each time point the detached and adherent cells were combined, collected by centrifugation, and resuspended in F10–Dulbecco's modified Eagle medium–0.5% fetal calf serum. Rhodamine 123 was added to 5 μM, and cells were incubated in the dark at room temperature for 15 min prior to flow analysis using a Coulter flow cytometer.
Inhibition of p53 function.
Dominant-negative p53 mutant p53-DD, which contains the C-terminal oligomerization domain of p53, was used to inhibit p53 function. N17Ras/Src cells were transfected with pCMV/p53-DD or pCMV empty vector, together with pEGFP-C1. At 24 h posttransfection the cells were transferred to glass coverslips in six-well plates (450,000 cells per well), at which time CdCl2 (2 μM) and ZnCl2 (100 μM) were added to the medium. Twelve hours later LY294002 (20 μM) was added. After 5 h, the cells remaining on the coverslips were fixed, permeabilized as described above, and stained with Hoechst 33258. The extent of apoptosis in the GFP-positive transfected cells was quantified by scoring nuclear fragmentation. More than 95% of the GFP-positive cells were found to express high levels of p53-DD, as judged by immunostaining with anti-p53 antibody (FL-393; Santa Cruz Biotechnology). To confirm that p53-DD blocks the activity of wild-type p53 in N17Ras/Src cells, the ability of p53-DD to block apoptosis induced by ectopic expression of wild-type p53 was tested. Cells were cotransfected with pCMV/p53wt and either pCMV/p53-DD or pCMV empty vector. At 24 h posttransfection, adherent cells were stained with a mouse monoclonal antibody (SC-98; Santa Cruz Biotechnology) that recognizes an epitope present in the N terminus of p53 that is deleted in p53-DD. The nuclear morphology of those cells expressing p53wt was used to score apoptosis induced by p53wt. The total level of p53 expression was monitored by immunostaining with the FL-393 antibody.
RESULTS
Apoptosis is induced in v-_src_-transformed Rat-2 cells by inhibition of Ras and PI 3-kinase.
Since Ras and PI 3-kinase are known to generate survival signals, we investigated whether inhibition of these proteins would result in apoptosis of v-_src_-transformed cells. To examine the effects of inhibiting Ras signaling, we used a Rat-2 fibroblast cell line expressing the dominant-negative H-ras Asn-17 mutant, N17Ras, under the control of the metal-inducible metallothionein promoter. Two derivatives of this line were generated, one coexpressing v-Src, designated N17Ras/Src, and the other coexpressing the empty vector, designated N17Ras/Vec. Treatment of N17Ras/Src and N17Ras/Vec cell lines with CdCl2 and ZnCl2 resulted in the expression of N17Ras to levels that were two- to fourfold higher than that of endogenous Ras (Fig. 1A). The induction of N17Ras inhibited the activity of the mitogen-activated protein kinase Erk2 in both cell lines (Fig. 1A) (2). The specific inhibitor LY294002 was used to inhibit PI 3-kinase. Treatment of N17Ras/Vec and N17Ras/Src cells with LY294002 reduced PI 3-kinase signaling, as indicated by the reduced activity of its downstream effector, Akt (Fig. 1A); the low residual levels of Akt activation were comparable in the two cell lines.
Simultaneous inhibition of Ras and PI 3-kinase in N17Ras/Src cells resulted in extensive cell shrinkage and loss of adhesion of the cells to the tissue culture dish (Fig. 1B). The detached cells displayed fragmented nuclei and elevated levels of chromosomal DNA degradation, as determined by a TUNEL assay (Fig. 1C), indicating that these cells were apoptotic. Inhibition of Ras and PI 3-kinase in N17Ras/Src cells led to greater than 50% cell death after 18 h (Fig. 1D). Similar treatment of nontransformed N17Ras/Vec cells resulted in 2.5-fold-lower levels of apoptosis, demonstrating that v-src transformation sensitizes these cells to apoptosis. Inhibition of Ras alone resulted in little increase in apoptosis compared to that for the DMSO carrier control, and inhibition of PI 3-kinase alone induced only moderate levels of apoptosis. The synergy between N17Ras and LY294002 suggests that the survival signaling downstream of either Ras or PI 3-kinase is sufficient to suppress the apoptosis induced by v-Src. These results suggest that v-src transformation generates a proapoptotic signal that is normally suppressed through activation of Ras and PI 3-kinase. Interestingly, the reduction in survival signaling produced by serum starvation was not sufficient to induce apoptosis in v-_src_-transformed cells (data not shown) (33).
Caspase 3 activity is induced in v-_src_-mediated apoptosis.
In other systems apoptosis results from activation of executioner caspases such as caspase 3. This activation involves proteolytic cleavage of a procaspase precursor. To examine the role of caspase 3 in v-Src-induced apoptosis, we monitored the cleavage of pro-caspase 3 in vivo by Western blot analysis. As shown in Fig. 2A, inhibition of Ras and PI 3-kinase in N17Ras/Src cells resulted in substantial cleavage of pro-caspase 3, as detected by the appearance of the p20 large subunit. In addition, these same conditions resulted in cleavage of the known caspase 3 substrate PARP from the full-length 116-kDa polypeptide to the 85-kDa fragment (Fig. 2B). Only minor processing of caspase 3 and PARP was seen in nontransformed N17Ras/Vec cells, consistent with the lower level of apoptosis induced by inhibition of Ras and PI 3-kinase in these cells. To confirm these findings, we used a synthetic peptide substrate to assay the activity of caspase 3 in lysates of N17Ras/Vec and N17Ras/Src cells. No caspase 3 activity was detected in untreated N17Ras/Vec or N17Ras/Src cells (Fig. 2C). Inhibition of Ras and PI 3-kinase in N17Ras/Vec cells led to a moderate induction of caspase 3 activity, whereas similar treatment of N17Ras/Src cells resulted in a fourfold increase in caspase 3 activity (Fig. 2C). Taken together, these results suggest that inhibition of survival pathways leads to v-Src-dependent activation of caspase 3.
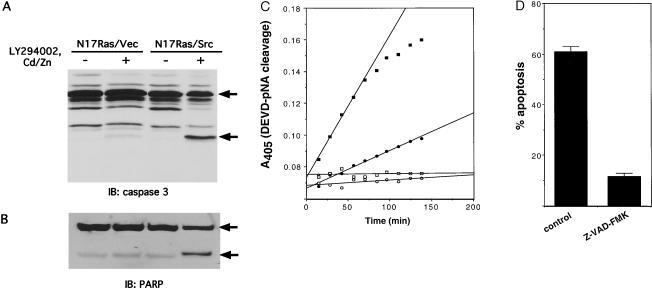
FIG. 2.
Caspase 3 mediates v-Src-induced apoptosis. (A) Cleavage of pro-caspase 3. Following N17Ras induction and inhibition of PI 3-kinase for 18 h, adherent and nonadherent N17Ras/Vec and N17Ras/Src cells were collected, pooled, and lysed. Lysates were examined by immunoblotting (IB) with anti-caspase 3 antibody. The positions of the 32-kDa pro-caspase 3 precursor and the 20-kDa active caspase 3 subunit are indicated. (B) Cleavage of PARP. Lysates were prepared as for panel A and analyzed by immunoblotting with anti-PARP antibody. The positions of intact PARP (116 kDa) and the 85-kDa fragment are indicated. (C) Activity of caspase 3. N17Ras/Vec and N17Ras/Src cells were treated either with DMSO carrier (open circles and squares, respectively) or with CdCl2 (2 μM), ZnCl2 (100 μM), and LY294002 (20 μM) (solid circles and squares, respectively) for 8 h. Lysates were prepared from pooled adherent and nonadherent cells and incubated at 37°C with the colorimetric caspase 3 substrate, DEVD-pNA. _A_405 was measured at 10-min intervals. (D) Effect of caspase inhibitor Z-VAD-FMK on induction of apoptosis by v-Src. N17Ras/Src cells were treated with CdCl2 (2 μM) and ZnCl2 (100 μM) for 18 h. Cells were then pretreated with either Z-VAD-FMK (125 μM) or DMSO carrier for 1 h before LY294002 (20 μM) was added. After 6 h the percentage of apoptosis was quantified as for Fig. 1.
To determine if caspase activation is required for v-Src-dependent apoptosis, we examined the effect of the broad-specificity caspase inhibitor Z-VAD-FMK. As shown in Fig. 2D, pretreatment of N17Ras/Src cells with Z-VAD-FMK almost completely blocked the apoptosis induced by inhibition of Ras and PI 3-kinase. The Z-VAD-FMK-treated N17Ras/Src cells remained attached to the tissue culture dish following inhibition of Ras and PI 3-kinase inhibition and did not undergo nuclear fragmentation (data not shown). However their morphology was notably different from that of untreated cells. The Z-VAD-FMK-treated cells were rounded, displayed extensive membrane blebbing, and could not reattach on subculture (data not shown). We conclude that caspase activation is necessary for v-Src-dependent apoptosis but that the cells die even when caspase activation is blocked. These findings are consistent with the report of McCarthy et al. (46), who showed that inhibition of caspases delayed the onset of _myc_-induced apoptosis but failed to protect cellular viability.
Involvement of mitochondria in v-Src-induced apoptosis.
To determine if release of cytochrome c from mitochondria occurs during the course of v-Src-induced apoptosis, we examined the subcellular location of cytochrome c following inhibition of Ras and PI 3-kinase by immunostaining with an anti-cytochrome c antibody. In untreated N17Ras/Src cells cytochrome c exhibited the punctate distribution characteristic of mitochondrial localization (Fig. 3). However, N17Ras/Src cells that were undergoing apoptosis following inhibition of Ras and PI 3-kinase, as judged by their fragmented nuclear morphology, displayed a diffuse pattern of cytochrome c staining (Fig. 3), while those cells that were not undergoing apoptosis did not. These results indicate that cytochrome c release occurs following inhibition of survival signaling in cells expressing v-Src.
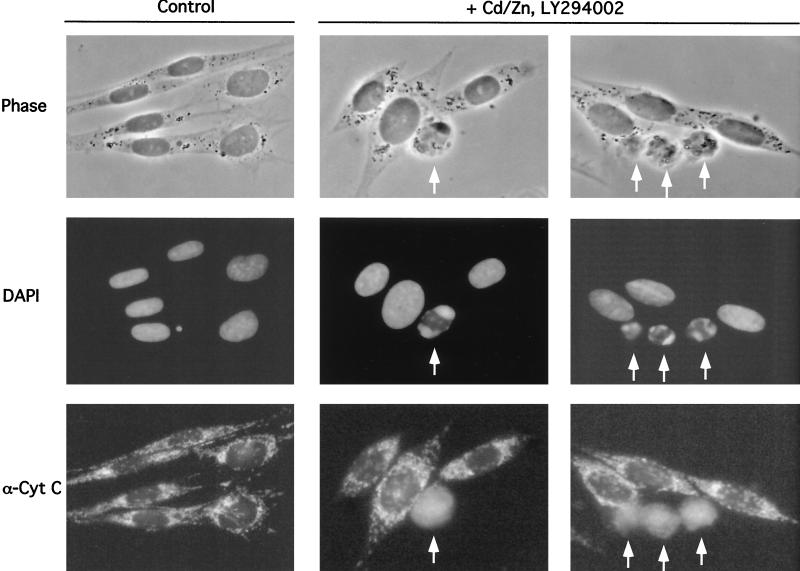
FIG. 3.
Inhibition of Ras and PI 3-kinase results in cytochrome c release in N17Ras/Src cells. (A) N17Ras/Src cells were incubated with CdCl2 (2 μM) and ZnCl2 (100 μM) for 18 h. LY294002 (20 μM) was added, and the cells were fixed after 3 h. Control N17Ras/Src cells were treated with DMSO vector alone. The cells were stained with a monoclonal anti-cytochrome c antibody and a FITC-conjugated antimouse secondary antibody. Nuclei were stained with DAPI. At this point in the induction of apoptosis, 10.4% of the treated cells displayed diffuse cytochrome c localization and 3.1% displayed fragmented nuclear morphology, compared to 0.4 and 0.25%, respectively, for control cells. Arrows indicate apoptotic cells with fragmented nuclei and diffuse cytochrome c localization.
We used the fluorescent dye rhodamine 123 to examine changes in the mitochondrial membrane potential in N17Ras/Src cells following inhibition of Ras and PI 3-kinase. Rhodamine 123 uptake was quantitated by flow cytometry (Fig. 4A). Untreated N17Ras/Src cells displayed a uniform distribution of cells with high rhodamine 123 content, indicative of healthy respiring mitochondria (Fig. 4A). Treatment of cells with carbonyl cyanide _p_-(trifluoromethoxy)-phenyl hydrazone (FCCP), an uncoupler of oxidative phosphorylation which dissipates the proton gradient across the inner membrane, substantially reduced rhodamine 123 uptake (data not shown), confirming that rhodamine 123 uptake is dependent on the mitochondrial membrane potential. Following inhibition of Ras and PI 3-kinase we observed the appearance of a population of cells exhibiting greatly reduced rhodamine 123 uptake, representing cells with decreased mitochondrial membrane potential. After 6 h, these cells represented approximately 40% of the total cell population, in agreement with the level of apoptosis determined by cell detachment, nuclear fragmentation, and TUNEL assays. Pretreatment with Z-VAD-FMK, which blocked apoptosis, did not block the loss of mitochondrial membrane potential (data not shown). Thus, inhibition of Ras and PI 3-kinase leads to mitochondrial membrane potential breakdown in v-_src_-transformed cells.
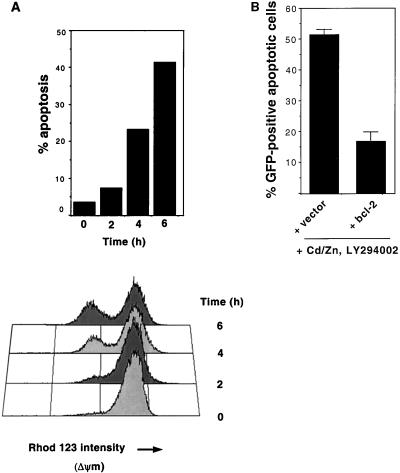
FIG. 4.
Role of mitochondria in v-Src-induced apoptosis. (A) Breakdown of the inner mitochondrial membrane potential in v-_src_-transformed cells following inhibition of Ras and PI 3-kinase. At the indicated times after N17Ras induction and inhibition of PI 3-kinase, detached and adherent cells were collected and the percentages of nonadherent cells were determined (top). Pooled cells were incubated with rhodamine 123 (5 μM) in the dark for 15 min. Cells were then analyzed by flow cytometry (bottom). (B) Ectopic expression of bcl-2 blocks apoptosis induced by inhibition of Ras and PI 3-kinase. N17Ras/Src cells were transfected with an empty vector (pSFFV.neo) or a vector containing the bcl-2 gene (pSFFV.neo/bcl-2), along with pEGFP-C1 as a transfection marker. Twenty-four hours after transfection, CdCl2 (2 μM), ZnCl2 (100 μM), and LY294002 (20 μM) were added to the medium. Adherent and nonadherent cells were collected and pooled after 9 h. Apoptosis was quantified as described for Fig. 1.
The antiapoptotic gene bcl-2 has been shown to block apoptosis induced by a number of stimuli, including the expression of oncogenes such as c-myc and E1A (23, 55, 66). Bcl-2 is localized to the outer mitochondrial membrane, and overexpression of Bcl-2 blocks cytochrome c release (39, 71). We therefore examined the effect of ectopic Bcl-2 expression on v-Src-dependent apoptosis. N17Ras/Src cells were transfected with a bcl-2 expression vector prior to N17Ras induction and inhibition of PI 3-kinase. Overexpression of Bcl-2 protected the N17Ras/Src cells from apoptosis (Fig. 4B). As the antiapoptotic effects of Bcl-2 are dependent on its mitochondrial localization, the ability of Bcl-2 to block v-Src-induced apoptosis provides further evidence that v-Src-dependent apoptosis is mediated by the mitochondrial pathway.
v-Src induces apoptosis independently of p53.
The tumor suppressor p53 plays an important role in mediating apoptosis in response to oncogenes such as c-myc and E1A (18, 28, 65). To determine if p53 is also involved in v-Src-induced apoptosis, we first examined whether the level of p53 protein in N17Ras/Src cells is elevated following inhibition of Ras and PI 3-kinase. As the level of p53 is known to be regulated through its degradation by the ubiquitin-proteasome machinery, the proteasome inhibitor MG115 was used as a positive control. MG115-treated cells accumulated significantly higher levels of p53 than control cells and underwent extensive apoptosis (Fig. 5A). In contrast, inhibition of Ras and PI 3-kinase in N17Ras/Src cells, while inducing comparable levels of apoptosis, did not lead to a detectable increase in the level of p53 protein (Fig. 5A). Thus v-Src-induced apoptosis occurs without detectable p53 accumulation.
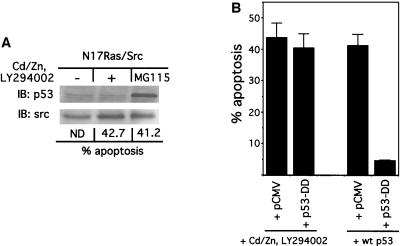
FIG. 5.
v-Src-induced apoptosis occurs independently of p53 function. (A) Immunoblot (IB) analysis of p53 protein levels. N17Ras/Src cells were treated with CdCl2 (2 μM), ZnCl2 (100 μM), and LY294002 (20 μM) for 12 h. Adherent and nonadherent cells were collected, and apoptosis was quantified as described in the legend to Fig. 1. Pooled cells were lysed, and lysates were subjected to immunoblot analysis using a monoclonal anti-p53 antibody. To confirm equal loading, the blot was reprobed with an antibody against Src. As a positive control for p53 protein accumulation, N17Ras/Src cells were treated with the proteasome inhibitor MG115 (25 μM) for 5 h, after which cells were processed as described above. ND, not determined. (B) Dominant-negative p53 does not block v-Src-induced apoptosis. (Left two bars) N17Ras/Src cells were transfected with either an empty pCMV vector (along with pEGFP-C1) or with pCMV/p53-DD, encoding a dominant-negative p53 mutant. At 24 h after transfection, cells were transferred onto coverslips. CdCl2 (2 μM) and ZnCl2 (100 μM) were added to the medium. After 12 h of incubation, LY294002 (20 μM) was added. Following 5 h of further incubation, the cells were fixed and permeabilized. Cells expressing p53-DD were identified using an FITC-conjugated antibody. For the pCMV-transfected cells, successfully transfected cells were identified by GFP fluorescence. The extent of apoptosis in transfected cells was quantitated by scoring cells with fragmented nuclear morphology. (Right two bars) The functionality of dominant-negative p53-DD was demonstrated by its ability to block apoptosis induced by expression of wild-type (wt) p53. N17Ras/Src cells were cotransfected with pCMV/p53wt and either pCMV vector or pCMV/p53-DD. At 24 h posttransfection adherent cells were stained with a monoclonal p53 antibody which recognizes wild-type p53 but not p53-DD, and the extent of apoptosis in these p53wt-expressing cells was quantified by scoring fragmented nuclear morphology. The reported values are the means of four independent experiments, with error bars showing the standard deviations. Comparable levels of p53-DD were expressed under both conditions (not shown).
To determine if p53 function is required for v-Src-induced apoptosis, we used a dominant-negative p53 mutant, p53-DD, to block the function of endogenous p53. p53-DD contains the first 14 amino acids of p53 fused to a segment containing the oligomerization domain (residues 302 to 390 of mouse p53). Expression of this construct has been shown to block p53 function by preventing the tetramerization required for p53 function (58). In N17Ras/Src cells p53-DD inhibited apoptosis induced by ectopic expression of wild-type p53 (Fig. 5B), confirming that this mutant acts in a dominant-negative manner in these cells. In contrast, the expression of p53-DD in N17Ras/Src cells failed to block the apoptosis induced by the inhibition of Ras and PI 3-kinase (Fig. 5B). These results indicate that apoptosis induced by v-Src occurs by a p53-independent mechanism.
v-Src signaling prevents p27 accumulation following inhibition of Ras and PI 3-kinase.
Deregulated cell cycle progression has increasingly been linked with apoptosis in a number of different systems (27). To address the mechanism of v-_src_-induced apoptosis, we examined the effects of inhibition of Ras and PI 3-kinase on the levels of cyclin-dependent kinase inhibitor p27 in N17Ras/Vec and N17Ras/Src cells. v-Src has previously been shown to perturb cell cycle regulation through downregulation of p27 (34). We found that inhibition of Ras and PI 3-kinase in N17Ras/Vec cells led to the accumulation of p27 protein to levels similar to those induced by serum starvation (Fig. 6). However, significant p27 accumulation was not detected in N17Ras/Src cells following inhibition of Ras and PI 3-kinase (Fig. 6). Thus, persistent downregulation of p27 in the absence of survival signaling may contribute to the apoptotic signal generated by v-Src.
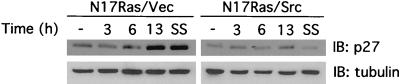
FIG. 6.
v-Src downregulates p27 following inhibition of Ras and PI 3-kinase. N17Ras/Vec and N17Ras/Src cells were treated with CdCl2 (2 μM) and ZnCl2 (100 μM) for 18 h. Cells were then treated with Z-VAD-FMK (100 μM for 1 h (to prevent apoptotic cells from detaching) before LY294002 (20 μM) was added. After 3, 6, and 13 h the cells were lysed and subjected to immunoblot (IB) analysis with an anti-p27 antibody. Control cells (−) were treated with DMSO. To confirm equal loading the blot was probed with an antitubulin antibody. To determine the effect of serum starvation on p27 accumulation, cells were incubated in serum-free medium for 18 h before lysing (SS).
DISCUSSION
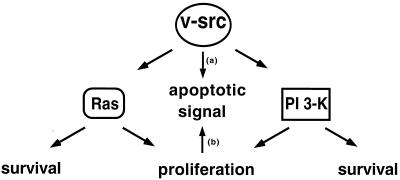
Evasion of apoptosis through activation of survival signaling has been shown to be a key determinant of tumorigenicity (47). To further explore the role of survival signaling in neoplastic transformation, we examined the significance of survival signaling in transformation by the oncogene v-src. Ras and PI 3-kinase have been shown to play important roles in mediating transformation by v-Src, and both Ras and PI 3-kinase regulate cell survival. We have shown here that simultaneous inhibition of Ras and PI 3-kinase results in high levels of apoptosis in v-_src_-transformed Rat-2 fibroblasts but considerably lower levels of apoptosis in nontransformed cells. These results indicate that v-Src generates a proapoptotic signal that under normal growth conditions is counterbalanced by survival signaling through Ras and PI 3-kinase but that is revealed when the activities of these proteins are inhibited (Fig. 7).
FIG. 7.
Possible mechanisms by which v-Src generates a proapoptotic signal. In one model, the proapoptotic signal is generated by a pathway that remains to be identified (arrow a). In the second model, persistent proliferative signals generated by Ras and PI 3-kinase produce the apoptotic signal following inhibition of these pathways (arrow b). See text for details.
The activation of both pro- and antiapoptotic pathways by individual signaling proteins or oncogenes may represent a general phenomenon. The paradigm of this phenomenon is signaling by death receptors (4, 43). The cell death receptor pathway is activated through ligand binding to the TNF family of receptors (TNFR), leading to recruitment and activation of caspase 8 through adapter proteins such as TRADD and FADD. At the same time the death receptors also activate antiapoptotic signaling via the same set of adapter proteins; thus, via the serine/threonine kinase RIP, FADD activates NF-κB, which in turn activates the expression of antiapoptotic genes such as bcl-2, bcl-xL, XIAP, TRAF1, TRAF2, c-IAP1, c-IAP2, and probably others (14, 16, 67, 73). Presumably the activation of both pro- and antiapoptotic pathways provides more opportunities for regulation. A similar situation appears to hold for oncoproteins such as activated Ras and v-Abl. Overexpression of oncogenic Ras (RasV12) elicits an apoptotic response in rat embryo fibroblasts, which can be suppressed by coexpression of activated Rac and activation of a survival pathway involving NF-κB (35). Likewise Raf provides protection from apoptosis induced by v-Abl signaling, as inhibition of Raf leads to apoptosis in v-_abl_-transformed cells (69). The results reported here, in conjunction with these earlier studies, suggest that oncoproteins, such as Src, Ras, and Abl, which regulate cytoplasmic signaling pathways, have both pro- and antiapoptotic effects and that the balance between the proapoptotic and antiapoptotic signals generated by these oncoproteins determines whether transformation or apoptosis occurs. The generation of a proapoptotic signal by v-Src may explain the sensitivity of v-_src_-transformed cells to apoptosis induced by various drug treatments (15, 44).
For v-Src, at least three signaling pathways appear to promote protection from apoptosis. In v-_src_-transformed Rat-2 cells, inhibition of Ras signaling alone had essentially no effect on cell viability, consistent with the dispensability of Ras for v-src transformation of these cells (2). Inhibition of PI 3-kinase alone induced modest levels of apoptosis. The synergistic apoptotic effect of simultaneously inhibiting Ras and PI 3-kinase suggests that both of these proteins mediate survival signals and that the survival signaling downstream of each is sufficient to override the proapoptotic signal generated by v-Src. Inhibition of Ras did not significantly affect PI 3-kinase activity (Fig. 1). This suggests that in these cells Ras generates an additional survival signal (or signals) that is distinct from activation of the PI 3-kinase/Akt pathway. This is consistent with our finding that the MEK inhibitor U01206 can also synergize with LY294002 in inducing apoptosis of v-_src_-transformed Rat-2 cells, although the effect of U01206 is not as dramatic as the effect of N17Ras induction (B. Webb, E. Green, and G. S. Martin, unpublished data). It should be noted that in Rat-2 cells constitutive expression of v-src results in at most a very slight increase in Ras-GTP level (2), so that the effect of N17Ras is primarily to reduce the basal level of signaling from Ras. While this paper was under review, Johnson et al. (33) reported that inhibition of PI 3-kinase induces apoptosis in serum-starved Src-transformed fibroblasts, while Odajima et al. (49) reported that inhibition of Ras induces apoptosis in Src-transformed Ba/F3 cells deprived of interleukin-3. Interestingly, inhibition of Ras and PI 3-kinase in v-_src_-transformed chicken embryo fibroblasts reverts morphological transformation rather than inducing apoptosis (53); it may be that other survival pathways are active in chicken embryo fibroblasts or that primary cells are more resistant to oncogene-induced apoptosis. In addition to Ras and PI 3-kinase, Stat3 mediates an antiapoptotic signal (9, 13, 37). Stat3 is activated by Src, either by direct tyrosine phosphorylation and serine phosphorylation by the p38 and JNK kinases (63) or through Btk-related kinase Etk (62). Stat3 activation is necessary for Src transformation of Rat-2 cells (8, 64), and Stat3 mediates an antiapoptotic signal through induction of bcl-xL (48). The present findings, in conjunction with these other reports, indicate that Ras, PI 3-kinase, and Stat3 all contribute to protection against v-Src-induced apoptosis.
Our finding that v-Src-dependent apoptosis is accompanied by loss of mitochondrial membrane potential and cytochrome c release and is inhibited by overexpression of bcl-2 indicates that, like the apoptosis induced by c-myc and E1A, v-Src-induced apoptosis is mediated by the mitochondrial pathway. However these observations do not exclude a role for the death receptor pathway, since the two pathways appear to interact in a variety of ways (30, 41). In particular, caspase 8 activated by the death receptor pathway can cleave Bid to generate a cleaved Bid product that induces cytochrome c release (42). Thus it is possible that the primary effect of Src might be on the death receptor pathway and that the mitochondrial pathway might be activated as a secondary consequence of this change; in this case the mitochondrial pathway might be required only to amplify the apoptotic signal.
The nature of the proapoptotic pathway activated by v-Src remains to be determined. Two models can be entertained (Fig. 7). In the first, the pro- and antiapoptotic signals are mediated by distinct pathways. Thus, while the antiapoptotic signal is generated by Ras, PI 3-kinase, and Stat3, the proapoptotic signal would be generated by another pathway that remains to be identified (Fig. 7). In the second model, the Ras and PI 3-kinase pathways mediate both pro- and antiapoptotic effects. According to this model, the proliferative signals generated by these pathways would lead to a proapoptotic signal (Fig. 7) that is more stable than the antiapoptotic signals. Thus, following inhibition of Ras and PI 3-kinase, the proapoptotic signals would persist longer than the antiapoptotic signals, leaving the proapoptotic signals unchecked to promote apoptosis.
Our results indicate that v-Src expression can downregulate the CDK inhibitor p27 even when Ras and PI 3-kinase signaling is suppressed, suggesting that deregulation of the cell cycle machinery by Src may be involved in the apoptotic response. One way in which proliferative signals can generate an apoptotic response is through p19ARF and tumor suppressor p53 (5). Cell cycle-regulated transcription factor E2F-1 and c-myc both induce the expression of p19ARF, which in turn inhibits the function of Mdm2 and stabilizes p53, leading to p53-dependent apoptosis (5, 72). However we found that inhibition of p53 function by a dominant-negative p53 mutant failed to block v-Src-induced apoptosis and that p53 did not accumulate during the course of v-Src-induced apoptosis. This indicates that, unlike c-myc, v-Src activates a cell death pathway that is independent of p53. Interestingly, the apoptosis induced by oncogenic Ras is also p53 independent (35). One potential mediator of p53-independent apoptosis is the p53-related gene p73, which has been shown to promote cisplatin-induced apoptosis independently of p53 (25). Another is E2F-1, which was recently shown to induce p53-independent apoptosis through the death receptor pathway by downregulating the expression of TRAF2 (54). As E2F-1 has been shown to be activated by v-Src and is required for v-Src-induced proliferation (51), it is possible that E2F-1 and the death receptor pathway are involved in mediating the apoptosis induced by v-Src.
Mutational activation of Src occurs in some metastatic colon cancers (31), and overexpression or activation of Src is believed to contribute to the progression of breast and colon cancer (6). The activation of Src may contribute to tumor progression by activating both proliferative and antiapoptotic pathways. It will be important to determine whether inhibition of survival pathways reveals a Src-dependent proapoptotic signal in these cells.
ACKNOWLEDGMENTS
We thank Eric Martens and Yen Sheng Hsu for technical assistance, Moshe Oren, Astar Winoto, and Gary Firestone for reagents, and members of the Martin laboratory for helpful comments on the manuscript.
This work was supported by NIH grant CA17542 and by the facilities of the Cancer Research Laboratory. B.L.W. was supported by NRSA fellowship F32 CA77915-03.
REFERENCES
- 1.Adams J M, Cory S. The Bcl-2 protein family: arbiters of cell survival. Science. 1998;281:1322–1326. doi: 10.1126/science.281.5381.1322. [DOI] [PubMed] [Google Scholar]
- 2.Aftab D T, Kwan J, Martin G S. Ras-independent transformation by v-Src. Proc Natl Acad Sci USA. 1997;94:3028–3033. doi: 10.1073/pnas.94.7.3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson S M, Carroll P M, Lee F D. Abrogation of IL-3 dependent growth requires a functional v-src gene product: evidence for an autocrine growth cycle. Oncogene. 1990;5:317–325. [PubMed] [Google Scholar]
- 4.Ashkenazi A, Dixit V M. Death receptors: signaling and modulation. Science. 1998;281:1305–1308. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- 5.Bates S, Phillips A C, Clark P A, Stott F, Peters G, Ludwig R L, Vousden K H. p14ARF links the tumour suppressors RB and p53. Nature. 1998;395:124–125. doi: 10.1038/25867. [DOI] [PubMed] [Google Scholar]
- 6.Biscardi J S, Tice D A, Parsons S J. c-Src, receptor tyrosine kinases, and human cancer. Adv Cancer Res. 1999;76:61–119. doi: 10.1016/s0065-230x(08)60774-5. [DOI] [PubMed] [Google Scholar]
- 7.Bonni A, Brunet A, West A E, Datta S R, Takasu M A, Greenberg M E. Cell survival promoted by the Ras-MAPK signaling pathway by transcription-dependent and -independent mechanisms. Science. 1999;286:1358–1362. doi: 10.1126/science.286.5443.1358. [DOI] [PubMed] [Google Scholar]
- 8.Bromberg J F, Horvath C M, Besser D, Lathem W W, Darnell J E., Jr Stat3 activation is required for cellular transformation by v-src. Mol Cell Biol. 1998;18:2553–2558. doi: 10.1128/mcb.18.5.2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bromberg J F, Wrzeszczynska M H, Devgan G, Zhao Y, Pestell R G, Albanese C, Darnell J E., Jr Stat3 as an oncogene. Cell. 1999;98:295–303. doi: 10.1016/s0092-8674(00)81959-5. [DOI] [PubMed] [Google Scholar]
- 10.Brunet A, Bonni A, Zigmond M J, Lin M Z, Juo P, Hu L S, Anderson M J, Arden K C, Blenis J, Greenberg M E. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 11.Budihardjo I, Oliver H, Lutter M, Luo X, Wang X. Biochemical pathways of caspase activation during apoptosis. Annu Rev Cell Dev Biol. 1999;15:269–290. doi: 10.1146/annurev.cellbio.15.1.269. [DOI] [PubMed] [Google Scholar]
- 12.Cardone M H, Roy N, Stennicke H R, Salvesen G S, Franke T F, Stanbridge E, Frisch S, Reed J C. Regulation of cell death protease caspase-9 by phosphorylation. Science. 1998;282:1318–1321. doi: 10.1126/science.282.5392.1318. [DOI] [PubMed] [Google Scholar]
- 13.Catlett-Falcone R, Landowski T H, Oshiro M M, Turkson J, Levitzki A, Savino R, Ciliberto G, Moscinski L, Fernández-Luna J L, Nuñez G, Dalton W S, Jove R. Constitutive activation of Stat3 signaling confers resistance to apoptosis in human U266 myeloma cells. Immunity. 1999;10:105–115. doi: 10.1016/s1074-7613(00)80011-4. [DOI] [PubMed] [Google Scholar]
- 14.Chen C, Edelstein L C, Gélinas C. The Rel/NF-B family directly activates expression of the apoptosis inhibitor Bcl-xL. Mol Cell Biol. 2000;20:2687–2695. doi: 10.1128/mcb.20.8.2687-2695.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen G, Shu J, Stacey D W. Oncogenic transformation potentiates apoptosis, S-phase arrest and stress-kinase activation by etoposide. Oncogene. 1997;15:1643–1651. doi: 10.1038/sj.onc.1201347. [DOI] [PubMed] [Google Scholar]
- 16.Chu Z L, McKinsey T A, Liu L, Gentry J J, Malim M H, Ballard D W. Suppression of tumor necrosis factor-induced cell death by inhibitor of apoptosis c-IAP2 is under NF-kappaB control. Proc Natl Acad Sci USA. 1997;94:10057–10062. doi: 10.1073/pnas.94.19.10057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Datta S R, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg M E. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 18.Debbas M, White E. Wild-type p53 mediates apoptosis by E1A which is inhibited by E1B. Genes Dev. 1993;7:546–554. doi: 10.1101/gad.7.4.546. [DOI] [PubMed] [Google Scholar]
- 19.Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher A M. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature. 1999;399:601–605. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- 20.Evan G. Cancer—a matter of life and cell death. Int J Cancer. 1997;71:709–711. doi: 10.1002/(sici)1097-0215(19970529)71:5<709::aid-ijc2>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 21.Evan G, Littlewood T. A matter of life and cell death. Science. 1998;281:1317–1322. doi: 10.1126/science.281.5381.1317. [DOI] [PubMed] [Google Scholar]
- 22.Evan G I, Wyllie A H, Gilbert C S, Littlewood T D, Land H, Brooks M, Waters C M, Penn L Z, Hancock D C. Induction of apoptosis in fibroblasts by c-myc protein. Cell. 1992;69:119–128. doi: 10.1016/0092-8674(92)90123-t. [DOI] [PubMed] [Google Scholar]
- 23.Fanidi A, Harrington E A, Evan G I. Cooperative interaction between c-myc and bcl-2 proto-oncogenes. Nature. 1992;359:554–556. doi: 10.1038/359554a0. [DOI] [PubMed] [Google Scholar]
- 24.Fulton D, Gratton J P, McCabe T J, Fontana J, Fujio Y, Walsh K, Franke T F, Papapetropoulos A, Sessa W C. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature. 1999;399:597–601. doi: 10.1038/21218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gong J G, Costanzo A, Yang H Q, Melino G, Kaelin W G, Jr, Levrero M, Wang J Y. The tyrosine kinase c-Abl regulates p73 in apoptotic response to cisplatin-induced DNA damage. Nature. 1999;399:806–809. doi: 10.1038/21690. [DOI] [PubMed] [Google Scholar]
- 26.Green D R, Reed J C. Mitochondria and apoptosis. Science. 1998;281:1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- 27.Guo M, Hay B A. Cell proliferation and apoptosis. Curr Opin Cell Biol. 1999;11:745–752. doi: 10.1016/s0955-0674(99)00046-0. [DOI] [PubMed] [Google Scholar]
- 28.Hermeking H, Eick D. Mediation of c-Myc-induced apoptosis by p53. Science. 1994;265:2091–2093. doi: 10.1126/science.8091232. [DOI] [PubMed] [Google Scholar]
- 29.Hueber A O, Evan G I. Traps to catch unwary oncogenes. Trends Genet. 1998;14:364–367. doi: 10.1016/s0168-9525(98)01520-0. [DOI] [PubMed] [Google Scholar]
- 30.Hueber A O, Zörnig M, Lyon D, Suda T, Nagata S, Evan G I. Requirement for the CD95 receptor-ligand pathway in c-Myc-induced apoptosis. Science. 1997;278:1305–1309. doi: 10.1126/science.278.5341.1305. [DOI] [PubMed] [Google Scholar]
- 31.Irby R B, Mao W, Coppola D, Kang J, Loubeau J M, Trudeau W, Karl R, Fujita D J, Jove R, Yeatman T J. Activating SRC mutation in a subset of advanced human colon cancers. Nat Genet. 1999;21:187–190. doi: 10.1038/5971. [DOI] [PubMed] [Google Scholar]
- 32.Ishizaki Y, Cheng L, Mudge A W, Raff M C. Programmed cell death by default in embryonic cells, fibroblasts, and cancer cells. Mol Biol Cell. 1995;6:1443–1458. doi: 10.1091/mbc.6.11.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johnson D, Agochiya M, Samejima K, Earnshaw W, Frame M, Wyke J. Regulation of both apoptosis and cell survival by the v-Src oncoprotein. Cell Death Differ. 2000;7:685–696. doi: 10.1038/sj.cdd.4400700. [DOI] [PubMed] [Google Scholar]
- 34.Johnson D, Frame M C, Wyke J A. Expression of the v-Src oncoprotein in fibroblasts disrupts normal regulation of the CDK inhibitor p27 and inhibits quiescence. Oncogene. 1998;16:2017–2028. doi: 10.1038/sj.onc.1201727. [DOI] [PubMed] [Google Scholar]
- 35.Joneson T, Bar-Sagi D. Suppression of Ras-induced apoptosis by the Rac GTPase. Mol Cell Biol. 1999;19:5892–5901. doi: 10.1128/mcb.19.9.5892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kane L P, Shapiro V S, Stokoe D, Weiss A. Induction of NF-kappaB by the Akt/PKB kinase. Curr Biol. 1999;9:601–604. doi: 10.1016/s0960-9822(99)80265-6. [DOI] [PubMed] [Google Scholar]
- 37.Karni R, Jove R, Levitzki A. Inhibition of pp60c-Src reduces Bcl-XL expression and reverses the transformed phenotype of cells overexpressing EGF and HER-2 receptors. Oncogene. 1999;18:4654–4662. doi: 10.1038/sj.onc.1202835. [DOI] [PubMed] [Google Scholar]
- 38.Kennedy S G, Wagner A J, Conzen S D, Jordán J, Bellacosa A, Tsichlis P N, Hay N. The PI 3-kinase/Akt signaling pathway delivers an anti-apoptotic signal. Genes Dev. 1997;11:701–713. doi: 10.1101/gad.11.6.701. [DOI] [PubMed] [Google Scholar]
- 39.Kluck R M, Bossy-Wetzel E, Green D R, Newmeyer D D. The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science. 1997;275:1132–1136. doi: 10.1126/science.275.5303.1132. [DOI] [PubMed] [Google Scholar]
- 40.Kops G J, de Ruiter N D, De Vries-Smits A M, Powell D R, Bos J L, Burgering B M. Direct control of the Forkhead transcription factor AFX by protein kinase B. Nature. 1999;398:630–634. doi: 10.1038/19328. [DOI] [PubMed] [Google Scholar]
- 41.Kuwana T, Smith J J, Muzio M, Dixit V, Newmeyer D D, Kornbluth S. Apoptosis induction by caspase-8 is amplified through the mitochondrial release of cytochrome c. J Biol Chem. 1998;273:16589–16594. doi: 10.1074/jbc.273.26.16589. [DOI] [PubMed] [Google Scholar]
- 42.Li H, Zhu H, Xu C J, Yuan J. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell. 1998;94:491–501. doi: 10.1016/s0092-8674(00)81590-1. [DOI] [PubMed] [Google Scholar]
- 43.Liu Z G, Hsu H, Goeddel D V, Karin M. Dissection of TNF receptor 1 effector functions: JNK activation is not linked to apoptosis while NF-kappaB activation prevents cell death. Cell. 1996;87:565–576. doi: 10.1016/s0092-8674(00)81375-6. [DOI] [PubMed] [Google Scholar]
- 44.Lu X, Fairbairn D W, Bradshaw W S, O'Neill K L, Ewert D L, Simmons D L. NSAID-induced apoptosis in Rous sarcoma virus-transformed chicken embryo fibroblasts is dependent on v-src and c-myc and is inhibited by bcl-2. Prostaglandins. 1997;54:549–568. doi: 10.1016/s0090-6980(97)00125-1. [DOI] [PubMed] [Google Scholar]
- 45.Marte B M, Downward J. PKB/Akt: connecting phosphoinositide 3-kinase to cell survival and beyond. Trends Biochem Sci. 1997;22:355–358. doi: 10.1016/s0968-0004(97)01097-9. [DOI] [PubMed] [Google Scholar]
- 46.McCarthy N J, Whyte M K, Gilbert C S, Evan G I. Inhibition of Ced-3/ICE-related proteases does not prevent cell death induced by oncogenes, DNA damage, or the Bcl-2 homologue Bak. J Cell Biol. 1997;136:215–227. doi: 10.1083/jcb.136.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Naik P, Karrim J, Hanahan D. The rise and fall of apoptosis during multistage tumorigenesis: down-modulation contributes to tumor progression from angiogenic progenitors. Genes Dev. 1996;10:2105–2116. doi: 10.1101/gad.10.17.2105. [DOI] [PubMed] [Google Scholar]
- 48.Nielsen M, Kaestel C G, Eriksen K W, Woetmann A, Stokkedal T, Kaltoft K, Geisler C, Röpke C, Odum N. Inhibition of constitutively activated Stat3 correlates with altered Bcl-2/Bax expression and induction of apoptosis in mycosis fungoides tumor cells. Leukemia. 1999;13:735–738. doi: 10.1038/sj.leu.2401415. [DOI] [PubMed] [Google Scholar]
- 49.Odajima J, Matsumura I, Sonoyama J, Daino H, Kawasaki A, Tanaka H, Inohara N, Kitamura T, Downward J, Nakajima K, Hirano T, Kanakura Y. Full oncogenic activities of v-Src are mediated by multiple signaling pathways. Ras as an essential mediator for cell survival. J Biol Chem. 2000;275:24096–24105. doi: 10.1074/jbc.m001606200. [DOI] [PubMed] [Google Scholar]
- 50.Ozes O N, Mayo L D, Gustin J A, Pfeffer S R, Pfeffer L M, Donner D B. NF-kappaB activation by tumour necrosis factor requires the Akt serine-threonine kinase. Nature. 1999;401:82–85. doi: 10.1038/43466. [DOI] [PubMed] [Google Scholar]
- 51.Pasteau S, Loiseau L, Brun G. Proliferation of chicken neuroretina cells induced by v-src, in vitro, depends on activation of the E2F transcription factor. Oncogene. 1997;15:17–28. doi: 10.1038/sj.onc.1201177. [DOI] [PubMed] [Google Scholar]
- 52.Pelengaris S, Rudolph B, Littlewood T. Action of Myc in vivo—proliferation and apoptosis. Curr Opin Genet Dev. 2000;10:100–105. doi: 10.1016/s0959-437x(99)00046-5. [DOI] [PubMed] [Google Scholar]
- 53.Penuel E, Martin G S. Transformation by v-Src: Ras-MAPK and PI3K-mTOR mediate parallel pathways. Mol Biol Cell. 1999;10:1693–1703. doi: 10.1091/mbc.10.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Phillips A C, Ernst M K, Bates S, Rice N R, Vousden K H. E2F-1 potentiates cell death by blocking antiapoptotic signaling pathways. Mol Cell. 1999;4:771–781. doi: 10.1016/s1097-2765(00)80387-1. [DOI] [PubMed] [Google Scholar]
- 55.Rao L, Debbas M, Sabbatini P, Hockenbery D, Korsmeyer S, White E. The adenovirus E1A proteins induce apoptosis which is inhibited by the E1B and Bcl-2 proteins. Proc Natl Acad Sci USA. 1992;89:7742–7746. doi: 10.1073/pnas.89.16.7742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Romashkova J A, Makarov S S. NF-kappaB is a target of AKT in anti-apoptotic PDGF signaling. Nature. 1999;401:86–90. doi: 10.1038/43474. [DOI] [PubMed] [Google Scholar]
- 57.Schlessinger J. New roles for Src kinases in control of cell survival and angiogenesis. Cell. 2000;100:293–296. doi: 10.1016/s0092-8674(00)80664-9. [DOI] [PubMed] [Google Scholar]
- 58.Shaulian E, Zauberman A, Ginsberg D, Oren M. Identification of a minimal transforming domain of p53: negative dominance through abrogation of sequence-specific DNA binding. Mol Cell Biol. 1992;12:5581–5592. doi: 10.1128/mcb.12.12.5581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Smith M R, DeGudicibus S J, Stacey D W. Requirement for c-ras proteins during viral oncogene transformation. Nature. 1986;320:540–543. doi: 10.1038/320540a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stacey D W, Roudebush M, Day R, Mosser S D, Gibbs J B, Feig L A. Dominant inhibitory Ras mutants demonstrate the requirement for Ras activity in the action of tyrosine kinase oncogenes. Oncogene. 1991;6:2297–2304. [PubMed] [Google Scholar]
- 61.Thomas S M, Brugge J S. Cellular functions regulated by Src family kinases. Annu Rev Cell Dev Biol. 1997;13:513–609. doi: 10.1146/annurev.cellbio.13.1.513. [DOI] [PubMed] [Google Scholar]
- 62.Tsai Y T, Su Y H, Fang S S, Huang T N, Qiu Y, Jou Y S, Shih H M, Kung H J, Chen R H. Etk, a Btk family tyrosine kinase, mediates cellular transformation by linking Src to STAT3 activation. Mol Cell Biol. 2000;20:2043–2054. doi: 10.1128/mcb.20.6.2043-2054.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Turkson J, Bowman T, Adnane J, Zhang Y, Djeu J Y, Sekharam M, Frank D A, Holzman L B, Wu J, Sebti S, Jove R. Requirement for Ras/Rac1-mediated p38 and c-Jun N-terminal kinase signaling in Stat3 transcriptional activity induced by the Src oncoprotein. Mol Cell Biol. 1999;19:7519–7528. doi: 10.1128/mcb.19.11.7519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Turkson J, Bowman T, Garcia R, Caldenhoven E, De Groot R P, Jove R. Stat3 activation by Src induces specific gene regulation and is required for cell transformation. Mol Cell Biol. 1998;18:2545–2552. doi: 10.1128/mcb.18.5.2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wagner A J, Kokontis J M, Hay N. Myc-mediated apoptosis requires wild-type p53 in a manner independent of cell cycle arrest and the ability of p53 to induce p21waf1/cip1. Genes Dev. 1994;8:2817–2830. doi: 10.1101/gad.8.23.2817. [DOI] [PubMed] [Google Scholar]
- 66.Wagner A J, Small M B, Hay N. Myc-mediated apoptosis is blocked by ectopic expression of Bcl-2. Mol Cell Biol. 1993;13:2432–2440. doi: 10.1128/mcb.13.4.2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang C Y, Mayo M W, Korneluk R G, Goeddel D V, Baldwin A S., Jr NF-kappaB antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science. 1998;281:1680–1683. doi: 10.1126/science.281.5383.1680. [DOI] [PubMed] [Google Scholar]
- 68.Wang H G, Rapp U R, Reed J C. Bcl-2 targets the protein kinase Raf-1 to mitochondria. Cell. 1996;87:629–638. doi: 10.1016/s0092-8674(00)81383-5. [DOI] [PubMed] [Google Scholar]
- 69.Weissinger E M, Eissner G, Grammer C, Fackler S, Haefner B, Yoon L S, Lu K S, Bazarov A, Sedivy J M, Mischak H, Kolch W. Inhibition of the Raf-1 kinase by cyclic AMP agonists causes apoptosis of v-abl-transformed cells. Mol Cell Biol. 1997;17:3229–3241. doi: 10.1128/mcb.17.6.3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wyke A W, Cushley W, Wyke J A. Mitogenesis by v-Src: a need for active oncoprotein both in leaving G0 and in completing G1 phases of the cell cycle. Cell Growth Differ. 1993;4:671–678. [PubMed] [Google Scholar]
- 71.Yang J, Liu X, Bhalla K, Kim C N, Ibrado A M, Cai J, Peng T I, Jones D P, Wang X. Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science. 1997;275:1129–1132. doi: 10.1126/science.275.5303.1129. [DOI] [PubMed] [Google Scholar]
- 72.Zindy F, Eischen C M, Randle D H, Kamijo T, Cleveland J L, Sherr C J, Roussel M F. Myc signaling via the ARF tumor suppressor regulates p53-dependent apoptosis and immortalization. Genes Dev. 1998;12:2424–2433. doi: 10.1101/gad.12.15.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zong W X, Edelstein L C, Chen C, Bash J, Gélinas C. The prosurvival Bcl-2 homolog Bfl-1/A1 is a direct transcriptional target of NF-kappaB that blocks TNFalpha-induced apoptosis. Genes Dev. 1999;13:382–387. doi: 10.1101/gad.13.4.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zou H, Henzel W J, Liu X, Lutschg A, Wang X. Apaf-1, a human protein homologous to C. elegans CED-4, participates in cytochrome c-dependent activation of caspase-3. Cell. 1997;90:405–413. doi: 10.1016/s0092-8674(00)80501-2. [DOI] [PubMed] [Google Scholar]