Differential Influence of Interleukin-12 in the Pathogenesis of Autoimmune and Virus-Induced Central Nervous System Demyelination (original) (raw)
Abstract
Experimental allergic encephalomyelitis (EAE) and Theiler’s murine encephalomyelitis virus (TMEV) disease are two demyelinating diseases of the central nervous system (CNS) that serve as animal models for multiple sclerosis. Th1 cells are thought to play a role in the pathogenesis of CNS demyelination in both these diseases. We show here the differential influence of interleukin 12, a critical cytokine for the development of Th1 cells in EAE and TMEV disease.
Experimental allergic encephalomyelitis (EAE) is an autoimmune disease induced following immunization with neural antigens or by adoptive transfer of neural antigen-specific T cells in susceptible animals (8). Theiler’s murine encephalomyelitis virus (TMEV) disease is induced following intracerebral inoculation of TMEV, a member of the picornavirus family, in susceptible animals (3). In both these diseases, the pathogenesis of paralytic syndrome is associated with the infiltration of inflammatory cells and destruction of myelin sheath in the central nervous system (CNS). Because of the close similarities in pathology and clinical syndrome, EAE and TMEV disease have been used as animal models for multiple sclerosis. Despite many similarities in the nature of the disease, the mechanisms involved in the pathogenesis of EAE and TMEV disease seem different. EAE is an archetypal autoimmune disease, mediated by major histocompatibility complex (MHC) class II-restricted, neural antigen-specific, CD4+ Th1 cells (8, 13), whereas in TMEV disease, the CD4+ Th1 cells play a role in the pathogenesis of CNS demyelination and in mediating protective antiviral immune responses (1, 3, 9, 11). However, the development of TMEV-induced CNS demyelination in both MHC class II and CD4 knockout mice suggests that the CD4+ Th1 cells play a less important role in the pathogenesis of TMEV disease (12, 13).
Cytokines produced by immune cells in the CNS are known to play a major role in determining the nature and extent of CNS demyelination in human and animal models. Interleukin-12 (IL-12) is a macrophage-derived, 70-kDa heterodimeric cytokine capable of inducing proliferation, gamma interferon production and the development of Th1 type immune response (14). Recent studies suggested that IL-12 plays a pivotal role in the pathogenesis of Th1 cell-mediated autoimmune diseases (2, 4, 14). We showed earlier that the expression of IL-12 in the CNS and lymphoid organs of mice with EAE (henceforth termed EAE mice) associates with the onset of paralytic syndrome (2). In this study, we compared the role of IL-12 in the pathogenesis of CNS demyelination in EAE and TMEV disease.
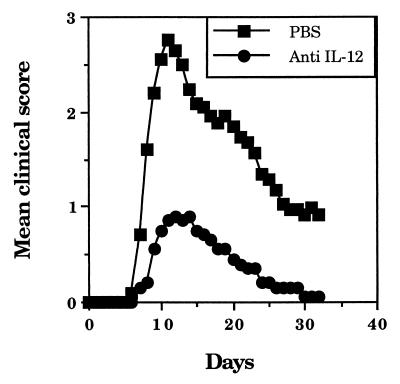
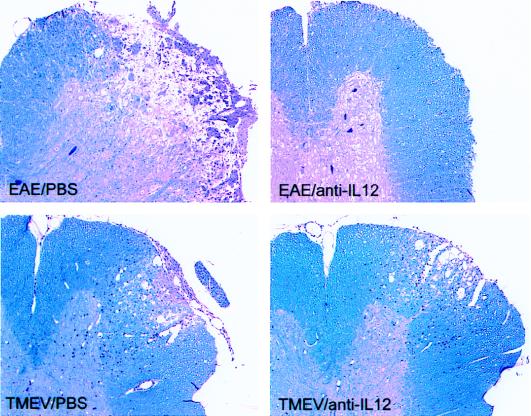
To examine the role of IL-12 in EAE, 4- to 6-week-old female SJL/J mice (National Institutes of Health, Bethesda, Md.) were treated intraperitoneally (i.p.) with 1 mg of neutralizing anti-IL-12 MAb (C17.15) (n = 10) or phosphate-buffered saline (PBS) (n = 10) on days 0, 3, 7, and 12 following adoptive transfer (i.p.) of 107 myelin basic protein (MBP)-specific T cells, and the clinical grades were scored as reported earlier (2). While eight mice that received PBS developed paralytic syndrome, none of the mice that received anti-IL-12 MAb developed hind limb paralysis (Fig. 1). The mean maximum clinical severity in mice treated with anti-IL-12 MAb was very low (0.9) compared to that in mice treated with PBS (2.75 [Student’s t test, P < 0.01]). To further determine the role of IL-12 in EAE, we examined the histology of CNS as described earlier (10). On day 25, the EAE mice treated with PBS showed extensive inflammation and demyelination in the spinal cord, whereas those treated with anti-IL-12 MAb showed only minimal inflammation and demyelination (Fig. 2). The decreases in the degree of parenchymal inflammation and demyelination in EAE mice following treatment with anti-IL-12 MAb were 94.3 and 96.9%, respectively (Student’s t test, P < 0.01) (Table 1).
FIG. 1.
Prevention of EAE by treatment with anti-IL-12 MAb. EAE was induced in 4- to 6-week-old female SJL/J mice by adoptive transfer of MBP-specific T lymphocytes. The donor mice were immunized (subcutaneously) with 350 μg of guinea pig MBP in complete Freund’s adjuvant on days 0 and 7. On day 14, lymph node cells were collected and cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum in the presence of 25 μg of MBP/ml. After 4 days of culture, the cells were harvested, and 1 × 107 T cell blasts were transferred (i.p.) into recipient mice. The mice were treated (i.p.) with 1 mg of neutralizing anti-IL-12 MAb C17.15 (gift of G. Trinchieri) or PBS on days 0, 3, 7, and 12. The clinical grades were scored as follows: loss of tail tone, 1; hind limb weakness, 2; hind limb paralysis, 3; moribund, 4; and death, 5. The y axis represents mean clinical score of 10 EAE mice per group from two experiments.
FIG. 2.
Histology of spinal cord showing demyelination and inflammation in EAE and TMEV-infected mice following treatment with anti-IL-12 MAb. EAE mice treated with anti-IL-12 MAb or PBS as described in the legend to Fig. 1 were sacrificed on day 25. The mice infected with TMEV were treated with 1 mg of anti-IL-12 MAb or PBS on days 0, 7, 14, 21, and 28 and sacrificed on day 35. After perfusion with intracardiac injection of PBS containing 4% paraformaldehyde and 1% glutaraldehyde, the brain and spinal cord were dissected. The spinal cord samples were fixed and sectioned coronally into 1-mm blocks, treated with 1% osmium tetroxide, and embedded in glycol-methacrylate. The photographs of 2-μm-thick spinal cord sections show inflammation and demyelination following staining with a modified erichrome-cresyl violet stain. The figures are representative of two experiments.
TABLE 1.
Pathological scores of spinal cord sections for EAE and TMEV-infected mice following treatment with anti-IL-12 MAba
| Disease/ treatment | No. of animals | Gray matter inflammation (%) | Parenchymal inflammation (%) | Demyelina-tion (%) |
|---|---|---|---|---|
| EAE/anti-IL-12 | 5 | 0.0 ± 0.0 | 1.4 ± 1.0 | 0.8 ± 0.8 |
| EAE/PBS | 5 | 0.0 ± 0.0 | 24.6 ± 7.1 | 25.5 ± 6.5 |
| TMEV/anti-IL-12 | 8 | 0.5 ± 0.5 | 21.3 ± 4.2 | 21.8 ± 4.6 |
| TMEV/PBS | 11 | 0.0 ± 0.0 | 20.7 ± 3.4 | 17.1 ± 2.3 |
To examine the role of IL-12 in the development of TMEV-induced disease, SJL/J mice were treated (i.p.) with 1 mg of neutralizing anti-IL-12 MAb C17.15 on days 0, 7, 14, 21, and 28 following intracerebral inoculation of TMEV (2 × 105 PFU in 10 μl of PBS). The histologic analyses of CNS showed that the degree of parenchymal inflammation in the spinal cord of 35-day TMEV-infected mice treated with anti-IL-12 MAb was comparable to that in mice treated with PBS (Fig. 2). The pathological scores for demyelination were greater for anti-IL-12 MAb-treated mice than for those treated with PBS (Table 1), but the difference was not statistically significant (Student’s t test, P < 0.05).
To further examine the differential influence of IL-12 in EAE and TMEV disease, the levels of serum IL-12 in naive, EAE and TMEV-infected mice were examined by enzyme-linked immunosorbent assay (ELISA) as described previously (2). Naive SJL/J mice showed detectable levels of circulating IL-12 (3.1 ± 1.7 ng/ml), which increased two- to threefold following induction of EAE or TMEV disease (Table 2). No detectable level of IL-12 was found in the sera from EAE or TMEV-infected mice after treatment with anti-IL-12 MAb, suggesting the development of TMEV disease but not EAE even after complete neutralization of IL-12 in circulation.
TABLE 2.
Levels of circulating IL-12 in the sera of EAE and TMEV-infected micea
| Mouse infection/treatment | IL-12 (ng/ml) |
|---|---|
| None (naive) | 3.1 ± 1.7 |
| 10-day EAE (onset of paralysis) | 6.5 ± 1.8 |
| 20-day EAE (recovery) | 3.5 ± 0.9 |
| 35-day TMEV infection | 8.5 ± 1.4 |
| 35-day TMEV infection/anti-IL-12 MAb treatment | 0.0 ± 0.0 |
| 10-day EAE/anti-IL-12 MAb treatment | 0.0 ± 0.0 |
Earlier studies showed the protective effects of anti-CD4 and anti-MHC class II MAb in animals with EAE but not TMEV disease (13). The development of TMEV-induced CNS demyelination in MHC class II and CD4 knockout mice suggested that the CD4+ T cells are not necessary for the pathogenesis of TMEV disease (6, 7, 12). The increased levels of IL-12 in the CNS and lymphoid organs of mice during the paralytic phase of EAE and the ability of anti-IL-12 MAb to reduce the incidence and severity of EAE suggest the central role of Th1 cells in the pathogenesis of EAE (2, 4). Although the increase in the levels of serum IL-12 correlated with the development of both EAE and TMEV disease, the lack of inhibition of TMEV disease after treatment with neutralizing anti-IL-12 MAb suggests that IL-12 is not critical in the pathogenesis of TMEV disease.
Viral infection of glial cells and autoimmune response to myelin antigens are the two perhaps mutually exclusive explanations for the mechanisms of CNS demyelination. The autoimmune process may be initiated by molecular mimicry between neural and viral antigens or by epitope spreading (5). Although our study does not refute the development of a Th1 response in SJL/J mice following infection with TMEV, it argues against an effector function for CD4+ Th1 cells in TMEV-induced CNS demyelination. Our study also suggests that a direct injury resulting from viral persistence in glial cells is the central mechanism of CNS demyelination in TMEV disease. Despite the many pathologic and clinical similarities, the immune mechanisms involved in the pathogenesis of EAE and TMEV disease are different.
REFERENCES
- 1.Borrow P, Welsh C J, Nash A A. Study of the mechanisms by which CD4+ T cells contribute to protection in Theiler’s murine encephalomyelitis. Immunology. 1993;80:502–506. [PMC free article] [PubMed] [Google Scholar]
- 2.Bright J J, Musuro B F, Du C, Sriram S. Expression of IL-12 in CNS and lymphoid organs of mice with experimental allergic encephalitis. J Neuroimmunol. 1998;82:22–30. doi: 10.1016/S0165-5728(97)00184-7. [DOI] [PubMed] [Google Scholar]
- 3.Drescher K M, Pease L R, Rodriguez M. Anti-viral immune responses modulate the nature of central nervous system (CNS) disease in a murine model of multiple sclerosis. Immunol Rev. 1997;159:177–193. doi: 10.1111/j.1600-065x.1997.tb01015.x. [DOI] [PubMed] [Google Scholar]
- 4.Leonard J P, Waldburger K E, Goldman S J. Prevention of experimental allergic encephalomyelitis by antibodies against interleukin 12. J Exp Med. 1995;181:381–386. doi: 10.1084/jem.181.1.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miller S D, Vanderlugt C L, Begolka W S, Pao W, Neville R L, Katz-Levy Y, Carrizosa A, Kim B S. Persistent infection with Theiler’s virus leads to CNS autoimmunity via epitope spreading. Nat Med. 1997;3:1133–1136. doi: 10.1038/nm1097-1133. [DOI] [PubMed] [Google Scholar]
- 6.Murray P D, Pavelko K D, Leibowitz J, Lin X, Rodriguez M. CD4+ and CD8+ T cells make discrete contributions to demyelination and neurologic disease in a viral model of multiple sclerosis. J Virol. 1998;72:7320–7329. doi: 10.1128/jvi.72.9.7320-7329.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Njenga M K, Pavelko K D, Baisch J, Lin X, David C, Leibowitz J, Rodriguez M. Theiler’s virus persistence and demyelination in major histocompatibility complex class II-deficient mice. J Virol. 1996;70:1729–1737. doi: 10.1128/jvi.70.3.1729-1737.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Owens T, Sriram S. The immunology of multiple sclerosis and its animal model, experimental allergic encephalomyelitis. Neurol Clin. 1995;13:51–73. [PubMed] [Google Scholar]
- 9.Peterson J D, Karpus W J, Clatch R J, Miller S D. Split tolerance of Th1 and Th2 cells in tolerance to Theiler’s murine encephalomyelitis virus. Eur J Immunol. 1993;23:46–55. doi: 10.1002/eji.1830230109. [DOI] [PubMed] [Google Scholar]
- 10.Pierce M, Rodriguez M. Erichrome stain for myelin on osmicated tissue embedded in glycol methacrylate plastic. J Histotechno. 1989;12:35–36. [Google Scholar]
- 11.Pope J G, Karpus W J, Vanderlugt C, Miller S D. Flow cytometric and functional analyses of central nervous system-infiltrating cells in SJL/J mice with Theiler’s virus-induced demyelinating disease: evidence for a CD4+ T cell-mediated pathology. J Immunol. 1996;156:4050–4058. [PubMed] [Google Scholar]
- 12.Rivera-Quinones C, McGavern D, Schmelzer J D, Hunter S F, Low P A, Rodriguez M. Absence of neurological deficits following extensive demyelination in a class I-deficient murine model of multiple sclerosis. Nat Med. 1998;4:187–193. doi: 10.1038/nm0298-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sriram S, Carroll L, Fortin S, Cooper S, Ranges G. In vivo immunomodulation by monoclonal anti-CD4 antibody. II. Effect on T-cell response to myelin basic protein and experimental allergic encephalomyelitis. J Immunol. 1998;141:464–468. [PubMed] [Google Scholar]
- 14.Trinchieri G. IL-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen specific adaptive immunity. Annu Rev Immunol. 1995;13:251–276. doi: 10.1146/annurev.iy.13.040195.001343. [DOI] [PubMed] [Google Scholar]