Nef-Induced CD4 and Major Histocompatibility Complex Class I (MHC-I) Down-Regulation Are Governed by Distinct Determinants: N-Terminal Alpha Helix and Proline Repeat of Nef Selectively Regulate MHC-I Trafficking (original) (raw)
Abstract
The Nef protein of primate lentiviruses triggers the accelerated endocytosis of CD4 and of class I major histocompatibility complex (MHC-I), thereby down-modulating the cell surface expression of these receptors. Nef acts as a connector between the CD4 cytoplasmic tail and intracellular sorting pathways both in the Golgi and at the plasma membrane, triggering the de novo formation of CD4-specific clathrin-coated pits (CCP). The downstream partners of Nef in this event are the adapter protein complex (AP) of CCP and possibly a subunit of the vacuolar ATPase. Whether Nef-induced MHC-I down-regulation stems from a similar mechanism is unknown. By comparing human immunodeficiency virus type 1 (HIV-1) Nef mutants for their ability to affect either CD4 or MHC-I expression, both in transient-transfection assays and in the context of HIV-1 infection, it was determined that Nef-induced CD4 and MHC-I down-regulation constitute genetically and functionally separate properties. Mutations affecting only CD4 regulation mapped to residues previously shown to mediate the binding of Nef to this receptor, such as W57 and L58, as well as to an AP-recruiting dileucine motif and to an acidic dipeptide in the C-terminal region of the protein. In contrast, mutation of residues in an alpha-helical region in the proximal portion of Nef and amino acid substitutions in a proline-based SH3 domain-binding motif selectively affected MHC-I down-modulation. Although both the N-terminal alpha-helix and the proline-rich region of Nef have been implicated in recruiting Src family protein kinases, the inhibitor herbimycin A did not block MHC-I down-regulation, suggesting that the latter process is not mediated through an activation of this family of tyrosine kinases.
The Nef protein of primate lentiviruses plays a multifaceted role in the life cycle of these pathogens (reviewed in reference 17). Produced in abundance from the earliest stage of viral gene expression, Nef associates with the membranes of infected cells by virtue of its N-terminal myristoylation (21, 36, 46), and it accomplishes several distinct functions. First, it down-regulates the cell surface expression of class I major histocompatibility complex (MHC-I), preventing the recognition and lysis of infected cells by cytotoxic lymphocytes (14, 48, 50, 66). Second, it decreases the surface expression of CD4, the primary viral receptor (1, 25, 36). Third, it stimulates virion infectivity by as yet ill-defined influences exerted during viral particle formation (3, 13, 54, 72, 73). Finally, it alters T-cell activation pathways, an effect that can be observed both in tissue culture and in transgenic mice (7, 9, 37, 51, 71).
Several lines of evidence indicate that Nef down-modulates CD4 by acting as a receptor-specific sorting adapter. The Nef effect is exerted at a posttranslational level and, unlike phorbol myristate acetate-induced down-regulation, does not require phosphorylation of the CD4 cytoplasmic tail (25). The membrane-proximal 20 amino acids of CD4, including an essential dileucine motif, are necessary for Nef-mediated down-modulation and can transfer Nef sensitivity to another integral membrane protein (1). Although difficult to detect in mammalian cells, an interaction between Nef and CD4 could be demonstrated in insect cells infected with baculovirus vectors, in the yeast two-hybrid system, and in vitro with recombinant Nef protein and CD4-derived peptide (35, 39, 64). In these last two settings, the importance of the CD4 dileucine motif for association with Nef was confirmed (35, 64). Nuclear magnetic resonance (NMR) analyses further defined the CD4 binding site of Nef (33, 35). A pocket formed of the noncontiguous amino acids WLE59, GGL97, R106, and L110 bound a peptide corresponding to the CD4 tail, albeit with a low affinity. Supporting the functional relevance of these data, a mutation targeting WL58 abrogated Nef-induced CD4 down-regulation (42). Additionally, human immunodeficiency virus type 1 (HIV-1), HIV-2, and simian immunodeficiency virus (SIV) Nef proteins require slightly different sequences in the CD4 cytoplasmic tail for efficient down-modulation, arguing against the existence of a cellular intermediate bridging Nef with CD4 (43).
While it now appears well established that Nef binds CD4, overwhelming evidence also indicates that the viral protein interacts with the endocytic machinery. HIV-1 Nef can trigger the de novo formation of clathrin-coated pits (CCP) that preferentially incorporate CD4 (20). Furthermore, a chimeric integral membrane protein composed of the extracellular and transmembrane domains of CD4 or CD8 with Nef as its cytoplasmic tail undergoes rapid internalization and causes an increase in the clathrin lattice on the inner side of the cell membrane (20, 53). Not strictly a cell surface phenomenon, Nef-induced CD4 down-regulation additionally reflects some degree of intracellular retention and rerouting from the Golgi to the endosomal compartment (53).
The model in which Nef acts as a connector between CD4 and CCP implies that the viral protein recognizes some component of the internalization machinery. Two such downstream partners have been recently proposed: the μ chain of the so-called adapter protein complexes (AP) (48, 60), and a subunit of the vacuolar ATPase, NBP1 (52). APs are heterotetrameric complexes which normally associate with receptor cytoplasmic tails containing tyrosine-based (8, 27, 56) and perhaps dileucine-based (40) signals and which recruit clathrin to induce the formation of CCP (24, 28, 69). AP-1 is present in the Golgi, and AP-2 is found at the plasma membrane (62). A third class of AP, AP-3, was recently identified and might be involved in lysosomal targeting (15, 18, 70). Nef proteins from HIV-1, HIV-2, and SIV were found to associate with the μ chain of both the Golgi (μ1) and plasma membrane (μ2) APs (48, 60). Mutating tyrosine residues at the N terminus of SIV Nef abrogated the Nef-μ interaction and prevented Nef-mediated CD4 down-regulation (60). In HIV-1 Nef, where these tyrosine-based motifs are absent, mutating a dileucine motif in a C-terminal disordered loop of the protein abrogated CD4 down-modulation (16). Furthermore, a 10- to 11-amino-acid sequence including this Nef-derived dileucine motif induced the accelerated internalization of a chimeric integral membrane protein (10, 16). Finally, the dileucine-dependent binding of HIV-1 Nef to APs could be demonstrated both in vitro and in tissue culture (16, 30). In another study, direct interactions between HIV-1 Nef and NBP1, the catalytic subunit of the vacuolar ATPase (V-ATPase), correlated with CD4 down-regulation (52). However, loss of interaction with NBP1 led to only a partial loss of the effect of Nef on CD4.
Although less information is available about the mechanisms of Nef-induced MHC-I down-regulation, this receptor also exhibits increased rates of internalization and lysosomal degradation in the presence of the viral protein (66). Furthermore, HLA-A and HLA-B accumulate in the Golgi and colocalize with clathrin-coated vesicles in this setting (31, 48). Whether the parallel between CD4 and MHC-I down-modulation can be extended further is, however, unknown.
To address this question, we analyzed the ability of a series of HIV-1 Nef mutants to down-regulate CD4 and MHC-I and to trigger in cis the accelerated endocytosis of a chimeric integral membrane protein. The results of our experiments support a model in which Nef uses distinct domains for connecting CD4 with cellular mediators of protein sorting and for down-modulating MHC-I. Additionally, we identify an N-terminal domain of Nef, shown by NMR to be an alpha-helix (5), as being crucial for MHC-I down-regulation.
MATERIALS AND METHODS
DNA constructions.
The HIV-1 nef alleles used in these experiments were derived from the R7 recombinant clone and have been described previously (1, 2). Nef and all surface markers were expressed from the cytomegalovirus (CMV) immediate-early promoter in the pCMX plasmid vector (75). The viral constructs were all based on the previously described R9 backbone, in which all HIV-1 genes are functional (23). All mutations were introduced by PCR and verified by sequencing.
Transfections and infections.
293T cells were grown in Dulbecco modified Eagle medium supplemented with 10% fetal calf serum and transfected by the calcium phosphate method (4). Confluent 10-cm plates were split 1:8 the day before the transfection. DNAs were mixed and combined with water for a final volume of 250 μl; 250 μl of 0.5 M CaCl2 was added, followed by 500 μl of 2× HEPES-buffered saline (pH 7.05) while vortexing vigorously. The mixture was allowed to sit for 20 min at room temperature before being added to the cells. After addition of the DNA, the cells were incubated in a 5% CO2 atmosphere overnight and were returned to 10% CO2 the next day after a change of medium. Fluorescence-activated cell sorter (FACS) analyses were performed 24 to 48 h later. A vector expressing a tailless variant of CD8 (called 88X), which is resistant to Nef-induced down-modulation, was cotransfected in all assays to ensure that analysis was performed only on transfected cells. CD4 was produced from the previously described CMV-based CMX-CD4 vector (1).
CEM-GFP cells were acutely infected with the different HIV variants by cocultivation for 24 h with 293T transfected cells as previously described (1). vesicular stomatitis virus G protein pseudotyped HIV-1 derivatives were generated by cotransfecting the pMD.G plasmid (55) with the HIV-1 variants in 293T cells. For protein analyses, pCMV-luciferase was cotransfected with the various Nef constructs.
Protein analyses.
Western blot analyses were performed as described previously (1) with an ECL kit (Amersham). Cytoplasmic proteins were extracted with lysis buffer (100 mM Tris [pH 8.0], 100 mM NaCl, 0.5% Nonidet P-40, 1 mM phenylmethylsulfonyl fluoride, 10 μg of aprotinin per ml, 2 μg of leupeptin per ml, 1 μg of pepstatin per ml), and the protein concentrations were determined by the bicinchoninic acid assay (Pierce). Normalization of lysates was performed by the luciferase assay (4). Phosphotyrosine blots were probed with a rabbit polyclonal antiphosphotyrosine antibody at 1:1,000 diluted in Tris-buffered saline with Tween 80 (TBST; Pharmingen). Nef blots were probed with a rabbit polyclonal anti-Nef serum at 1:1,000 in TBST as previously described (12).
Flow cytometry.
Flow cytometry was performed on a Becton-Dickinson FACSCalibur, or FACStar, with fluorescein isothiocyanate- and R-phycoerythrin-conjugated monoclonal antibodies against CD4 and CD8 (Dako) or the MHC-I heavy chain (Dako). A second antibody specific to HLA-A2, BIH0648 (One Lambda Inc.), was used to confirm the results of the MHC-I down-regulation assays. Live-cell–dead-cell discrimination was accomplished by staining with propidium iodide. Data analysis was performed with Becton-Dickinson software. HIV-infected cells were fixed for 1 to 24 h in 3% formaldehyde–phosphate-buffered saline (PBS) before analysis. After fixation, well-preserved cells were gated by using forward and side scatters.
Endocytosis assays.
The FACS-based endocytosis assay (10) used an R-phycoerythrin-conjugated monoclonal antibody to CD4 (Pharmingen). A total of 107 cells were incubated with the antibody for 30 min at 4°C in tissue culture medium containing 10% fetal calf serum. Following several washes to remove unbound antibody, total bound antibody and background signal were measured by FACS by transferring 1.5 × 106 cells in 50 μl into a fivefold excess of PBS or a buffered saline solution at pH 2, respectively. The cells were then incubated at 37°C, and aliquots were removed at various times for analysis following addition of a fivefold excess of the acidic saline solution. The fraction of CD4 internalized was calculated by subtracting the mean fluorescence of the initial time zero acid wash from all values and then dividing the mean fluorescence of the acid wash (internalized) by the mean fluorescence of the total bound antibody (surface plus internalized). Of note, it was previously shown that Fab fragments and divalent antibodies could be interchangeably used to study CD4 endocytosis (57, 58).
Hydrogen peroxide treatment of infected cells.
Hydrogen peroxide treatment to block tyrosine phosphatase activity was performed as previously described (38, 67) by treating cells for 15 min at 37°C with 5 mM hydrogen peroxide. A 3-μl volume of 30% H2O2 was added to 530 μl of cells in complete medium at 37°C. After treatment, the cells were washed in PBS twice and subsequently lysed as described above.
RESULTS
CD4 and MHC-I down-regulation are genetically separable properties of Nef.
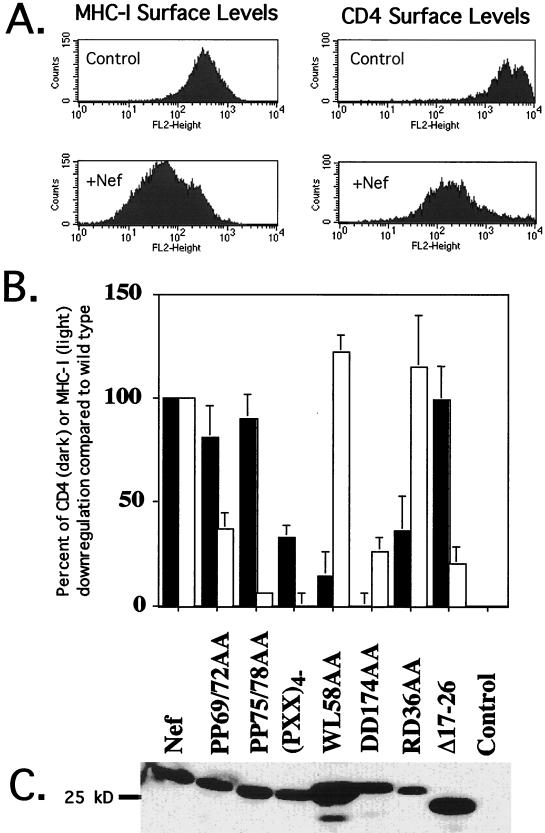
To assess the ability of nef mutants to down-regulate CD4 or MHC-I, a previously described 293T fibroblast transient-transfection assay was used (1, 2). In this system, both CD4 and MHC-I were down-regulated by wild-type HIV-1 Nef (Fig. 1A), although MHC-I down-modulation was much less dramatic, as previously observed (31, 48, 66).
FIG. 1.
Nef-induced CD4 and MHC-I down-regulation are genetically separate properties. (A) CD4 and MHC-I down-regulation by wild-type HIV-1 Nef. Cells were transfected with 35 μg of a Nef expression vector or an empty control vector, 2 μg of a CD4 expression vector, and 2 μg of a plasmid expressing either blue fluorescent protein or a tailless variant of CD8. All vectors used the CMV immediate-early promoter-enhancer. Only cells judged transfected by blue fluorescence or CD8 expression were analyzed. Cells were stained with phycoerythrin-conjugated anti-MHC-I or anti-CD4 antibodies for analysis. Fluorescein isothiocyanate-conjugated anti-CD8 antibody was used to select transfected cells when required. (B) Relative activities of Nef mutants. Solid bars, percent Nef wild-type CD4 down-modulation; open bars, percent Nef wild-type MHC-I down-modulation. Wild-type Nef activity was set at 100%, and the activity of the empty vector was set at 0%. Error bars represent the standard deviation for at least three experiments. (C) Western blot analysis. Lysates were normalized for transfection efficiency by measurement of luciferase activity (triplicate determination) before loading. A polyclonal anti-Nef serum diluted 1:1,000 in TBST served as the primary antibody (2).
A recent NMR study implicated residues WLE59, GGL97, R106, and L110 in the binding of Nef to CD4 (35). In agreement with this proposal, mutating amino acids WL58 of Nef to AA abrogated CD4 down-regulation. However, the protein remained fully active on MHC-I (Fig. 1B). When residue R106 or L110 was altered, the protein was partly impaired in its ability to affect both CD4 and MHC-I (data not shown). Another mutant, containing the DD174-to-AA mutation (the DD174AA mutant), was completely defective for CD4 down-modulation, as previously described (2). The two aspartate residues targeted by this mutation lie in a disordered loop adjacent to the proposed CD4-binding pocket of Nef (34, 35, 47), and perhaps assist in binding CD4. Changing these residues decreased the efficiency of MHC-I down-modulation to 35% of wild type in our system, whereas a similar mutation was previously reported not to affect this function of Nef. Allelic differences in Nef and the use of different cells (293T rather than Jurkat) might explain this difference. Another mutant impaired solely for CD4 down-regulation was RD36AA, although its phenotype was not as dramatic as that of WL58AA and DD174AA (Fig. 1B).
Mutants that failed to down-regulate MHC-I while retaining the ability to modulate CD4 levels were also identified (Fig. 1B). Substituting alanine for all four prolines of the central proline repeat of HIV-1 Nef, in a mutant called (PXX)4−, resulted in a protein with such a discrepant phenotype. A more refined analysis revealed that the C-terminal two prolines were most important for MHC-I down-modulation (compare PP69/72AA to PP75/78AA in Fig. 1B). Alterations in a predicted alpha-helix near the N terminus of Nef, in the V10EΔ17–26 variant, did not block the effect of Nef on CD4 but greatly decreased the efficiency of MHC-I down-regulation.
A Western blot of the Nef mutant proteins showed levels of expression equivalent to that of wild-type Nef, with the exception of the RD36AA mutant, which exhibited lower levels, and the WL58AA and V10EΔ17–26 variants, which exhibited slightly higher steady-state levels after normalization for transfection efficiency by luciferase (Fig. 1C). The lower expression levels of the Nef RD36AA mutant did not seem to block its ability to down-regulate MHC-I, suggesting that the down-regulation pathway is saturable, at least in fibroblasts. Accordingly, the better expressed WL58AA mutant gained little extra MHC-I down-regulation as a result of its higher expression.
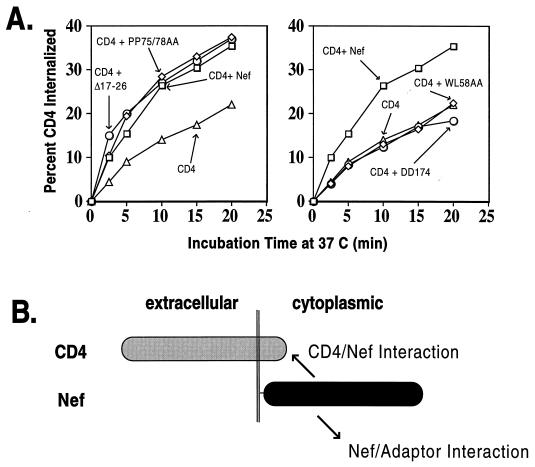
An analysis of some of the mutants in an endocytosis assay confirmed that Nef variants that did not down-regulate CD4 also failed to accelerate its internalization (Fig. 2A). The smaller magnitude of Nef-induced MHC-I down-modulation precluded a similar analysis for this receptor. Mutant PP75/78AA triggered rates of CD4 endocytosis indistinguishable from those induced by wild-type Nef, while the WL58AA and DD174AA mutants did not increase CD4 internalization at all. It is noteworthy that in this type of assay, the rates of CD4 endocytosis reflect the efficiency of at least two interactions: that between CD4 and Nef and that between Nef and components of CCP (Fig. 2B).
FIG. 2.
Correlation between Nef-induced CD4 down-regulation and endocytosis. (A) Internalization of CD4 in response to coexpression of wild-type or mutant Nef in 293T cells. CD4 was expressed alone or together with wild-type Nef or various Nef mutants as noted on the graphs. The results are representative of two independent experiments. Only the Nef mutants which down-modulate CD4 surface levels trigger its accelerated endocytosis in trans. (B) Diagram of interactions measured in this _trans_-endocytosis assay. Myristoylated Nef associates with the cytoplasmic tail of CD4 and also with the cell surface AP-2, a component of CCP.
Discordant Nef mutant phenotypes in HIV-1-infected cells.
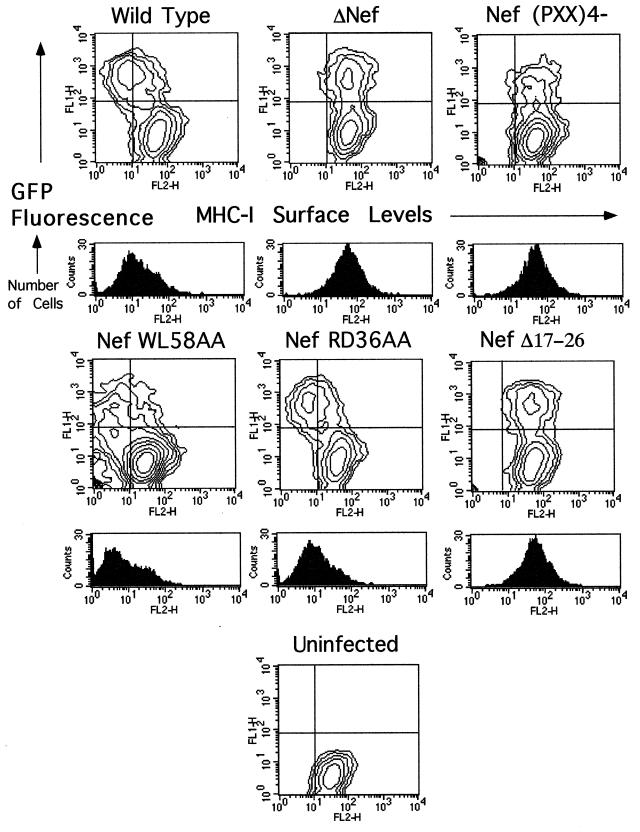
To ascertain whether the effects observed with Nef alone were relevant to events taking place during HIV-1 infection, selected mutants were analyzed within the context of HIV-1-infected CEM-GFP cells. CEM-GFP is a human T-lymphoid cell line that contains an HIV-1 long terminal repeat-driven copy of the green fluorescent protein cDNA (GFP) (26). As such, GFP expression is inducible by Tat, allowing infected and uninfected cells to be distinguished by flow cytometry. CEM-GFP cells were inoculated by cocultivation with 293T cells producing either wild-type or _nef_-mutated HIV-1 and, after a few days in culture to allow the virus to spread, were analyzed by FACS analysis for GFP expression and for surface levels of MHC-I (Fig. 3) and CD4 (data not shown). Sorting GFP-positive cells permitted the selective analysis of infected cells present in the population (histograms below the two-dimensional contour plots in Fig. 3). These analyses confirmed and extended the results obtained with Nef alone, in that the WL58AA and RD36AA mutants down-regulated MHC-I as efficiently as the wild type did, while the four-proline and V10EΔ17–26 mutants did not affect this receptor. No significant differences were observed in the cell surface levels of CD4 between cells infected with wild-type and _nef_-mutated viruses, probably due to the concurrent effects of Env and Vpu.
FIG. 3.
An N-terminal alpha-helix and the proline repeat element of Nef are required for efficient MHC-I down-regulation in HIV-1-infected cells. Two-dimensional FACS analysis of HIV-1-infected CEM-GFP cells. The horizontal axis shows phycoerythrin-conjugated anti-HLA-ABC (MHC-I). The vertical axis shows GFP fluorescence. The LTR-GFP reporter responds to Tat expression by increasing GFP production, thus allowing selection of GFP-positive infected cells for analysis. Cells were infected by coculture with virus-producing 293T cells. Wild-type and mutant Nef alleles were expressed within the context of the R9 virus, which expresses all HIV-1 accessory genes. A total of 2,000 cells were collected per condition. For the histograms, GFP-positive (infected) cells were collected for analysis of the MHC-I levels in infected cells; 5,000 cells were collected per condition. The results are representative of two independent experiments.
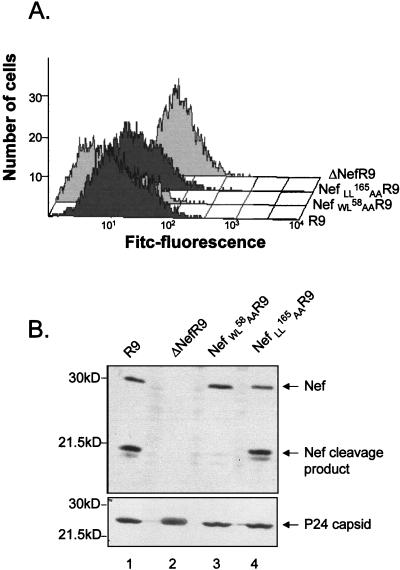
To assess whether Nef interaction with APs is necessary for MHC-I downregulation, the same analysis was also performed with a virus expressing a dileucine-mutated version of Nef. This Nef variant, NefLL165AA, is unable to bind APs and is completely defective for the acceleration of CD4 endocytosis, either in trans or within the context of a CD4-Nef chimera (references 10 and 30 and data not shown). In this experiment, cells infected with wild-type Nef HIV-1 exhibited 5- to 10-fold-lower surface levels of MHC-I than did cells inoculated with a virus with nef deleted (Fig. 4A). Cells infected with a virus expressing the CD4 binding-defective NefWL58AA derivative exhibited even greater levels of MHC-I down-regulation. The virus producing the NefLL165AA protein was as efficient as the wild type at down-modulating MHC-I. Virions were analyzed by Western blotting after normalization through an exogenous reverse transcription assay (Fig. 4B). HIV-1 virions were previously shown to contain significant amounts of Nef, partly in a cleaved form due to the action of the viral protease between residues 58 and 59 of Nef. Wild-type Nef and NefLL165AA viruses exhibited the expected pattern of Nef content, with full-length and cleaved products present in an approximately 1:1 ratio. As previously reported, the NefWL58AA virus harbored mostly uncleaved Nef (12). Probing the blot with p24 capsid antibodies ascertained that equal amounts of viruses had been loaded onto the gel.
FIG. 4.
The AP-binding dileucine-based motif of Nef is dispensable for MHC-I down-regulation in HIV-1 infected cells. (A) Surface-level analysis of MHC-I in CEM-GFP cells infected by virions produced from 293T cells. GFP-positive cells were selected and analyzed for cell surface expression of MHC-I with a monoclonal antibody against the MHC-I heavy chain. (B) Immunoblot analysis of viral particles produced from 293T cells transfected with wild-type virus (R9) or the indicated _nef_-mutated variants, using antibodies against Nef (top) or p24 CA (bottom).
Of particular interest, _vpu_-deleted and _env_-defective VSV-G pseudotyped HIV-1 derivatives were as effective as the wild type at down-regulating MHC-I (data not shown). Together with the finding that MHC-I levels were normal in cells infected with the Nef(PXX)4− virus, these data demonstrate that, at least in the system used here, no other viral protein besides Nef plays a significant role in HIV-1-mediated MHC-I down-modulation.
CCP interaction of Nef mutants singly defective for either CD4 or MHC-I down-modulation.
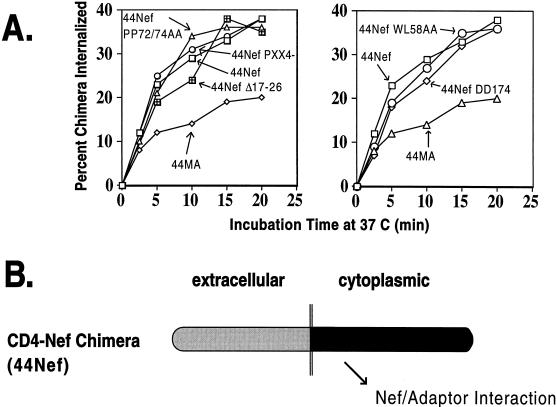
To examine the ability of the various Nef mutants used in this study to interact with CCP, we took advantage of a CD4-based chimeric integral membrane protein harboring Nef as its cytoplasmic domain. The rates of internalization of such a molecule provide an estimate of the affinity of Nef for CCP (53, 60). Mutations corresponding to those analyzed for CD4 and MHC-I down-regulation in trans were introduced in the Nef region of a fusion protein composed of the extracellular and transmembrane regions of CD4 and Nef as its cytoplasmic tail. All the resulting chimeras exhibited increased rates of endocytosis similar to those of a fusion protein between wild-type Nef and CD4 and markedly above those of a control molecule which contained the HIV-1 matrix (MA) protein as its cytoplasmic tail (Fig. 5). These CD4-Nef variants included the RD36AA, WL58AA, PP69/72AA, PP75/78AA, (PXX)4−, V10EΔ17–26, and DD174AA mutants (Fig. 4 and data not shown). Therefore, among this set of mutations, neither those affecting CD4 down-modulation alone (RD36AA, WL58AA, and DD174AA) nor those preventing only MHC-I regulation [PP75/78AA, (PXX)4− and V10EΔ17–26] resulted in disruption of the interaction of Nef with CCP. In contrast, the LL165AA substitution, which prevents the interaction between Nef and APs, abrogated the accelerated internalization of a corresponding CD4-Nef chimera (data not shown), as recently reported (10, 16, 30).
FIG. 5.
Interactions of Nef mutants with the endocytic apparatus. (A) Internalization rates of chimeras composed of the extracellular and transmembrane domains of CD4 and Nef as the cytoplasmic domain in 293T fibroblasts. Graphs are representative of two independent experiments. To combine the results of different experiments, results were normalized to the 44Nef and 44MA controls. (B) Diagram of the interaction measured in this _cis_-endocytosis assay. The chimeric integral membrane protein with Nef as the cytoplasmic domain is internalized at a rate that reflects its association the cell surface AP-2, a component of CCP.
The activation of Src family tyrosine kinases previously shown to bind Nef is not required for MHC-I down-modulation.
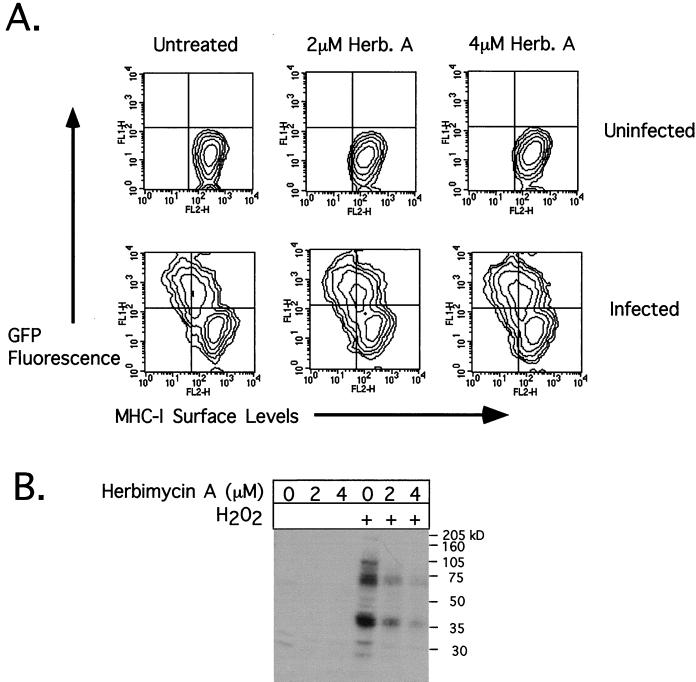
Both the proline-rich region and amino acids 17-26 of HIV-1 Nef have been implicated in the binding of Src family kinases (6, 47, 65). This raised the possibility that tyrosine kinase activity was required for Nef-mediated MHC-I down-modulation. The forced expression of either Lck or constitutively active, inactive, or wild-type forms of Hck did not affect MHC-I down-modulation (data not shown). To probe further a potential role of Nef binding to tyrosine kinases in MHC-I down-regulation, herbimycin A was used. CEM-GFP cells infected 3 days earlier with wild-type HIV-1 by coculture with transfected 293T fibroblasts were treated with increasing concentrations of herbimycin A for an additional 24 h and subsequently analyzed for MHC-I and GFP levels. Herbimycin A inhibits many tyrosine kinases, including those of the Src family (45, 74), by decreasing their catalytic activity and triggering their degradation in the proteasome (67). At concentrations of 2 and 4 μM, herbimycin A had no appreciable effect on the extent of Nef-induced MHC-I down-modulation (Fig. 6A). We confirmed that the drug was active in the infected cells by treating them briefly with H2O2, an inhibitor of tyrosine phosphatases, which normally results in a high degree of tyrosine phosphorylation in cell lysates. A phosphotyrosine Western blot of the treated cells revealed that tyrosine phosphorylation was efficiently blocked by herbimycin A (Fig. 6B).
FIG. 6.
An inhibitor of Src family tyrosine kinases does not block Nef-induced MHC-I down-regulation. (A) CEM-GFP cells were infected by a 24-h coculture with virus-producing 293T cells. Several days later, both infected and uninfected cells were treated for 24 h with herbimycin A (Herb. A) at increasing concentrations and then analyzed for MHC-I levels (horizontal axis) by monitoring infection through GFP production. A minor downward shift in MHC-I levels was noted for both infected and uninfected cells following herbimycin A treatment. (B) Phosphotyrosine Western blot of infected cells before (lanes 1 to 3) and after (lanes 4 to 6) a 15-min treatment with 5 mM hydrogen peroxide (a tyrosine phosphatase inhibitor), which reveals that herbimycin A efficiently inhibits tyrosine kinase activity at both 2 and 4 μM.
DISCUSSION
This work demonstrates that the down-regulation of CD4 and MHC-I are genetically and functionally separate properties of Nef and defines regions of the viral protein that are critical for either one of these two effects. Our results are consistent with a model in which Nef uses distinct functional domains for recruiting CD4, for targeting this receptor to the cell-sorting machinery, and for down-regulating MHC-I. This study delineates two regions of Nef critical for MHC-I down-modulation: an N-terminal alpha-helix and a previously identified proline-rich motif in the core of the protein. This adds to the recent demonstration that a proximally located acidic stretch (EEEE65 in our Nef allele) also participates in this process (31).
The involvement of the HIV-1 Nef N terminus in MHC-I down-regulation had not yet been reported. This region apparently forms an amphipathic helix, whose hydrophobic face associates with the cellular membrane (5). This Nef sequence has been implicated in Lck association (6, 32), as well as in the Nef-associated enhancement of viral infectivity (6). The mutations that were introduced in the present analyses not only shorten this helix but also substitute a charged residue for a hydrophobic one, potentially disrupting its orientation with respect to the membrane. Importantly, these changes do not affect CD4 down-modulation by Nef.
Our functional measurements indicate that amino acids RD36, WL58, and DD174 of Nef are important primarily for CD4 down-regulation. Mutating these residues does not abrogate MHC-I down-regulation or impair the accelerated endocytosis of a chimeric integral membrane protein harboring Nef as its cytoplasmic tail. Supporting our proposal that the WL58 region is involved in binding CD4 but not the endocytic apparatus are the results of an NMR analysis of the Nef-CD4 complex (35) and the demonstration that this domain is dispensable for the rapid endocytosis of a CD8-Nef fusion protein (52). In apparent contradiction with these results, a recent study proposed that both WL58 and the region encompassing the DD174 acidic dipeptide participate in the interaction of Nef with the endocytic machinery, because Nef-GFP fusion proteins altered in these residues failed to colocalize with β-adaptin at the cell periphery (29). However, we previously showed that the Nef-induced de novo formation of CCP depends on the proper tethering of Nef at the plasma membrane, either by coexpression of CD4 or within the context of an integral membrane protein (20). Therefore, it is conceivable that Nef mutants that do not bind CD4 might also fail to colocalize with AP-2. Additionally, there is not a complete correlation between the ability to down-modulate CD4 and colocalization with adaptins: a Nef mutant, G29R/D36G, colocalizes with adaptins like wild-type Nef yet fails to down-regulate CD4 (29).
Greenberg et al. also observed that a mutation in the C-terminal acidic domain of Nef (DD174 in our Nef allele) abrogates Nef-induced CD4 but not MHC-I down-modulation and does not prevent Nef colocalization with AP-1 in the trans Golgi (31). This corroborates our finding that the DD174 motif is dispensable for the accelerated endocytosis of a Nef-CD4 fusion protein, and together these data suggest that these residues are not required for interaction with the endocytic machinery. This seems difficult to reconcile with the results of a recent study indicating that this domain plays a major role in connecting Nef with the NBP1 subunit of V-ATPase, a proposed downstream partner of the viral protein in CD4 endocytosis (52). However, chimeric integral membrane proteins carrying in their cytoplasmic domain an HIV-1 Nef-derived 11-amino-acid segment encompassing the dileucine repeat undergo dileucine-dependent accelerated endocytosis in the absence of the NBP1-binding domain (references 10 and 16 and our unpublished results). We therefore conclude that NBP1 recruitment plays at best a minor stimulatory role in Nef-induced CD4 down-regulation. This corroborates our previous demonstration that the corresponding acidic domain of SIV Nef is important neither for interacting with μ adaptins nor for MHC-I down-regulation (reference 60 and unpublished data).
It is noteworthy that the LL165 dileucine motif, which mediates the interaction of HIV-1 Nef with APs, is necessary for accelerating CD4 endocytosis yet completely dispensable for MHC-I down-modulation. Therefore, while Nef triggers the internalization of CD4-Nef complexes by acting as a physical connector between CD4 and APs, the viral protein instead appears to reveal a cryptic endocytosis motif in MHC-I, as was recently suggested (48). The mechanism of this latter process has yet to be elucidated.
Nef residues essential for MHC-I down-modulation map to at least four distinct motifs: the myristoylation signal (unpublished), an acidic stretch in the proximal region of HIV-1 Nef (EEEE65) (31), the C-terminal two of four prolines which are part of an SH3-binding motif in the Nef core domain (reference 31 and this study), and a conserved α-helix near the N terminus of the protein (this study). The last two regions of Nef have been shown to participate in mediating interactions between the viral protein and members of the Src family of protein tyrosine kinases (6, 65). Based on the finding that a tyrosine-based motif in the cytoplasmic tail of HLA-A and HLA-B is essential for the response to Nef (31, 48), it is tempting to postulate that Nef-induced MHC-I down-regulation involves phosphotyrosine-based signaling pathways. Our demonstration that herbimycin A does not block the effect of Nef argues against such a model. However, the activity of the drug depends upon its irreversible binding to SH groups in the vicinity of the kinase active center, and herbimycin A is most effective against a subset of tyrosine kinases with catalytic domains closely related to the Src family (22). Although these include Lck, Hck, and Lyn, the kinases previously shown to interact with Nef, one cannot rule out the possibility that a drug-resistant SH3 containing tyrosine kinase is the effector of Nef-mediated MHC-I down-regulation. Alternatively, and perhaps more likely, since no tyrosine phosphorylation of MHC-I is observed in response to Nef (48), an SH3-containing protein devoid of tyrosine kinase activity might be the downstream partner of Nef in this process.
Greenberg et al. recently suggested that the Nef-associated blockade of CD69 up-regulation, observed in some T-lymphoid cells following anti-CD3 stimulation, might be somehow connected to MHC-I down-modulation since both activities require an intact proline repeat and a nearby acidic stretch (31, 44). However, the N-terminal region of Nef extending between amino acids 10 and 26 is dispensable for the CD69 phenotype (44), while our analyses demonstrate that it plays a crucial role in MHC-I down-regulation.
Nef-induced MHC-I down-regulation protects HIV-infected cells against cytotoxic T-lymphocyte-mediated killing (14). During the early phase of the infection, inhibiting Nef action may allow for a more vigorous cytotoxic T-lymphocyte response and, in turn, may lessen the viral burden on CD4+ cells. This might facilitate the development of a CD4-specific antiviral response that could be crucial for averting progression to the symptomatic stages of HIV infection (63). Furthermore, although the in vivo life span of the majority of T lymphocytes productively infected with HIV-1 appears to be approximately 2 days (41, 59, 76), there is a very long-lived population of CD45RO+ memory cells which harbor latent, replication-competent virus (19, 77). This population represents an obstacle to the eradication of the virus in patients on combination antiretroviral therapy. It is possible that Nef, which, as the earliest HIV gene product, is expressed in some modes of viral latency (61, 68), participates in hiding this reservoir from the cellular arm of the immune system. Drugs aimed at blocking Nef-induced MHC-I down-regulation may thus help to eradicate the virus from HIV-infected individuals.
ACKNOWLEDGMENTS
A.M. and V.P. contributed equally to this work.
We thank A. Gervaix for the gift of the CEM-GFP cell line, T. Smithgall for the Hck expression vectors, and Bart Sefton for the Lck expression vector.
This study was supported by grants from the Swiss National Foundation and from the Fondation Giorgi-Cavaglieri and by award AI34306 from the National Institutes of Health to D.T. V.P. is the recipient of an M.D./Ph.D. scholarship from the Swiss National Science Foundation.
REFERENCES
- 1.Aiken C, Konner J, Landau N R, Lenburg M E, Trono D. Nef induces CD4 endocytosis: requirement for a critical dileucine motif in the membrane-proximal CD4 cytoplasmic domain. Cell. 1994;76:853–864. doi: 10.1016/0092-8674(94)90360-3. [DOI] [PubMed] [Google Scholar]
- 2.Aiken C, Krause L, Chen Y L, Trono D. Mutational analysis of HIV-1 Nef: identification of two mutants that are temperature-sensitive for CD4 downregulation. Virology. 1996;217:293–300. doi: 10.1006/viro.1996.0116. [DOI] [PubMed] [Google Scholar]
- 3.Aiken C, Trono D. Nef stimulates human immunodeficiency virus type 1 proviral DNA synthesis. J Virol. 1995;69:5048–5056. doi: 10.1128/jvi.69.8.5048-5056.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ausubel F, Brent R, Kingston R, Moore D, Smith J, Seidman J, Struhl K. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons, Inc.; 1987. [Google Scholar]
- 5.Barnham K J, Monks S A, Hinds M G, Azad A A, Norton R S. Solution structure of a polypeptide from the N terminus of the HIV protein Nef. Biochemistry. 1997;36:5970–5980. doi: 10.1021/bi9629945. [DOI] [PubMed] [Google Scholar]
- 6.Baur A S, Sass G, Laffert B, Willbold D, Cheng M C, Peterlin B M. The N-terminus of Nef from HIV-1/SIV associates with a protein complex containing Lck and a serine kinase. Immunity. 1997;6:283–291. doi: 10.1016/s1074-7613(00)80331-3. [DOI] [PubMed] [Google Scholar]
- 7.Baur A S, Sawai E T, Dazin P, Fantl W J, Cheng M C, Peterlin B M. HIV-1 Nef leads to inhibition or activation of T cells depending on its intracellular localization. Immunity. 1994;1:373–384. doi: 10.1016/1074-7613(94)90068-x. [DOI] [PubMed] [Google Scholar]
- 8.Boll W, Ohno H, Songyang Z, Rapoport I, Cantley L, Bonifacino J, Kirchhausen T. Sequence requirements for the recognition of tyrosine-based endocytic signals by clathrin AP-2 complexes. EMBO J. 1996;15:5789–5795. [PMC free article] [PubMed] [Google Scholar]
- 9.Brady H J, Pennington D J, Miles C G, Dzierzak E A. CD4 cell surface downregulation in HIV-1 Nef transgenic mice is a consequence of intracellular sequestration. EMBO J. 1993;12:4923–4932. doi: 10.1002/j.1460-2075.1993.tb06186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bresnahan P A, Yonemoto W, Ferrell S, Williams-Herman D, Geleziunas R, Greene W C. A dileucine motif in HIV-1 Nef acts as an internalization signal for CD4 downregulation and binds the AP-1 clathrin adaptor. Curr Biol. 1998;8:1235–1238. doi: 10.1016/s0960-9822(07)00517-9. [DOI] [PubMed] [Google Scholar]
- 11.Chambers J, Simon S, Berger E, Sklar L, Arfors K-I. Endocytosis of β2 integrins by stimulated human neutrophils analyzed by flow cytometry. J Leukoc Biol. 1993;120:37–45. doi: 10.1002/jlb.53.4.462. [DOI] [PubMed] [Google Scholar]
- 12.Chen Y L, Trono D, Camaur D. The proteolytic cleavage of human immunodeficiency virus type 1 Nef does not correlate with its ability to stimulate virion infectivity. J Virol. 1998;72:3178–3184. doi: 10.1128/jvi.72.4.3178-3184.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chowers M Y, Spina C A, Kwoh T J, Fitch N J, Richman D D, Guatelli J C. Optimal infectivity in vitro of human immunodeficiency virus type 1 requires an intact nef gene. J Virol. 1994;68:2906–2914. doi: 10.1128/jvi.68.5.2906-2914.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Collins K L, Chen B K, Kalams S A, Walker B D, Baltimore D. HIV-1 Nef protein protects infected primary cells against killing by cytotoxic T lymphocytes. Nature. 1998;391:397–401. doi: 10.1038/34929. [DOI] [PubMed] [Google Scholar]
- 15.Cowles C, Odorizzi G, Payne G, SD E. The AP-3 adaptor complex is essential for cargo-selective transport to the yeast vacuole. Cell. 1997;91:109–118. doi: 10.1016/s0092-8674(01)80013-1. [DOI] [PubMed] [Google Scholar]
- 16.Craig H M, Pandori M W, Guatelli J C. Interaction of HIV-1 nef with the cellular dileucine-based sorting pathway is required for CD4 down-regulation and optimal viral infectivity. Proc Natl Acad Sci USA. 1998;95:11229–11234. doi: 10.1073/pnas.95.19.11229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cullen B R. HIV-1 Auxiliary proteins: making connections in a dying cell. Cell. 1998;93:685–692. doi: 10.1016/s0092-8674(00)81431-2. [DOI] [PubMed] [Google Scholar]
- 18.Dell’Angelica E, Ohno H, Ooi C, Rabinovich E, Roche K, Bonifacino J. AP-3: an adaptor-like protein complex with ubiquitous expression. EMBO J. 1997;16:917–928. doi: 10.1093/emboj/16.5.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Finzi D, Hermankova M, Pierson T, Carruth L, Buck C, Chaisson R, Quinn T, Chadwick K, Margolick J, Brookmeyer R, Gallant J, Markowitz M, Ho D, Richman D, Siliciano R. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science. 1998;278:1295–1300. doi: 10.1126/science.278.5341.1295. [DOI] [PubMed] [Google Scholar]
- 20.Foti M, Mangasarian A, Piguet V, Lew D, Krause K, Trono D, Carpentier J. Nef-mediated clathrin coated pit formation. J Cell Biol. 1997;139:37–47. doi: 10.1083/jcb.139.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Franchini G, Robert-Guroff M, Ghrayeb J, Chang N, Wong-Staal F. Cytoplasmic localization of the HTLV-III 3′ orf protein in cultured T-cells. Virology. 1986;155:593–599. doi: 10.1016/0042-6822(86)90219-9. [DOI] [PubMed] [Google Scholar]
- 22.Fukazawa H, Uehara Y, Murakami Y, Mizuno S, Hamada M, Takeuchi T. Labeling of v-Src and BCR-ABL tyrosine kinases with [14C]herbimycin A and its use in the elucidation of the kinase inactivation mechanism. FEBS Lett. 1994;340:155–158. doi: 10.1016/0014-5793(94)80127-4. [DOI] [PubMed] [Google Scholar]
- 23.Gallay P, Hope T, Chin D, Trono D. HIV-1 infection of nondividing cells through the recognition of integrase by the importin/karyopherin pathway. Proc Natl Acad Sci USA. 1997;94:9825–9835. doi: 10.1073/pnas.94.18.9825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gallusser A, Kirchhausen T. The β1 and β2 subunits of the AP complexes are the clathrin coat assembly components. EMBO J. 1993;12:5237–5244. doi: 10.1002/j.1460-2075.1993.tb06219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garcia J, Miller A. Serine phosphorylation independent downregulation of cell surface CD4 by Nef. Nature. 1991;350:508–511. doi: 10.1038/350508a0. [DOI] [PubMed] [Google Scholar]
- 26.Gervaix A, West D, Leoni L, Richman D, Wong-Staal F, Corbeil J. A new cell line to monitor HIV-1 infection and drug susceptibility in vitro. Proc Natl Acad Sci USA. 1997;94:4653–4658. doi: 10.1073/pnas.94.9.4653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Glickman J, Conibear E, Pearse B. Specificity of binding clathrin adapters to signals on the mannose-6-phosphate/insulin-like growth factor II receptor. EMBO J. 1989;8:1041–1047. doi: 10.1002/j.1460-2075.1989.tb03471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goodman O, Keen J. The α-chain of the AP-2 adaptor is a clathrin binding subunit. J Biol Chem. 1995;270:23768–23773. doi: 10.1074/jbc.270.40.23768. [DOI] [PubMed] [Google Scholar]
- 29.Greenberg M, Bronson S, Lock M, Neumann M, Pavlakis G, Skowronski J. Co-localization of HIV-1 Nef with the AP-2 adaptor protein complex correlates with Nef-induced CD4 down-regulation. EMBO J. 1997;16:6964–6976. doi: 10.1093/emboj/16.23.6964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Greenberg M, DeTulleo L, Rapoport I, Skowronski J, Kirchhausen T. A dileucine motif in HIV-1 Nef is essential for sorting into clathrin-coated pits and for downregulation of CD4. Curr Biol. 1998;8:1239–1242. doi: 10.1016/s0960-9822(07)00518-0. [DOI] [PubMed] [Google Scholar]
- 31.Greenberg M E, Iafrate A J, Skowronski J. The SH3 domain-binding surface and an acidic motif in HIV-1 Nef regulate trafficking of class I MHC complexes. EMBO J. 1998;17:2777–2789. doi: 10.1093/emboj/17.10.2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Greenway A, Azad A, McPhee D. Human immunodeficiency virus type 1 Nef protein inhibits activation pathways in peripheral blood mononuclear cells and T-cell lines. J Virol. 1995;69:1842–1850. doi: 10.1128/jvi.69.3.1842-1850.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grzesiek S, Bax A, Hu J, Kaufman J, Palmer I, Stahl S, Tjandra N, Wingfield P. Refined solution structure and backbone dynamics of HIV-1 Nef. Protein Sci. 1997;6:1248–1263. doi: 10.1002/pro.5560060613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grzesiek S, Bax A, Hu J S, Kaufman J, Palmer I, Stahl S J, Tjandra N, Wingfield P T. Refined solution structure and backbone dynamics of HIV-1 Nef. Protein Sci. 1997;6:1248–1263. doi: 10.1002/pro.5560060613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grzesiek S, Stahl S J, Wingfield P T, Bax A. The CD4 determinant for downregulation by HIV-1 Nef directly binds to Nef. Mapping of the Nef binding surface by NMR. Biochemistry. 1996;35:10256–10261. doi: 10.1021/bi9611164. [DOI] [PubMed] [Google Scholar]
- 36.Guy B, Kieny M, Riviere Y, Peuch C, Dott K, Girard M, Montagnier L, Lecocq J. HIV F/3′ orf encodes a phosphorylated GTP-binding protein resembling an oncogene product. Nature. 1987;330:266–269. doi: 10.1038/330266a0. [DOI] [PubMed] [Google Scholar]
- 37.Hanna Z, Kay D G, Rebai N, Guimond A, Jothy S, Jolicoeur P. Nef harbors a major determinant of pathogenicity for an AIDS-like disease induced by HIV-1 in transgenic mice. Cell. 1998;95:163–175. doi: 10.1016/s0092-8674(00)81748-1. [DOI] [PubMed] [Google Scholar]
- 38.Hardwick J S, Sefton B M. Activation of the Lck tyrosine protein kinase by hydrogen peroxide requires the phosphorylation of Tyr-394. Proc Natl Acad Sci USA. 1995;92:4527–4531. doi: 10.1073/pnas.92.10.4527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harris M P, Neil J C. Myristoylation-dependent binding of HIV-1 Nef to CD4. J Mol Biol. 1994;241:136–142. doi: 10.1006/jmbi.1994.1483. [DOI] [PubMed] [Google Scholar]
- 40.Heilker R, Manning-Krieg U, Zuber J, Spiess M. In vitro binding of clathrin adaptors to sorting signals correlates with endocytosis and basolateral sorting. EMBO J. 1996;15:2893–2899. [PMC free article] [PubMed] [Google Scholar]
- 41.Ho D, Neumann A, Perelson A, Chen W, Leonard J, Markowitz M. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature. 1995;373:123–126. doi: 10.1038/373123a0. [DOI] [PubMed] [Google Scholar]
- 42.Hua J, Blair W, Truant R, Cullen B R. Identification of regions in HIV-1 Nef required for efficient downregulation of cell surface CD4. Virology. 1997;231:231–238. doi: 10.1006/viro.1997.8517. [DOI] [PubMed] [Google Scholar]
- 43.Hua J, Cullen B. Human immunodeficiency virus types 1 and 2 and simian immunodeficiency virus Nef use distinct but overlapping target sites for downregulation of cell surface CD4. J Virol. 1997;71:6742–6748. doi: 10.1128/jvi.71.9.6742-6748.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Iafrate A J, Bronson S, Skowronski J. Separable functions of Nef disrupt two aspects of T cell receptor machinery: CD4 expression and CD3 signaling. EMBO J. 1997;16:673–684. doi: 10.1093/emboj/16.4.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.June C, Fletcher M, Ledbetter J, Schieven G, Siegel J, Phillips A, Samuelson L. Inhibition of tyrosine phosphorylation prevents T-cell receptor-mediated signal transduction. Proc Natl Acad Sci USA. 1990;87:7722–7726. doi: 10.1073/pnas.87.19.7722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kaminchik J, Bashan N, Sarver A I, Gorecki N, Panet A. Genetic characterization of human immunodeficiency virus type 1 nef gene productas in vitro and expressed in mammalian cells. J Virol. 1991;65:583–588. doi: 10.1128/jvi.65.2.583-588.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee C H, Leung B, Lemmon M A, Zheng J, Cowburn D, Kuriyan J, Saksela K. A single amino acid in the SH3 domain of Hck determines its high affinity and specificity in binding to HIV-1 Nef protein. EMBO J. 1995;14:5006–5015. doi: 10.1002/j.1460-2075.1995.tb00183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Le Gall S, Erdtmann L, Benichou S, Berlioz-Torrent C, Liu L, Benarous R, Heard J-M, Schwarz O. Nef interacts with the mu subunit of clathrin adapter complexes and reveals a cryptic sorting signal in MHC-I molecules. Immunity. 1998;8:483–495. doi: 10.1016/s1074-7613(00)80553-1. [DOI] [PubMed] [Google Scholar]
- 49.Le Gall S, Heard J M, Schwartz O. Analysis of Nef-induced MHC-I endocytosis. Res Virol. 1997;148:43–47. doi: 10.1016/s0923-2516(97)81912-7. [DOI] [PubMed] [Google Scholar]
- 50.Le Gall S, Prevost M C, Heard J M, Schwartz O. Human immunodeficiency virus type I Nef independently affects virion incorporation of major histocompatibility complex class I molecules and virus infectivity. Virology. 1997;229:295–301. doi: 10.1006/viro.1996.8417. [DOI] [PubMed] [Google Scholar]
- 51.Lindemann D, Wilhelm R, Renard P, Althage A, Zinkernagel R, Mous J. Severe immunodeficiency associated with a human immunodeficiency virus 1 NEF/3′-long terminal repeat transgene. J Exp Med. 1994;179:797–807. doi: 10.1084/jem.179.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lu X, Yu H, Liu S, Brodsky F M, Peterlin B M. Interactions between HIV1 Nef and vacuolar ATPase facilitate the internalization of CD4. Immunity. 1998;8:647–656. doi: 10.1016/s1074-7613(00)80569-5. [DOI] [PubMed] [Google Scholar]
- 53.Mangasarian A, Foti M, Aiken C, Chin D, Carpentier J L, Trono D. The HIV-1 Nef protein acts as a connector with sorting pathways in the Golgi and at the plasma membrane. Immunity. 1997;6:67–77. doi: 10.1016/s1074-7613(00)80243-5. [DOI] [PubMed] [Google Scholar]
- 54.Miller M D, Feinberg M B, Greene W C. The HIV-1 nef gene acts as a positive viral infectivity factor. Trends Microbiol. 1994;2:294–298. doi: 10.1016/0966-842x(94)90007-8. [DOI] [PubMed] [Google Scholar]
- 55.Naldini L, Blomer U, Gallay P, Ory D, Mulligan R, Gage F, Verma I, Trono D. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science. 1996;272:263–267. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]
- 56.Pearse B. Receptors compete for adaptors found in plasma membrane coated pits. EMBO J. 1988;7:3331–3336. doi: 10.1002/j.1460-2075.1988.tb03204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pelchen-Matthews A, Armes J, Griffiths G, Marsh M. Differential endocytosis of CD4 in lymphocytic and non-lymphocytic cells. J Exp Med. 1991;173:575–587. doi: 10.1084/jem.173.3.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pelchen-Matthews A, Armes J, Marsh M. Internalization and recycling of CD4 transfected into HeLa and NIH3T3 cells. J Exp Med. 1989;8:3641–3649. doi: 10.1002/j.1460-2075.1989.tb08538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Perelson A, Neumann A, Markowitz M, Leonard J, Ho D. HIV-1 dynamics in vivo: virion clearance rate, infected cell life span, and viral generation time. Science. 1996;271:1582–1586. doi: 10.1126/science.271.5255.1582. [DOI] [PubMed] [Google Scholar]
- 60.Piguet V, Chen Y-L, Mangasarian A, Foti M, Carpentier J, Trono D. Mechanism of Nef induced CD4 endocytosis: Nef connects CD4 with the mu chain of adaptor complexes. EMBO J. 1998;17:2472–2481. doi: 10.1093/emboj/17.9.2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pomerantz R, Trono D, Feinberg M, Baltimore D. Cells nonproductively infected with HIV-1 exhibit an aberrant pattern of viral RNA expression: a molecular model for latency. Cell. 1990;61:1271–1276. doi: 10.1016/0092-8674(90)90691-7. [DOI] [PubMed] [Google Scholar]
- 62.Robinson M, Pearse B. Immunofluorescent localization of 100k coated vesicle proteins. J Cell Biol. 1986;102:48–52. doi: 10.1083/jcb.102.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rosenberg E S, Billingsley J M, Caliendo A M, Boswell S L, Sax P E, Kalams S A, Walker B D. Vigorous HIV-1 specific CD4+ T-cell responses associated with control of viremia. Science. 1997;278:1447–1450. doi: 10.1126/science.278.5342.1447. [DOI] [PubMed] [Google Scholar]
- 64.Rossi F, Gallina A, Milanesi G. Nef-CD4 physical interaction sensed with the yeast two-hybrid system. Virology. 1996;217:397–403. doi: 10.1006/viro.1996.0130. [DOI] [PubMed] [Google Scholar]
- 65.Saksela K, Cheng G, Baltimore D. Proline-rich (PxxP) motifs in HIV-1 Nef bind to SH3 domains of a subset of Src kinases and are required for the enhanced growth of Nef+ viruses but not for down-regulation of CD4. EMBO J. 1995;14:484–491. doi: 10.1002/j.1460-2075.1995.tb07024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schwartz O, Maréchal V, Le Gall S, Lemonnier F, Heard J M. Endocytosis of major histocompatibility complex class I molecules is induced by the HIV-1 Nef protein. Nat Med. 1996;2:338–342. doi: 10.1038/nm0396-338. [DOI] [PubMed] [Google Scholar]
- 67.Sepp-Lorenzino L, Ma Z, Lebwohl D, Vinitsky A, Rosen N. Herbimycin A induces the 20S proteasome- and ubiquitin-dependent degradation of receptor tyrosine kinases. J Biol Chem. 1995;270:16580–16587. doi: 10.1074/jbc.270.28.16580. [DOI] [PubMed] [Google Scholar]
- 68.Seshamma T, Bagasra O, Trono D, Baltimore D, Pomerantz R J. Blocked early-stage latency in the peripheral blood cells of certain individuals infected with human immunodeficiency virus type 1. Proc Natl Acad Sci USA. 1992;89:10663–10667. doi: 10.1073/pnas.89.22.10663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shih W, Gallusser A, Kirchhausen T. A clathrin binding site in the hinge of the beta-2 chain of mammalian AP-2 complexes. J Biol Chem. 1995;270:31083–31090. doi: 10.1074/jbc.270.52.31083. [DOI] [PubMed] [Google Scholar]
- 70.Simpson F, Peden A, Christopoulou L, Robinson M. Characterization of the adaptor-related protein complex AP-3. J Cell Biol. 1997;137:835–845. doi: 10.1083/jcb.137.4.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Skowronski J, Parks D, Mariani R. Altered T cell activation and development in transgenic mice expressing the HIV-1 nef gene. EMBO J. 1993;12:703–713. doi: 10.1002/j.1460-2075.1993.tb05704.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Spina C A, Kwoh T J, Chowers M Y, Guatelli J C, Richman D D. The importance of nef in the induction of human immunodeficiency virus type 1 replication from primary quiescent CD4 lymphocytes. J Exp Med. 1994;179:115–123. doi: 10.1084/jem.179.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Swingler S, Gallay P, Camaur D, Song J, Abo A, Trono D. The Nef protein of human immunodeficiency virus type 1 enhances serine phosphorylation of the viral matrix. J Virol. 1997;71:4372–4377. doi: 10.1128/jvi.71.6.4372-4377.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Uehara Y, Fukazawa H. Use and selectivity of herbimycin A as inhibitor of protein-tyrosine kinases. Methods Enzymol. 1991;201:370–379. doi: 10.1016/0076-6879(91)01033-x. [DOI] [PubMed] [Google Scholar]
- 75.Umesono K, Murakami K, Thompson C, Evans R. Direct repeats as selective response elements for the thyroid hormone, retinoic acid and vitamin D3 receptors. Cell. 1991;65:1255–1266. doi: 10.1016/0092-8674(91)90020-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wei X, Hhosh S, Taylor M, Johnson V, Emini E, Deutsch P, Lifson J, Nonhoeffer S, Nowak M, Hahn B, Saag M, Shaw G. Viral dynamics in human immunodeficiency virus type 1 infection. Nature. 1995;373:117–122. doi: 10.1038/373117a0. [DOI] [PubMed] [Google Scholar]
- 77.Wong J, Hezareh M, Gunthard H, Havlir D, Ignacio C, Spina C, Richman D. Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science. 1998;278:1291–1295. doi: 10.1126/science.278.5341.1291. [DOI] [PubMed] [Google Scholar]
- 78.Yurchak L K, Hardwick J S, Amrein K, Pierno K, Sefton B M. Stimulation of phosphorylation of Tyr394 by hydrogen peroxide reactivates biologically inactive, non-membrane-bound forms of Lck. J Biol Chem. 1996;271:12549–12554. doi: 10.1074/jbc.271.21.12549. [DOI] [PubMed] [Google Scholar]