Protection of Macaques against Pathogenic Simian/Human Immunodeficiency Virus 89.6PD by Passive Transfer of Neutralizing Antibodies (original) (raw)
Abstract
The role of antibody in protection against human immunodeficiency virus (HIV-1) has been difficult to study in animal models because most primary HIV-1 strains do not infect nonhuman primates. Using a chimeric simian/human immunodeficiency virus (SHIV) based on the envelope of a primary isolate (HIV-89.6), we performed passive-transfer experiments in rhesus macaques to study the role of anti-envelope antibodies in protection. Based on prior in vitro data showing neutralization synergy by antibody combinations, we evaluated HIV immune globulin (HIVIG), and human monoclonal antibodies (MAbs) 2F5 and 2G12 given alone, compared with the double combination 2F5/2G12 and the triple combination HIVIG/2F5/2G12. Antibodies were administered 24 h prior to intravenous challenge with the pathogenic SHIV-89.6PD. Six control monkeys displayed high plasma viremia, rapid CD4+-cell decline, and clinical AIDS within 14 weeks. Of six animals given HIVIG/2F5/2G12, three were completely protected; the remaining three animals became SHIV infected but displayed reduced plasma viremia and near normal CD4+-cell counts. One of three monkeys given 2F5/2G12 exhibited only transient evidence of infection; the other two had marked reductions in viral load. All monkeys that received HIVIG, 2F5, or 2G12 alone became infected and developed high-level plasma viremia. However, compared to controls, monkeys that received HIVIG or MAb 2G12 displayed a less profound drop in CD4+ T cells and a more benign clinical course. These data indicate a general correlation between in vitro neutralization and protection and suggest that a vaccine that elicits neutralizing antibody should have a protective effect against HIV-1 infection or disease.
Passive or active antibody-based immunity is important for protection against numerous virus diseases of humans (1–3, 13, 14, 16, 19, 21, 41, 43, 48, 50) and for several animal retroviral diseases (11, 15, 17, 18, 24, 35, 37, 55). Despite many years of study, the role of antibody in protection against the transmission of human immunodeficiency virus type 1 (HIV-1) remains unclear. Several studies have shown that polyclonal HIVIG (9, 36) or an anti-V3 monoclonal antibody (10) can protect chimpanzees against HIV-1 challenge. However, the virus used in these experiments was the neutralization-sensitive T-cell line-adapted (TCLA) strain HIV-IIIB; primary HIV-1 isolates are substantially more difficult to neutralize in vitro (8, 31–33, 57). This has called into question the relevance of challenge models using TCLA viruses and has emphasized the need for HIV-1 animal models that readily support the replication of primary isolates in attempts to more accurately define humoral correlates of protection. One small study with chimpanzees evaluated a primary isolate challenge. Conely et al. (6) administered the anti-gp41 virus-neutralizing monoclonal antibody (MAb) 2F5 to two animals prior to intravenous challenge with the primary virus HIV-5016. Compared with two controls, both passively immunized animals exhibited delayed plasma viremia and one had a reduced viral load in plasma through 1 year of follow-up (6). Unfortunately, the chimpanzee model is limited by the availability of animals and by the virus strains that can be used. Protection against primary virus challenge has also been found in a mouse model that uses mice of the severe combined immunodeficiency (SCID) phenotype reconstituted with human peripheral blood mononuclear cells. In this model, the neutralizing MAb IgG1b12 protected against primary HIV-1 isolates JR-CSF and AD6 (12).
The recent construction of chimeric simian/human immunodeficiency virus (SHIV) isolates that express env genes derived from HIV-1 has made it possible to use the macaque animal model to study anti-Env HIV-1 responses (26, 29, 44, 45). Initial constructs used the env gene of HXBc2, a clone of the TCLA HIV-IIIB isolate, but several groups have recently made SHIV isolates based on the env genes from primary HIV-1 strains (38, 40, 46). Reimann and colleagues constructed a SHIV strain with the HIV-1 env gene of a macrophage-tropic clone of a patient isolate, HIV-89.6 (40). HIV-89.6 was obtained from a patient with advanced immunodeficiency by cocultivation of patient peripheral blood mononuclear cells (PBMC) with donor PBMC; the virus replicated in phytohemagglutinin (PHA)-stimulated T cells and monocyte-derived macrophages and was not passaged in T-cell lines (5, 20). Studies of coreceptor entry showed that HIV-89.6 utilizes CCR5, CCR2b, CCR3, and CXCR4 (4, 7, 47). In rhesus macaques, SHIV-89.6 initiated a systemic infection, although evidence of disease was not observed (40). After two serial in vivo passages, an isolate (SHIV-89.6PD) was obtained which causes high plasma viremia and rapid CD4+-T-cell decline in macaques (28, 39, 49).
We used the SHIV-89.6PD rhesus macaque model to study the protective effect of passive transfer of two well-characterized human HIV-1-neutralizing MAbs and a polyclonal HIVIG. Previous in vitro data from our laboratory demonstrated that double or triple combinations of these antibodies produced additive or synergistic neutralization against primary HIV-1 isolates (30). Therefore, we compared each antibody administered alone to a double- and triple-antibody combination. The data demonstrate that anti-HIV-1 neutralizing antibodies can protect against a pathogenic SHIV strain based on a primary HIV-1 envelope. While sterile protection required high levels of neutralizing antibodies, partial protection was afforded by substantially lower antibody levels. Future passive-transfer studies with intravenous or intravaginal SHIV challenge could add important new information to our understanding of the role of antibody in protection against HIV-1.
MATERIALS AND METHODS
Antibodies.
HIVIG (manufactured as HIV-IG by NABI, Boca Raton, Fla.) is a preparation of purified polyclonal IgG derived from the plasma of multiple HIV-1-positive donors as previously described (22). The product is a 50-mg/ml solution that contains 98% monomeric IgG. MAb 2F5 recognizes the gp41 sequence ELDKWA that is conserved among many HIV-1 strains (34, 51). MAb 2G12 binds to a conformationally sensitive epitope in the C3-V4 region of gp120 (52). Both human MAbs are isotype IgG1 and were produced by recombinant expression in CHO cells. Control human intravenous immunoglobulin (IVIG) was purchased from the manufacturer (Gamimune N 5%; Miles Pharmaceuticals, Elkhart, Ind.).
Passive antibody transfer and virus challenge.
Animals used in this study were captive-bred juvenile rhesus macaques and were housed in accordance with the American Association for Accreditation of Laboratory Animal Care standards. The viral challenge stock (SHIV-89.6PD) was grown in rhesus PBMC as previously described (28). Detailed descriptions of the derivation of SHIV-89.6PD and its pathogenicity have recently been published (28, 39, 40). Antibodies were infused intravenously 24 h prior to virus challenge. For all experiments, the doses were as follows: HIVIG, 400 mg/kg; 2F5, 15 mg/kg; and 2G12, 15 mg/kg. Monkeys were challenged with 40 50% tissue culture infectious doses (∼40 animal infectious doses) of SHIV-89.6PD by the intravenous route and were monitored clinically and by routine hematologic testing, lymphocyte subset measurements, and blood chemistry measurements. Inguinal lymph node biopsies were done on all monkeys 2 weeks after virus challenge. All the monkeys were euthanized and full necropsies were performed 14 weeks postchallenge.
Assessment of SHIV infection in macaques.
SHIV infection was assessed by measuring the SHIV-specific antibody response, plasma viremia, virus isolation from PBMC and viable necropsy tissues and by PCR-based detection of SHIV gag DNA in PBMC and necropsy tissues. For viral cultures, monkey PBMC (or single-cell tissue suspensions) were stimulated with phytohemagglutinin (PHA) and interleukin-2 (IL-2) for 3 days prior to the addition of PHA- and IL-2-stimulated donor PBMC. Cultures were monitored for expression of p27 antigen by enzyme-linked immunosorbent assay (ELISA). In our experience, this method is highly sensitive for detection of virus infection (25). Viral RNA levels in plasma were determined by an externally controlled reverse transcription-PCR assay as previously described (25, 53, 56). This assay has a lower limit of detection of 200 RNA copies/ml. A semiquantitative PCR assay was used to detect proviral gag DNA in PBMC and tissue specimens (23, 25, 42). The development of SHIV-specific antibody responses was assessed by serial ELISA measurement of serum antibody reactivity to p27 antigen. MAbs 2F5 and 2G12 do not bind to p27 antigen. HIVIG displays some reactivity in the p27 antigen ELISA, and therefore, for monkeys that received HIVIG, seroconversion to p27 antigen was defined as a rising titer of anti-p27 antibody over the 14-week period of the study. Virus replication in inguinal lymph nodes removed 2 weeks after virus challenge was assessed by in situ hybridization (ISH). ISH was performed on formalin-fixed, paraffin-embedded tissues essentially as described elsewhere (27), using digoxigenin-labeled antisense RNA probes generated by SP6 or T7 polymerase transcription from 1- to 2-kb subclones spanning the entire SIVmac239 genome. Lack of signal with the sense probes indicated the specificity of hybridization.
Antibody concentration in serum and pharmacokinetics.
The concentrations of HIVIG and MAbs 2F5 and 2G12 in serum were determined by ELISA with standard curves generated from the corresponding MAb or HIVIG. In each case, ELISA plates were incubated with serial twofold dilutions of monkey serum or the standard control antibody. The concentration of MAb 2F5 in serum was determined with the peptide GGGLELDKWAASL as the capture antigen and detected with goat anti-human IgG antibody conjugated with horseradish peroxidase. For MAb 2G12, plates were coated with gp120LAI and serum antibody was detected with a highly specific biotinylated 2G12 anti-idiotypic antibody. HIVIG concentration was determined by using plates coated with oligomeric gp140451 as previously described (30, 54); therefore, the measured concentrations of HIVIG were based on gp140-reactive antibodies. Since some monkeys received HIVIG and MAbs together, we performed mixing studies to determine if HIVIG would bind to the ligand for 2F5 or 2G12 in the ELISA and therefore produce artificially elevated values for MAb concentration. High concentrations of HIVIG did display slight reactivity with the 2F5 peptide, but mixing studies showed that the maximum concentrations of HIVIG achieved in vivo (10,000 μg/ml) did not significantly affect the measurement of MAb 2F5 or 2G12 concentrations in serum. The concentrations of HIVIG, 2F5, and 2G12 in serum were measured 15 min postinfusion, on days 1, 2, 5, and 7, and approximately weekly for 4 to 6 weeks. Pharmacokinetic analysis of the half-life of MAbs in serum was based on a two-compartment model and was calculated with a computer software package (MEDI∖WARE, Groningen, The Netherlands).
Virus neutralization assays.
Virus neutralization assays were performed with PHA- and IL-2-stimulated PBMC targets and a p24 or p27 antigen ELISA read out as previously described (30). The HIV-89.6 isolate was obtained from Ronald Collman via the National Institutes of Health AIDS Research and Reference Reagent Program (5). Briefly, antibody was incubated in quadruplicate wells of a microtiter plate with 100 50% tissue culture infectious doses of virus for 30 min at 37°C prior to the addition of 1.5 × 105 PHA- and IL-2-stimulated human PBMC. After overnight incubation, the cells were washed to remove virus and antibody. Virus p27 antigen (for SHIV) or p24 antigen (for HIV) in culture supernatants was measured by ELISA (Coulter, Miami, Fla.) during the early virus growth phase (day 4 or 5). For double-combination experiments, MAbs 2F5 and 2G12 were serially diluted at equal concentrations starting at 200 μg/ml. In the triple-combination experiments, the concentration of polyclonal HIVIG at each dilution was 100-fold greater than that of the MAbs (i.e., the starting concentration of MAbs was 200 μg/ml each and that of HIVIG was 20,000 μg/ml). In all experiments, the antibody concentrations were defined as the final concentration after PBMC were added to the antibody-virus mixture. The fraction of remaining virus was determined by the ratio of the mean level of p24 or p27 antigen in antibody wells to that in control wells with no antibody. Thus, a value of 0.1 indicated that 90% of infectious virus was neutralized. Values lower than 0.001 (99.9% neutralization) could not be measured due to the lower limit of sensitivity for p27 antigen (∼25 pg/ml). For experiments with monkey plasma, undiluted heat-inactivated plasma was incubated with SHIV-89.6PD and the p27 antigen concentration was monitored every 3 to 4 days for 30 days.
RESULTS
In vitro neutralization of SHIV-89.6PD.
Previous studies in our laboratory demonstrated that combinations of HIVIG and MAbs 2F5 and 2G12 produced additive or synergistic neutralization against a panel of primary HIV-1 isolates (30). Similar neutralization experiments were performed with the challenge virus SHIV-89.6PD. To simulate the planned in vivo experiments, each antibody was used alone and compared to the double combination 2F5/2G12 and the triple combination HIVIG/2F5/2G12. Since the envelope sequence of SHIV-89.6PD is not identical to the parental HIV-89.6 (39), HIV-89.6 was included for comparison. As shown in Fig. 1, neutralization sensitivity to HIVIG, 2F5, and 2G12 was similar for HIV-89.6 and SHIV-89.6PD. HIVIG and MAb 2F5 each displayed potent neutralization of SHIV-89.6PD (i.e., 99%), while MAb 2G12 approached 90% neutralization only at the highest concentration tested (i.e., 100 to 200 μg/ml). The combinations of 2F5/2G12 and HIVIG/2F5/2G12 produced highly potent (>99.9%) virus neutralization. For analysis of synergy, dose-response curves were fit with a two-parameter logistic function as previously described (30). The combinations of 2F5/2G12 and HIVIG/2F5/2G12 each produced neutralization greater than the predicted additive effect of the antibodies. An example of this synergy is found by inspection of the 99% inhibitory concentration (IC99) values in Table 1. The IC99 of both MAb 2F5 and 2G12 was greater than 200 μg/ml, yet when the two MAbs were used together, the IC99 was 21 μg/ml.
FIG. 1.
Neutralization curves for individual antibodies and combinations. Dose-response data were generated by serial dilutions of antibody starting at 200 μg/ml for MAbs 2F5 and 2G12 and 20,000 μg/ml for HIVIG. Double- and triple-antibody combinations were diluted in a fixed ratio (e.g., the initial dilution of HIVIG/2F5/2G12 contained a final concentration of 20,000 μg of HIVIG per ml and 200 μg of each MAb per ml). Note that the x axis is labeled to display the MAb concentration; for HIVIG, the corresponding concentration was 100-fold greater than the MAb concentration.
TABLE 1.
Comparison of antibody inhibitory concentration and level in serum
| Antibody | Inhibitory concn (μg/ml)a: | Concn in serum (μg/ml)b | ||
|---|---|---|---|---|
| IC99 | IC90 | IC50 | ||
| HIVIG | >20,000 | 2,398 | 465 | 5,346 |
| 2F5 | >200 | 17 | 2 | 192 |
| 2G12 | >200 | 185 | 7 | 175 |
| 2F5/2G12 | 21 | 4 | 1 | 216, 244 |
| HIVIG/2F5/2G12 | 12 | 2 | 1 | 7,712, 200, 224 |
Initial passive-transfer experiment.
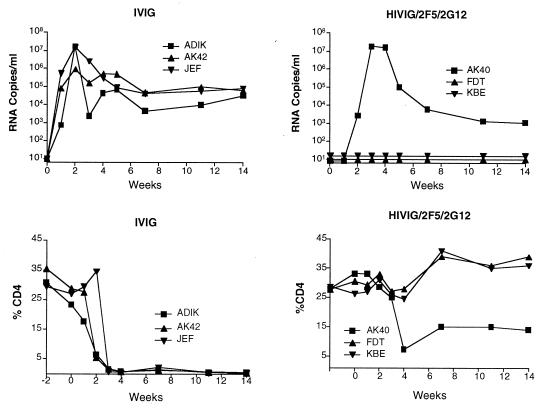
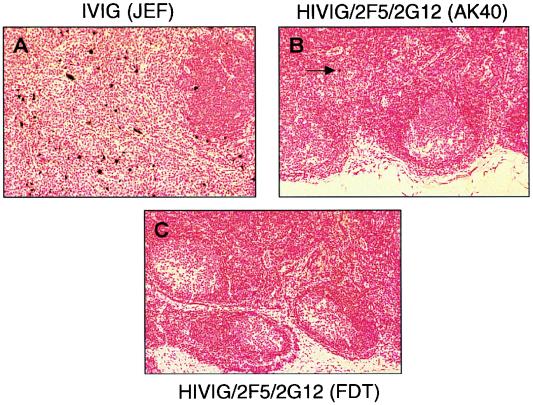
To determine if protection against SHIV-89.6PD could be achieved, we performed an initial passive-transfer experiment with the triple combination HIVIG/2F5/2G12. Three monkeys received all three antibodies, and three controls received IVIG. Figure 2 shows the plasma viral load and percentage of CD4+ T cells over the course of 14 weeks after SHIV-89.6PD challenge. All three control monkeys became SHIV infected and had high viral loads and a rapid decline in the percentage of CD4+ T cells. Consistent with data from other studies (28), these monkeys did not seroconvert to SHIV p27 antigen. At necropsy, all three controls had evidence of generalized wasting, lymphoid depletion, and opportunistic infections. In contrast, two of three monkeys given HIVIG/2F5/2G12 were completely protected. Their viral loads were <200 copies/ml of plasma at all time points, and the percentage of CD4+ T cells remained in the normal range. PBMC cocultures were negative, and evaluation of viable tissues taken at necropsy displayed no evidence of virus by PCR for SHIV gag DNA (Table 2). One monkey (AK40) in the HIVIG/2F5/2G12 group became SHIV infected and reached a peak viral load (∼107 copies/ml of plasma) about 1 week later than the control monkeys. At 14 weeks, the viral load had declined to ∼103 copies/ml, about 1 log10 unit lower than the mean viral load for the three monkeys given IVIG (Fig. 2). Unlike the IVIG controls, AK40 maintained a CD4+-T-cell count between 10 and 15% and seroconverted to SHIV p27 antigen; necropsy revealed normal lymphoid tissue and no evidence of opportunistic infection. Figure 3 shows representative results of ISH on inguinal lymph nodes removed 2 weeks after virus challenge. The lymph node from monkey AK40 showed rare RNA-positive cells and a normal histologic appearance compared to the IVIG controls, which had multiple RNA-positive cells and follicular depletion and involution.
FIG. 2.
Viral load in plasma (RNA copies per milliliter) and peripheral CD4+-T-cell values (% CD4) for three control (IVIG) and three treated (HIVIG/2F5/2G12) animals in the initial passive-transfer experiment.
TABLE 2.
Results of PBMC coculture, PCR for viral DNA, and seroconversion to p27 antigen
| Monkey | Antibody received | PBMC coculturea at wk: | Virus in necropsy PCRb of: | Anti-p27 Abc | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 7 | 9 | 12 | Ing | Ax | Spl | Mes | Thy | |||
| JEF | IVIG | − | + | + | + | + | NDd | + | + | + | + | + | + | + | + | − |
| ADIK | IVIG | − | + | + | + | + | ND | + | + | + | ND | + | + | + | + | − |
| AK42 | IVIG | − | + | + | + | + | ND | + | + | + | ND | ND | + | + | ND | − |
| FDT | HIVIG/2F5/2G12 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| KBE | HIVIG/2F5/2G12 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| AK40 | HIVIG/2F5/2G12 | − | − | + | + | + | + | + | + | + | + | + | + | + | + | + |
| 400 | IVIG | − | + | + | + | + | + | + | + | + | ND | ND | + | + | ND | − |
| 401 | IVIG | − | + | + | + | + | + | + | + | + | ND | ND | + | + | ND | − |
| 403 | IVIG | − | + | + | + | + | + | + | + | + | ND | ND | + | + | ND | − |
| 402 | HIVIG/2F5/2G12 | − | − | + | + | + | + | + | + | + | + | + | + | + | + | + |
| 404 | HIVIG/2F5/2G12 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| 405 | HIVIG/2F5/2G12 | − | − | − | + | + | + | + | + | + | + | + | + | + | + | + |
| 406 | HIVIG | − | + | + | + | + | + | + | + | + | + | + | + | ND | + | + |
| 407 | HIVIG | − | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| 408 | HIVIG | − | + | + | + | + | + | + | + | + | ND | + | + | + | + | + |
| 409 | 2F5 | − | + | + | + | + | + | + | + | + | ND | ND | + | ND | ND | − |
| 410 | 2F5 | − | + | + | + | + | + | + | + | + | ND | ND | + | + | ND | − |
| 411 | 2F5 | − | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| 412 | 2G12 | − | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| 413 | 2G12 | − | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| 414 | 2G12 | − | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| 423 | 2F5/2G12 | − | − | + | + | + | + | + | + | + | + | + | + | + | + | + |
| 425 | 2F5/2G12 | − | − | + | + | + | + | + | + | + | ND | + | + | + | − | + |
| 426 | 2F5/2G12 | − | − | + | − | + | − | − | − | − | − | ND | − | − | − | − |
FIG. 3.
ISH on inguinal lymph nodes removed 2 weeks after virus challenge. (A) Multiple RNA-positive cells and follicular involution in a control animal. (B and C) Rare or no infected cells in treated animals that were partially (B) or completely (C) protected against infection.
Second passive-transfer experiment.
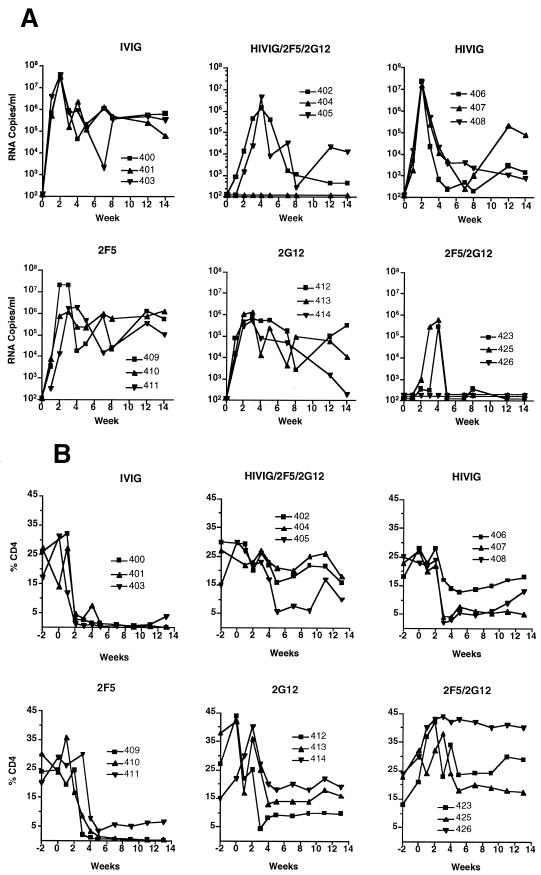
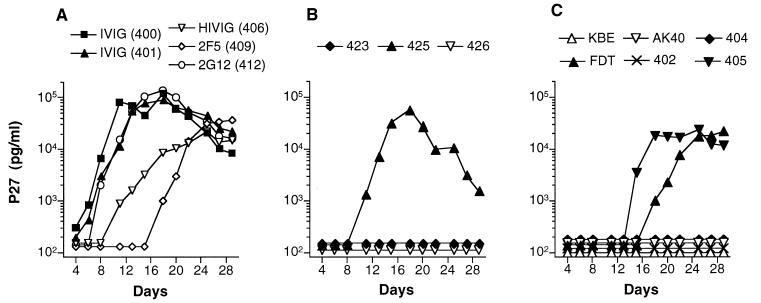
Since the initial experiment had demonstrated that protection against SHIV-89.6PD could be achieved, subsequent experiments were performed to compare each antibody alone to the double- and triple-antibody combinations. In groups of three, monkeys were given IVIG, HIVIG, 2F5, or 2G12 alone, the double combination 2F5/2G12, or the triple combination HIVIG/2F5/2G12. Table 2 and Figure 4 display the results for these 18 monkeys. One monkey in the triple-combination group was protected against infection (animal 404). Similar to the first experiment, PBMC cocultures were negative, no virus was detected in plasma or from tissues at necropsy, and there was no seroconversion to p27 antigen in this animal. The other two animals in the HIVIG/2F5/2G12 group displayed a virologic and clinical course similar to monkey AK40 in the first experiment (see above). Peak viral loads in plasma were slightly delayed and by 8 to 14 weeks were 1 to 2 log10 units lower than in control IVIG animals (Fig. 4A). Both animals seroconverted to SHIV p27 antigen (Table 2). While all three monkeys in the 2F5/2G12 group became SHIV infected, two animals displayed markedly reduced viral loads in plasma that became undetectable by 12 weeks. The percentage of CD4+ T cells in both monkeys remained in the normal range. At necropsy, viral gag DNA was detected in multiple tissues (Table 2) but lymphatic tissues were histologically normal and there was no evidence of opportunistic infection. In the third monkey (animal 426), all measures of virus detection were negative with the exception of two time points (weeks 2 and 4) by PBMC coculture. No virus was detected in plasma or in tissues at necropsy, and antibody to p27 antigen was not detected. One of the two positive cultures was repeated from cryopreserved PBMC and confirmed to be positive. Thus, this animal appears to have experienced a low-level transient SHIV infection.
FIG. 4.
Viral load in plasma (A) and percentage of CD4+ T cells (B) in 18 monkeys after SHIV challenge. The passively administered antibody is noted at the top of each graph.
All nine monkeys that received a single antibody became SHIV infected. In contrast to the six IVIG controls, the monkeys that received HIVIG or 2G12 alone did not exhibit complete loss of peripheral CD4+ cells and did make antibody to SHIV p27 antigen. The viral loads in these animals were variable and generally lower than for the control monkeys (Fig. 4A). At necropsy, none of these animals had evidence of opportunistic infections, although one monkey given HIVIG (animal 407) had mild chronic interstitial pneumonia. Two of the three monkeys given 2F5 exhibited rapid and complete loss of CD4+ cells. Similar to the IVIG controls, these two monkeys did not seroconvert to p27 antigen and had clinical immunodeficiency by 12 to 14 weeks. One 2F5-treated monkey (animal 411) maintained a low percentage of CD4+ T cells and seroconverted to p27. No evidence of immunodeficiency was found in this animal at necropsy.
Antibody concentration in serum and pharmacokinetics.
The mean concentration of each antibody in serum on the day of virus challenge (i.e., 24 h after antibody infusion) is shown in Table 1. Antibody concentrations on the day of challenge were compared to in vitro measurements of IC50, IC90, and IC99 of each antibody and antibody combination. For monkeys that received 2F5/2G12 or HIVIG/2F5/2G12, the antibody concentrations in serum were in excess of the IC99s of each antibody (e.g., for HIVIG/2F5/2G12 monkeys, the IC99 of MAbs was 12 μg/ml, while MAb concentrations in serum were >150 μg/ml at the time of challenge). Since three of six monkeys given HIVIG/2F5/2G12 were completely protected from infection, sterile protection was associated with antibody levels that produce >99% in vitro neutralization. In contrast, for single antibodies, the concentrations in serum were below the respective IC99, and none of these monkeys was completely protected.
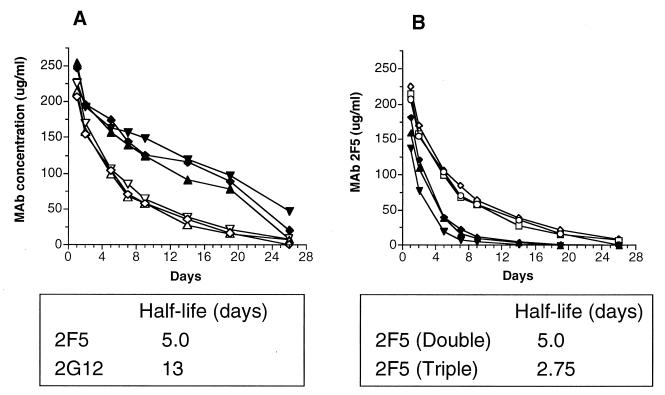
While sterile protection required high levels of neutralizing antibodies, partial protection was mediated by substantially lower antibody levels. Both HIVIG and MAb 2G12 given alone ameliorated the decline in the percentage of CD4+ T cells and prevented clinical AIDS during the 14 weeks of observation. In both cases, the antibody concentration in serum was close to the in vitro IC90 but was well below the IC99. The reason that MAb 2F5 conferred less clinical benefit than MAb 2G12 despite displaying significantly greater in vitro neutralizing activity is not clear but may be related to the antibody half-life in serum. Figure 5A shows the pharmacokinetics of MAbs 2F5 and 2G12 in the three monkeys that received both MAbs. While both MAbs are isotype IgG1, the half-life of 2G12 was 13 days compared to 5 days for 2F5. The reason for this difference is not clear, and it is important to note that we evaluated antibody pharmacokinetics in monkeys that were SHIV infected; this may not reflect the half-life in uninfected animals. Nonetheless, in each of the three 2F5/2G12-treated animals, the half-life of 2F5 was clearly shorter than that of 2G12. Also of note is that the half-life of MAb 2F5 was reduced to 2.75 days when administered as part of the triple combination HIVIG/2F5/2G12 (Fig. 5B). Thus, the half-life of MAb 2F5 in serum was approximately 5 days when administered alone (data not shown) or with 2G12, but was reduced to 2.75 days when administered together with HIVIG and 2G12. The half-life of MAb 2G12 was not significantly affected by coadministration with other antibodies (data not shown). Pharmacokinetic analysis was not performed for HIVIG because its concentrations in plasma were simply estimated based on the anti-gp140-reactive antibody component of HIVIG.
FIG. 5.
Pharmacokinetics of infused antibody. (A) Three monkeys infused with the double combination 2F5/2G12. Open symbols represent the concentration of 2F5, and solid symbols represent the concentration of 2G12. Note the substantially longer half-life of MAb 2G12. (B) Comparison of MAb 2F5 concentration in monkeys that received 2F5/2G12 (open symbols) and those that received HIVIG/2F5/2G12 (solid symbols). Note the longer half-life of 2F5 in monkeys that received the double-antibody combination.
Plasma-mediated neutralizing activity.
Neutralizing activity against the challenge virus SHIV-89.6PD was measured by using plasma from the day of virus challenge. The plasma displayed neutralizing activity consistent with the known levels of passively transferred antibody. Thus, plasma from animals that received double or triple antibody combinations displayed neutralizing activity that was >99% at the first dilution tested (1:8), giving an IC90 at a dilution of approximately 1:50 (data not shown). In contrast, plasma from monkeys that received a single antibody was substantially less potent. To determine if the plasma neutralization could completely prevent infection of PHA- and IL-2-stimulated PBMC (i.e., 100% neutralization), we performed neutralization assays with undiluted plasma. After exposure to the virus-plasma mixture for 24 h, the cells were washed to remove antibody and cultures were monitored for expression of p27 antigen for 30 days. As shown in Fig. 6, plasma from four of six monkeys that received HIVIG/2F5/2G12 and plasma from one of three that received 2F5/2G12 were able to completely prevent PBMC infection. However, 100% in vitro neutralization did not correlate exactly with in vivo protection (e.g., plasma from monkey AK40 displayed 100% in vitro neutralization but this monkey became SHIV infected). This lack of specific correlation may result from the inherent biologic variation in an assay attempting to measure the difference between 99 and 100% neutralization of infectious virions. Data was similar in repeat experiments, although in some cases the complete neutralizing activity was seen in two of three independent experiments. Thus, similar to the in vivo protection, the level of antibody present in the plasma appeared to be on the threshold for attaining complete neutralization in vitro. None of the plasma samples from animals given a single antibody demonstrated complete neutralization (Fig. 6A; only one representative serum sample from each single antibody is shown).
FIG. 6.
SHIV-89.6PD neutralization by undiluted monkey plasma obtained on the day of virus challenge. Results are shown for representative plasma samples from monkeys infused with IVIG, HIVIG, 2F5, or 2G12 (A), three monkeys that received 2F5/2G12 (B), and six monkeys that received HIVIG/2F5/2G12.
DISCUSSION
The quantitative and qualitative nature of the antibody response required to protect against initial HIV-1 infection has been difficult to study because of the limited animal models for HIV-1. The recent construction of SHIV isolates based on primary HIV-1 envelope genes allows the evaluation of anti-envelope antibodies in a nonhuman primate model system. We studied three well-characterized antibodies, alone and in combination, to correlate in vitro neutralizing activity with protection. HIVIG, 2F5, and 2G12 were chosen because their anti-HIV-1 neutralizing activities have been well described (30, 51) and because they were available in sufficient quantities to perform passive-transfer studies. Since our in vitro data showed that double and triple combinations of these antibodies were substantially more potent than any single antibody (30), these data could be compared to the protective effect in vivo.
The passive-transfer data showed that sterile protection against intravenous challenge with SHIV-89.6PD could be achieved by preexisting antibody but that a high level of neutralizing antibodies was required. Even with the triple-combination HIVIG/2F5/2G12, sterile protection was achieved in only three of six animals. Thus, the level and potency of infused antibody appeared to be below the threshold for complete protection. This corresponded to our in vitro neutralization data for these monkeys; the neutralizing activity in plasma at the time of challenge was greater than 99% but not consistently 100%. The association between in vitro neutralization and in vivo effect was further strengthened by the data showing that sterile protection was achieved only in monkeys infused with the triple-antibody combination (which was most potent in vitro) and that the double combination (2F5/2G12) was more effective than any single antibody. Despite this association of neutralizing activity and sterile protection, extrapolation of the absolute amount of neutralizing antibody in serum required to protect against human HIV-1 infection should be approached with caution. SHIV-89.6PD is highly pathogenic, causing AIDS in 12 to 14 weeks, and it was administered by the intravenous route at a relatively high inoculum. Thus, the level of antibody required to protect in this model may not be the same as that required to protect against a low-dose mucosal exposure to HIV-1 in humans. Additionally, there are several antibody-mediated mechanisms that may play a role in protection. Our experiments sought only to correlate virus neutralization, measured against activated PBMC targets, with in vivo protection.
It is important to note that antibody levels far below those required for sterile protection had a substantial effect on the viral load in plasma, the CD4+ cell count, and the clinical outcome. In contrast to IVIG controls, animals that received HIVIG or MAb 2G12 alone did not suffer complete CD4+ cell loss, were able to mount an anti-SHIV antibody response, and had little or no evidence of lymphoid-cell depletion or opportunistic infections at necropsy. Our unpublished data and data published by others (28, 49) suggest that such animals can often control SHIV-infection through 1 to 2 years of follow up. The fact that the concentrations of HIVIG and 2G12 in serum at the time of challenge were only in the IC90 range against the challenge virus (and well below the IC99 level) suggests that moderate levels of preexisting neutralizing antibody could be an important component of protective immunity. Compared to MAb 2G12, the lack of protective effect by MAb 2F5 (Fig. 4) was unexpected because its in vitro neutralizing activity was substantially greater than that of 2G12. At the time of challenge, 2F5 concentrations in serum were 10 times the IC90 while 2G12 concentrations were approximately equal to the IC90 (Table 1). Thus, in this case, the magnitude of preexisting neutralizing activity did not correlate with the level of partial protection. One possible explanation was the shorter half-life of 2F5 than of 2G12 (5 days versus 13 days). While the almost threefold shorter half-life of 2F5 does not appear to completely account for the lack of protective effect by 2F5, it is likely that a sustained neutralizing-antibody level contributes to protection by reducing early plasma viremia. In future experiments, we could address the role of antibody half-life by reinfusing antibody shortly after virus challenge. Also of note, the half-life of 2F5 was further shortened (to 2.75 days) when administered as part of the triple combination HIVIG/2F5/2G12. This may have been due to some combination of antibody agglutination and/or increased clearance, but the specific cause is not clear. In vitro experiments did not reveal any inhibitory effect on the ELISA measurement of 2F5 concentration when the three antibodies were combined. This effect was not seen with 2G12 (i.e., the pharmacokinetics were similar when it was administered alone and in combination). A comparison of viral loads in SHIV-infected monkeys in the HIVIG/2F5/2G12 and 2F5/2G12 groups reveals lower viral loads in the double-antibody group. This further supports the hypothesis that antibody half-life influences the overall protective effect. Given the potent beneficial effect of the double-MAb combination on the CD4+ cell count, viral load, and clinical disease, future studies could titrate down the infusion dose to investigate the lowest level of neutralizing antibodies that can attenuate the SHIV-89.6PD disease course.
In summary, our data from the SHIV-macaque model show a general correlation between in vitro neutralization and protection. Sterile protection was achieved only with the triple antibody combination, which demonstrated the most potent virus neutralization in vitro. Similarly, the double-antibody combination, which was highly synergistic in vitro, was substantially more effective in vivo than either MAb alone. While no individual antibody provided sterile protection, there were beneficial effects on viral load, CD4+ cell count, and clinical outcome. The data suggest that a vaccine that induces neutralizing antibody could have a protective effect against HIV-1 infection or disease. Since current HIV-1 vaccine candidates do not elicit the high levels of neutralizing antibody that were achieved in our passive-transfer experiments (32), one interpretation of the data is that these vaccines will fail to protect against HIV-1. However, in the setting of active immunization and natural mucosal exposure to HIV-1, numerous factors could influence protection. Our passive-transfer experiments do not account for the role of cellular responses, and active immunization should elicit amnestic responses with raising rather than falling antibody levels. This latter point is of particular interest in light of the partial protective effect afforded by HIVIG and MAb 2G12, despite modest in vitro neutralizing activity. Since the SHIV-macaque animal model appears to be useful for the study of anti-envelope antibody responses, future passive-transfer experiments evaluating intravenous and intravaginal SHIV challenge should provide further data on the role of antibody in protection against HIV-1.
ACKNOWLEDGMENTS
We thank Larry Loomis-Price, Ralph Steinman, and Francine McCutchan for helpful discussions and review of the manuscript and Jake Yalley-Ogunro, Jack Greenhouse, Holly Beary, and Michael Eller for technical assistance.
This work was supported in part by grant HL59718 (to J.R.M.) from the National Heart Lung and Blood Institute and by Cooperative Agreement DAMD17-93-V 3004 between the U.S. Army Medical Research and Materiel Command and the Henry M. Jackson Foundation for the Advancement of Military Medicine.
REFERENCES
- 1.Bahmanyar M, Fayaz A, Nour-Salehi S, Mohammadi M, Koprowski H. Successful protection of humans exposed to rabies infection: postexposure treatment with the new human diploid cell rabies vaccine and antirabies serum. JAMA. 1976;236:2751–2754. [PubMed] [Google Scholar]
- 2.Beasley R P, Hwang L Y, Steven C E. Efficacy of hepatitis B immune globulin for prevention of perinatal transmission of the hepatitis B virus carrier state: final report of a randomized double-blind, placebo-controlled trial. Hepatology. 1983;3:135–141. doi: 10.1002/hep.1840030201. [DOI] [PubMed] [Google Scholar]
- 3.Casadevall A, Scharff M D. Return to the past: the case for antibody-based therapies in infectious diseases. J Infect Dis. 1995;21:150–161. doi: 10.1093/clinids/21.1.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choe H, Farzan M, Sun Y, Sullivan N, Rollins B, Ponath P D, Wu L J, Mackay C R, Larosa G, Newman W, Gerard N, Gerard C, Sodroski J. The β-chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates. Cell. 1996;85:1135–1148. doi: 10.1016/s0092-8674(00)81313-6. [DOI] [PubMed] [Google Scholar]
- 5.Collman R, Balliet J W, Gregory S A, Friedman H, Kolson D L, Nathanson N, Srinivasan A. An infectious molecular clone of an unusual macrophage-tropic and highly cytopathic strain of human immunodeficiency virus type 1. J Virol. 1992;66:7517–7521. doi: 10.1128/jvi.66.12.7517-7521.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Conley A J, Kessler II J A, Boots L J, McKenna P M, Schleif W A, Emini E A, Mark III G E, Katinger H, Cobb E K, Lunceford S M, Rouse S R, Murthy K K. The consequence of passive administration of an anti-human immunodeficiency virus type 1 neutralizing monoclonal antibody before challenge of chimpanzees with a primary virus isolate. J Virol. 1996;70:6751–6758. doi: 10.1128/jvi.70.10.6751-6758.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doranz B J, Rucker J, Yi Y J, Smyth R J, Samson M, Peiper S C, Parmentier M, Collman R G, Doms R W. A dual-tropic primary HIV-1 isolate that uses fusin and the β-chemokine receptors CKR-5, CKR-3, and CKR-2b as fusion cofactors. Cell. 1996;85:1149–1158. doi: 10.1016/s0092-8674(00)81314-8. [DOI] [PubMed] [Google Scholar]
- 8.D’Souza M P, Milman G, Bradac J A, McPhee D, Hanson C V, Hendry R M, Corcoran T, Stott J, Fung M, Hanson C, Laman J, Mascola J, Rasheed S, Richman D, Schuitemaker H, Thiriart C, Wainberg M, Weber J, Beddows S, Tilley S, Robinson J, Zolla-Pazner S, Katinger H. Neutralization of primary HIV-1 isolates by anti-envelope monoclonal antibodies. AIDS. 1995;9:867–874. doi: 10.1097/00002030-199508000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Eichberg J W, Murthy K K, Ward R H R, Prince A M. Prevention of HIV infection by passive immunization with HIVIG or CD4-IgG. AIDS Res Hum Retroviruses. 1992;8:1515. doi: 10.1089/aid.1992.8.1515. [DOI] [PubMed] [Google Scholar]
- 10.Emini E A, Schleif W A, Nunberg J H, Conley A J, Eda Y, Tokiyoshi S, Putney S D, Matsushita S, Cobb K E, Jett C M, Eichberg J W, Murthy K K. Prevention of HIV-1 infection in chimpanzees by gp120 V3 domain-specific monoclonal antibody. Nature. 1992;355:728–730. doi: 10.1038/355728a0. [DOI] [PubMed] [Google Scholar]
- 11.Gardner M, Rosenthal A, Jennings M, Yee J, Antipa L, Robinson J. Passive immunization of rhesus macaques against SIV infection and disease. AIDS Res Hum Retroviruses. 1995;11:843–854. doi: 10.1089/aid.1995.11.843. [DOI] [PubMed] [Google Scholar]
- 12.Gauduin M C, Parren P W H I, Weir R, Barbas C F, Burton D R, Koup R A. Passive immunization with a human monoclonal antibody protects hu-PBL-SCID mice against challenge by primary isolates of HIV-1. Nat Med. 1997;3:1389–1393. doi: 10.1038/nm1297-1389. [DOI] [PubMed] [Google Scholar]
- 13.Gellis S S, McGuinnes A C, Peters M. Study of prevention of mumps orchitis by gamma globulin. Am J Med Sci. 1945;210:661–664. [Google Scholar]
- 14.Groothius J R, Simoes E A F, Levin M J, Hall C B, Long C E, Rodriguez W J, Arrobio J, Meissner H C, Fulton D R, Welliver R C, Tristram D A, Siber G R, Prince G A, Van Raden M, Hemming V G. Prophylactic administration of respiratory syncytial virus immune globulin to high-risk infants and young children. N Engl J Med. 1993;329:1524–1530. doi: 10.1056/NEJM199311183292102. [DOI] [PubMed] [Google Scholar]
- 15.Haigwood N L, Watson A, Sutton W F, McClure J, Lewis A, Ranchalis J, Travis B, Voss G, Letvin N L, Hu S L, Hirsch V M, Johnson P R. Passive immune globulin therapy in the SIV/macaque model: early intervention can alter disease profile. Immunol Lett. 1996;51:107–114. doi: 10.1016/0165-2478(96)02563-1. [DOI] [PubMed] [Google Scholar]
- 16.Hammon W M, Coriel L L, Wehrle P F. Evaluation of Red Cross gamma globulin as a prophylactic agent for poliomyelitis. JAMA. 1953;151:1272–1285. [PubMed] [Google Scholar]
- 17.Hasenkrug K J, Chesebro B. Immunity to retroviral infection: the Friend virus model. Proc Natl Acad Sci USA. 1997;94:7811–7816. doi: 10.1073/pnas.94.15.7811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hohdatsu T, Pu R, Torres B A, Trujillo S, Gardner M B, Yamamoto J K. Passive antibody protection of cats against feline immunodeficiency virus infection. J Virol. 1993;67:2344–2348. doi: 10.1128/jvi.67.4.2344-2348.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Janeway C A. Use of concentrated human serum gamma-globulin in the prevention and treatment of measles. Bull N Y Acad Med. 1945;21:202–220. [PMC free article] [PubMed] [Google Scholar]
- 20.Kim F M, Kolson D L, Balliet J W, Srinivasan A, Collman R G. V3-independent determinants of macrophage tropism in a primary human immunodeficiency virus type 1 isolate. J Virol. 1995;69:1755–1761. doi: 10.1128/jvi.69.3.1755-1761.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Korns R F. Prevention of german measles with immune serum globulin. J Infect Dis. 1952;90:183–189. doi: 10.1093/infdis/90.2.183. [DOI] [PubMed] [Google Scholar]
- 22.Lambert J S, Mofenson L M, Fletcher C V, Moye J, Jr, Stiehm E R, Meyer III W A, Nemo G J, Mathieson B J, Hirsch G, Sapan C V, Cummins L M, Jimenez E, O’neil E, Kovacs A, Stek A. Safety and pharmacokinetics of hyperimmune anti-human immunodeficiency virus (HIV) immunoglobulin administered to HIV-infected pregnant women and their newborns. J Infect Dis. 1997;175:283–291. doi: 10.1093/infdis/175.2.283. [DOI] [PubMed] [Google Scholar]
- 23.Lewis M G, Bellah S, McKinnon K, Yalley-Ogunro J, Zack P M, Elkins W R, Desrosiers R C, Eddy G A. Titration and characterization of two rhesus-derived SIVmac challenge stocks. AIDS Res Hum Retroviruses. 1994;10:213–220. doi: 10.1089/aid.1994.10.213. [DOI] [PubMed] [Google Scholar]
- 24.Lewis M G, Elkins W R, McCutchan F E, Benveniste R E, Lai C Y, Montefiori D C, Burke D S, Eddy G A, Shafferman A. Passively transferred antibodies directed against conserved regions of SIV envelope protect macaques from SIV infection. Vaccine. 1993;11:1347–1355. doi: 10.1016/0264-410x(93)90106-8. [DOI] [PubMed] [Google Scholar]
- 25.Lewis M G, Yalley-Ogunro J, Greenhouse J J, Brennan T P, Bo Hiang J, VanCott T C, Lu Y, Eddy G A, Birx D L. Limited protection from a pathogenic chimeric simian-human immunodeficiency virus challenge following immunization with attenuated simian immunodeficiency virus. J Virol. 1999;73:1262–1270. doi: 10.1128/jvi.73.2.1262-1270.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li J, Lord C I, Haseltine W, Letvin N L, Sodroski J. Infection of cynomolgus monkeys with a chimeric HIV-1/SIV virus that expresses the HIV-1 envelope glycoproteins. J Acquired Immune Defic Syndr. 1992;5:639–646. [PubMed] [Google Scholar]
- 27.Lifson J D, Nowak M A, Goldstein S, Rossio J L, Kinter A, Vasquez G, Wiltrout T A, Brown C, Schneider D, Wahl L, Lloyd A L, Williams J, Elkins W R, Fauci A S, Hirsch V M. The extent of early viral replication is a critical determinant of the natural history of simian immunodeficiency virus infection. J Virol. 1997;71:9508–9514. doi: 10.1128/jvi.71.12.9508-9514.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu Y C, Pauza C D, Lu X S, Montefiori D C, Miller C J. Rhesus macaques that become systemically infected with pathogenic SHIV 89.6-PD after intravenous, rectal, or vaginal inoculation and fail to make an antiviral antibody response rapidly develop AIDS. J Acquired Immune Defic Syndr Hum Retrovirol. 1998;19:6–18. doi: 10.1097/00042560-199809010-00002. [DOI] [PubMed] [Google Scholar]
- 29.Luciw P A, Pratt-Lowe E, Shaw K E S, Levy J A, Cheng-Mayer C. Persistent infection of rhesus macaques with T-cell-line-tropic and macrophage-tropic clones of simian/human immunodeficiency viruses (SHIV) Proc Natl Acad Sci USA. 1995;92:7490–7494. doi: 10.1073/pnas.92.16.7490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mascola J R, Louder M K, VanCott T C, Sapan C V, Lambert J S, Muenz L R, Bunow B, Birx D L, Robb M L. Potent and synergistic neutralization of human immunodeficiency virus (HIV) type 1 primary isolates by hyperimmune anti-HIV immunoglobulin combined with monoclonal antibodies 2F5 and 2G12. J Virol. 1997;71:7198–7206. doi: 10.1128/jvi.71.10.7198-7206.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mascola J R, Louwagie J, McCutchan F E, Fischer C L, Hegerich P A, Wagner K F, Fowler A K, McNeil J G, Burke D S. Two antigenically distinct subtypes of human immunodeficiency virus type 1: viral genotype predicts neutralization serotype. J Infect Dis. 1994;169:48–54. doi: 10.1093/infdis/169.1.48. [DOI] [PubMed] [Google Scholar]
- 32.Mascola J R, Snyder S W, Weislow O S, Belay S M, Belshe R B, Schwartz D H, Clements M L, Dolin R, Graham B S, Gorse G J, Keefer M C, McElrath M J, Walker M C, Wagner K F, McNeil J G, McCutchan F E, Burke D S. Immunization with envelope subunit vaccine products elicits neutralizing antibodies against laboratory-adapted but not primary isolates of human immunodeficiency virus type 1. J Infect Dis. 1996;173:340–348. doi: 10.1093/infdis/173.2.340. [DOI] [PubMed] [Google Scholar]
- 33.Matthews T J. Dilemma of neutralization resistance of HIV-1 field isolates and vaccine development. AIDS Res Hum Retroviruses. 1994;10:631–632. doi: 10.1089/aid.1994.10.631. [DOI] [PubMed] [Google Scholar]
- 34.Muster T, Steindl F, Purtscher M, Trkola A, Klima A, Himmler G, Rüker F, Katinger H. A conserved neutralizing epitope on gp41 of human immunodeficiency virus type 1. J Virol. 1993;67:6642–6647. doi: 10.1128/jvi.67.11.6642-6647.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pincus S H, Cole R, Ireland R, McAtee F, Fujisawa R, Portis J. Protective efficacy of nonneutralizing monoclonal antibodies in acute infection with murine leukemia virus. J Virol. 1995;69:7152–7158. doi: 10.1128/jvi.69.11.7152-7158.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prince A M, Reesink H, Pascual D, Horowitz B, Hewlett I, Murthy K K, Cobb K E, Eichberg J W. Prevention of HIV infection by passive immunization with HIV immunoglobulin. AIDS Res Hum Retroviruses. 1991;7:971–973. doi: 10.1089/aid.1991.7.971. [DOI] [PubMed] [Google Scholar]
- 37.Putkonen P, Thorstensson R, Ghavamzadeh L, Albert J, Hild K, Biberfeld G, Norrby E. Prevention of HIV-2 and SIVsm infection by passive immunization in cynomolgus monkeys. Nature. 1991;352:436–438. doi: 10.1038/352436a0. [DOI] [PubMed] [Google Scholar]
- 38.Ranjbar S, Jones S, Stott E J, Almond N. The construction and evaluation of SIV/HIV chimeras that express the envelope of European HIV type 1 isolates. AIDS Res Hum Retroviruses. 1997;13:797–800. doi: 10.1089/aid.1997.13.797. [DOI] [PubMed] [Google Scholar]
- 39.Reimann K A, Li J T, Veazey R, Halloran M, Park I W, Karlsson G B, Sodroski J, Letvin N L. A chimeric simian/human immunodeficiency virus expressing a primary patient human immunodeficiency virus type 1 isolate env causes an AIDS-like disease after in vivo passage in rhesus monkeys. J Virol. 1996;70:6922–6928. doi: 10.1128/jvi.70.10.6922-6928.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reimann K A, Li J T, Voss G, Lekutis C, Tenner-Racz K, Racz P, Lin W Y, Montefiori D C, Lee-Parritz D E, Lu Y C, Collman R G, Sodroski J, Letvin N L. An env gene derived from a primary human immunodeficiency virus type 1 isolate confers high in vivo replicative capacity to a chimeric simian human immunodeficiency virus in rhesus monkeys. J Virol. 1996;70:3198–3206. doi: 10.1128/jvi.70.5.3198-3206.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Robbins J B, Schneerson R, Szu S C. Perspective: hypothesis: serum IgG antibody is sufficient to confer protection against infectious diseases by inactivating the inoculum. J Infect Dis. 1995;171:1387–1398. doi: 10.1093/infdis/171.6.1387. [DOI] [PubMed] [Google Scholar]
- 42.Rosenberg Y J, Zack P M, Leon E C, White B D, Papermaster S F, Hall E, Greenhouse J J, Eddy G A, Lewis M G. Immunological and virological changes associated with decline in CD4/CD8 ratios in lymphoid organs of SIV-infected macaques. AIDS Res Hum Retroviruses. 1994;10:863–872. doi: 10.1089/aid.1994.10.863. [DOI] [PubMed] [Google Scholar]
- 43.Ross A H. Modification of chicken pox in family contacts by administration of gamma globulin. N Engl J Med. 1962;267:369–376. doi: 10.1056/NEJM196208232670801. [DOI] [PubMed] [Google Scholar]
- 44.Sakuragi S, Shibata R, Mukai R, Komatsu T, Fukasawa M, Sakai H, Sakuragi J-I, Kawamura M, Ibuki K, Hayami M, Adachi A. Infection of macaque monkeys with a chimeric human and simian immunodeficiency virus. J Gen Virol. 1992;73:2983–2987. doi: 10.1099/0022-1317-73-11-2983. [DOI] [PubMed] [Google Scholar]
- 45.Shibata R, Kawamura M, Sakai H, Hayami M, Ishimoto A, Adachi A. Generation of a chimeric human and simian immunodeficiency virus infectious to monkey peripheral blood mononuclear cells. J Virol. 1991;65:3514–3520. doi: 10.1128/jvi.65.7.3514-3520.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shibata R, Maldarelli F, Siemon C, Matano T, Parta M, Miller G, Fredrickson T, Martin M A. Infection and pathogenicity of chimeric simian-human immunodeficiency viruses in macaques: determinants of high virus loads and CD4 cell killing. J Infect Dis. 1997;176:362–373. doi: 10.1086/514053. [DOI] [PubMed] [Google Scholar]
- 47.Smyth R J, Yi Y J, Singh A, Collman R G. Determinants of entry cofactor utilization and tropism in a dualtropic human immunodeficiency virus type 1 primary isolate. J Virol. 1998;72:4478–4484. doi: 10.1128/jvi.72.5.4478-4484.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Snydman D R, Werner B G, Heinze-Lacey B, Berardi V P, Tilney N L, Kirkman R L, Milford E L, Cho S I, Bush H L, Jr, Levey A S. Use of cytomegalovirus immune globulin to prevent cytomegalovirus disease in renal-transplant recipients. N Engl J Med. 1987;317:1049–1054. doi: 10.1056/NEJM198710223171703. [DOI] [PubMed] [Google Scholar]
- 49.Steger K K, Dykhuizen M, Mitchen J L, Hinds P W, Preuninger B L, Wallace M, Thomson J, Montefiori D C, Lu Y C, Pauza C D. CD3+-T-cell and CD20+-B-cell changes predict rapid disease progression after simian-human immunodeficiency virus infection in macaques. J Virol. 1998;72:1600–1605. doi: 10.1128/jvi.72.2.1600-1605.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stokes J, Jr, Neefe J R. The prevention and attenuation of infectious hepatitis by gamma globulin. JAMA. 1945;127:144–145. [Google Scholar]
- 51.Trkola A, Pomales A B, Yuan H, Korber B, Maddon P J, Allaway G P, Katinger H, Barbas III C F, Burton D R, Ho D D, Moore J P. Cross-clade neutralization of primary isolates of human immunodeficiency virus type 1 by human monoclonal antibodies and tetrameric CD4-IgG. J Virol. 1995;69:6609–6617. doi: 10.1128/jvi.69.11.6609-6617.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Trkola A, Purtscher M, Muster T, Ballaun C, Buchacher A, Sullivan N, Srinivasan K, Sodroski J, Moore J P, Katinger H. Human monoclonal antibody 2G12 defines a distinctive neutralization epitope on the gp120 glycoprotein of human immunodeficiency virus type 1. J Virol. 1996;70:1100–1108. doi: 10.1128/jvi.70.2.1100-1108.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vahey M T, Wong M T. Quantitative liquid hybridization PCR method employing storage phosphor technology. In: Dieffenback C W, Dveksler G S, editors. PCR primer: a laboratory manual. Plainview, N.Y: Cold Spring Harbor Laboratory Press; 1995. pp. 313–338. [Google Scholar]
- 54.VanCott T C, Kaminski R W, Mascola J R, Kalyanaraman V S, Wassef N M, Alving C R, Ulrich J T, Lowell G H, Birx D L. HIV-1 neutralizing antibodies in the genital and respiratory tracts of mice intranasally immunized with oligomeric gp160. J Immunol. 1998;160:2000–2012. [PubMed] [Google Scholar]
- 55.Van Rompay K K A, Berardi C J, Dillard-Telm S, Tarara R P, Canfield D R, Valverde C R, Montefiori D C, Cole K S, Montelaro R C, Miller C J, Marthas M L. Passive immunization of newborn rhesus macaques prevents oral simian immunodeficiency virus infection. J Infect Dis. 1998;177:1247–1259. doi: 10.1086/515270. [DOI] [PubMed] [Google Scholar]
- 56.Wong M T, Dolan M J, Kozlow E, Doe R, Melcher G P, Burke D S, Boswell R N, Vahey M. Patterns of virus burden and T cell phenotype are established early and are correlated with the rate of disease progression in human immunodeficiency virus type 1-infected persons. J Infect Dis. 1996;173:877–887. doi: 10.1093/infdis/173.4.877. [DOI] [PubMed] [Google Scholar]
- 57.Wrin T, Loh T P, Vennari J C, Schuitemaker H, Nunberg J H. Adaptation to persistent growth in the H9 cell line renders a primary isolate of human immunodeficiency virus type 1 sensitive to neutralization by vaccinee sera. J Virol. 1995;69:39–48. doi: 10.1128/jvi.69.1.39-48.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]