Induction of CD4+CD25+ regulatory T cells by copolymer-I through activation of transcription factor Foxp3 (original) (raw)
Abstract
Copolymer-I (COP-I) has unique immune regulatory properties and is a treatment option for multiple sclerosis (MS). This study revealed that COP-I induced the conversion of peripheral CD4+CD25- to CD4+CD25+ regulatory T cells through the activation of transcription factor Foxp3. COP-I treatment led to a significant increase in Foxp3 expression in CD4+ T cells in MS patients whose Foxp3 expression was reduced at baseline. CD4+CD25+ T cell lines generated by COP-I expressed high levels of Foxp3 that correlated with an increased regulatory potential. Furthermore, we demonstrated that the induction of Foxp3 in CD4+ T cells by COP-I was mediated through its ability to produce IFN-γ and, to a lesser degree, TGF-β1, as shown by antibody blocking and direct cytokine induction of Foxp3 expression in T cells. It was evident that in vitro treatment and administration with COP-I significantly raised the level of Foxp3 expression in CD4+ T cells and promoted conversion of CD4+CD25+ regulatory T cells in wild-type B6 mice but not in IFN-γ knockout mice. This study provides evidence for the role and mechanism of action of COP-I in the induction of CD4+CD25+ regulatory T cells in general and its relevance to the treatment of MS.
Keywords: IFN-γ, multiple sclerosis
Copolymer-I (COP-I) is a random polymer of four amino acids (glutamic acid, lysine, alanine, and tyrosine) enriched in myelin basic protein (MBP) and has proven treatment efficacy for multiple sclerosis (MS) (1-3). Although COP-I has been approved as a treatment option for MS since 1995, the mechanism of action of COP-I in relation to its treatment efficacy is unclear (4). COP-I has been found to activate T cells in human and animal experimental systems (5-7). The relevance of this unique property of COP-I to its efficacy in MS is thought to partially involve a biased induction of T helper (Th)2 immunity. There is evidence suggesting that human T cell lines generated by COP-I initially secrete Th1 (IL-2 and IFN-γ) and Th2 cytokines (IL-4, IL-6, and IL-10) in response to COP-I (8). However, repeated in vitro stimulation of these T cell lines progressively shifts cytokine production toward the Th2 response (9, 10). Similarly, repeated COP-I injections may lead to deviation from Th1 to Th2 response in patients with MS (11, 12). Studies reported by other investigators, however, indicate that the effect of COP-I on the induction of T cell activation is not entirely selective for Th2 cells and that it consistently activates the production of Th1 and Th2 cytokines in MS (13). Other plausible mechanisms have been proposed that include its inhibitory property on the T cell responses to MBP containing the four frequently appearing amino acids that COP-I comprises (14, 15). This effect is thought to involve competition between COP-I and MBP for binding sites on MHC class II molecules (16, 17). However, recent studies suggest that the inhibition of COP-I on T cells is not entirely specific for MBP because COP-I also affects the activation of T cells specific for two other myelin antigens, proteolipid protein and myelin oligodendrocyte glyco-protein, as well as the binding of these antigens to MHC class II molecules (18). Alternatively, this inhibitory effect of COP-I is attributed to “bystander” suppression of unknown mechanism. To date, the exact mechanism of action of COP-I remains elusive.
In this study, we examined the proposed hypothesis that the unique properties of COP-I in the activation of T cells may induce CD4+CD25+ regulatory T cell responses. The hypothesis was prompted based on our initial discovery that COP-I was able to induce the expression of transcription factor Foxp3 in CD4+ T cells, which is associated with CD4+CD25+ regulatory T cells (19-21). A potential role of COP-I in the induction of CD4+CD25+ regulatory T cell response is particularly relevant to MS because they have been recognized recently as an important regulatory component that keeps autoreactive T cells in check (19-22). Significant deficiencies in the number or function of these regulatory T cells have been found to correlate with several autoimmune conditions, including MS (23-25). CD4+CD25+ regulatory T cells can be distinguished from other CD4+ activated T cells of nonregulatory functions present in the CD4+CD25+ T cell pool by the expression of transcription factor Foxp3 (26). Gene transfer of Foxp3 converts naive T cells toward a regulatory T cell phenotype similar to that of naturally occurring CD4+ regulatory T cells (19, 27). Experiments were performed here to investigate whether COP-I was able to induce conversion of peripheral CD4+CD25- T cells to CD4+CD25+ regulatory T cells through the activation of Foxp3 in human and animal systems. Foxp3 expression and regulatory function of T cells were also analyzed ex vivo in MS patients with or without COP-I treatment and in mice administered with COP-I. Human COP-I-specific, short-term T cell lines were generated and characterized. The study described here has provided evidence indicating the role of COP-I in the induction of CD4+CD25+ regulatory T cells through the activation of Foxp3.
Materials and Methods
Cell Stimulation. Fresh peripheral blood mononuclear cells (PBMC) were isolated from blood specimens by Ficoll hypaque separation. PBMC (2 × 105) or purified T cells (1 × 105) were cultured in the presence or absence of 40 μg/ml COP-I alone or 40 μg/ml COP-I with irradiated T cell-depleted PBMC as a source of antigen-presenting cells (2 × 105) in complete RPMI medium 1640 in U-bottom 96-well plates at 37°C in 5% CO2.On day 4 of the culture, an aliquot of T cells was harvested for Foxp3 gene expression by real-time PCR analysis. Supernatants were collected for cytokine detection, and 1-μCi (1 Ci = 37 GBq) [3H]thymidine (Amersham Pharmacia) was added to the remaining cultures in the last 6 h of culture before cell harvesting.
Foxp3 mRNA Expression by Real-Time PCR. Quantitative real-time RT-PCR was performed on a Prism 7000 sequence detection system (Applied Biosystems). Hypoxanthine phosphoribosyltransferase was used as a reference for sample normalization. Total RNA isolated from PBMC or purified T cells were reverse-transcribed into cDNA by using random hexamer. Human Foxp3 primers (forward, 5′-CAC CTG GCT GGG AAA ATG G-3′; reverse, 5′-GGA GCC CTT GTC GGA TGA T-3′) and TaqMan minor groove binder probe (5′-FAM-ACT GAC CAA GGC TTC AT-3′) sequences were designed with the primer express application program (Applied Biosystems). Mouse Foxp3 primers and probe and all hypoxanthine phosphoribosyltransferase primers and probe were purchased as forms of Assay-on-Demand (Applied Biosystems). The detailed amplification protocol used here is described in Supporting Materials and Methods, which is published as supporting information on the PNAS web site. A representative specific amplification of Foxp3 mRNA derived from a human CD4+CD25+ T cell preparation is shown in Fig. 8, which is published as supporting information on the PNAS web site.
Isolation of Human CD4+CD25+ Regulatory T Cells. Isolation of human CD4+, CD4+CD25+, and CD4+CD25- T cells was performed by using a human regulatory T cell isolation kit (Miltenyi Biotec, Auburn, CA) according to manufacturer's instructions. Briefly, CD4+ T cells were first isolated through negative selection by removing all other cell types. Preisolated CD4+ T cells were incubated with 10 μl of magnetic beads conjugated with anti-CD25 antibody (for 107 cells) to separate CD4+CD25+ and CD4+CD25- T cell populations. The purity of the resulting T cell populations was confirmed to be >97% by flow cytometry.
Generation of Specific T Cell Lines. PBMC were initially seeded out in 96-well round-bottom plates at 20,000 cells per well with irradiated autologous PBMC (100,000 cells per well) as accessory cells in 10% FBS RPMI medium 1640 in the presence of 40 μg/ml COP-I. Cultures were supplemented with IL-2 at 50 units/ml after 48 h. After 7 days, all cultures per wells were assayed for specific reactivity to COP-I in proliferation assays. Briefly, each well was split into four aliquots (≈104 cells per aliquot) and cultured in duplicate with 105 irradiated autologous PBMC in the presence and absence of COP-I (40 μg/ml). Cultures were kept for 3 days and pulsed with [3H]thymidine at 1 μCi per well during the last 16 h of culture. A positive T cell line was defined as specific for COP-I when cpm were at 1,500 and exceeded the reference cpm (in the absence of COP-I) at least 3-fold. COP-I reactive T cell lines were expanded by restimulation with COP-I under the same conditions described above.
Inhibition Assay. CD4+CD25- T cells (responder) and CD4+CD25+ T cells (inhibitor) were cocultured at 5 × 103 per well in the presence or absence of anti-CD3 and anti-CD28 monoclonal antibodies in U-bottom 96-well plates at a responder to inhibitor ratio of 1:1. All cells were cultured in the presence of 105 irradiated accessory cells. At day 7, cell proliferation was measured as described above. The inhibition was calculated as follows: [1 - (experimental cpm per control cpm)] × 100%.
Antibody Blocking Experiments. PBMC were plated out in 96-well plates at 105 cells per well and stimulated with 40 μg/ml COP-I in the presence or absence of the indicated blocking antibodies to various cytokines. The final concentrations of the antibodies as suggested by the manufacturers were as follows: 1 μg/ml for antibodies to IL-8, TNF-α, IFN-γ, and IL-10, 50 ng/ml for IL-1β antibody and 10 μg/ml for TGF-β1 antibody. Cells were harvested on day 4 for Foxp3 expression.
Immunization and Mouse T Cell Preparation. Wild-type and IFN-γ gene knockout C57BL/6 mice were obtained from The Jackson Laboratory. Wild-type and IFN-γ gene knockout mice were injected s.c. with 1.6 mg of COP-I per injection at day 0, day 2, and day 4 for a total of three injections. Mice were killed at a 4-day interval for the isolation of CD4+CD25+ T cells. Spleens were gently minced in complete medium containing 10% FBS and CD4+ T cells were isolated by using a mouse CD4+ T cell negative selection kit (Miltenyi Biotec). T cell-depleted splenocytes of wild-type mice were used as antigen-presenting cells as indicated. To isolate CD4+CD25- T cells, anti-CD25 antibody (10 μl for 107 T cells) conjugated with microbeads was incubated with preselected CD4+ T cells before separation, to yield a purity of >95% of CD4+CD25- T cells. For recovery of CD4+CD25+ T cells, the column bound T cells were flushed off with cold medium. The purity of CD4+CD25+ T cells was always >95%.
Supporting Information. For further information, see Supporting Materials and Methods and Fig. 8; see also Figs. 9 and 10, which are published as supporting information on the PNAS web site.
Results
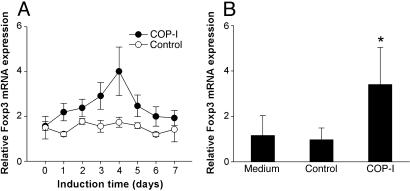
Induction of Transcription Factor Foxp3 Expression in Human CD4+ T Cells by COP-I. After the initial discovery that COP-I induced the expression of transcription factor Foxp3 in human PBMC, the time course of induction of Foxp3 expression in PBMC derived from healthy individuals was evaluated in response to COP-I stimulation by real-time PCR analysis. As shown in Fig. 1_A_, the peak expression of Foxp3 occurred ≈4 days after exposed to COP-I. The effect of COP-I on the induction of Foxp3 expression correlated closely with the rate of cell proliferation in PBMC induced by COP-I (data not shown). Furthermore, the observed effect appeared specific for COP-I as the control peptides, although stimulatory to some PBMC preparations, did not alter Foxp3 expression in the same PBMC preparations (Fig. 1 A). Similar results were obtained when purified CD4+ T cells were treated with COP-I under the same experimental conditions (Fig. 1_B_). It was shown that COP-I induced the expression of Foxp3 predominantly in CD4+ T cell population of both CD45RA and CD45RO phenotypes (Fig. 9).
Fig. 1.
Induction of Foxp3 mRNA expression by COP-I as a function of time and proliferation rate of PBMC in response to COP-I. (A) PBMC preparations derived from 10 randomly selected healthy individuals were cultured, respectively, in the presence or absence of 40 μg/ml COP-I in complete RPMI medium 1640. Cells were collected at the indicated induction time over a span of 7 days and analyzed for mRNA expression of Foxp3 by real-time PCR. (B) CD4+ T cell preparations purified from the same PBMC specimens described above were cultured in the presence or absence (medium control) of COP-I, respectively, for 4 days. Mixed irrelevant peptides (see Materials and Methods) were used at the same concentration as the control. Cells were harvested for Foxp3 expression by real-time PCR. Data are given as relative mRNA expression. *, Significant statistical differences between COP-I-treated cells and controls (P < 0.01).
The potential in vivo effect of COP-I on the expression of Foxp3 in T cells was evaluated in MS patients treated with COP-I by using untreated MS patients and healthy volunteers as controls. To this end, PBMC prepared from MS patients with or without standard COP-I treatment and healthy controls were analyzed ex vivo for the expression of Foxp3. As illustrated in Fig. 10, Foxp3 expression was significantly decreased in untreated MS patients compared with that of healthy individuals. The expression of Foxp3 was significantly higher in PBMC preparations derived from nine different MS patients that had been on COP-I treatment for 10-12 months, even though the Foxp3 expression level did not reach that seen in healthy controls. Among nine patients examined, three pretreatment specimens were available for self-paired analyses that indicated a significant high level of Foxp3 expression in posttreatment PBMC.
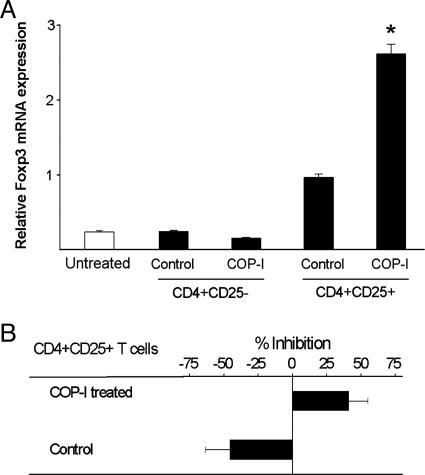
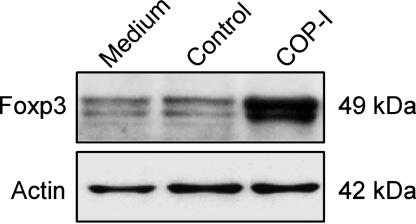
Conversion of Peripheral CD4+CD25- T Cells to CD4+CD25+ Regulatory T Cells by COP-I Through the Activation of Foxp3. We then examined whether the induction of Foxp3 expression in CD4+ T cells by COP-I represented conversion of peripheral CD4+CD25- T cells to CD4+CD25+ regulatory T cells. To this end, human CD4+CD25-T cells were purified by magnetic bead separation and exposed to COP-I. CD4+CD25+ T cells rose from background value of 7.99 ± 0.28% to 23.36 ± 0.69% after CD4+CD25- T cell preparations were exposed to COP-I compared with 16.08 ± 0.31% in those treated with the control (P < 0.05, data not shown). In vitro treatment of CD4+CD25- T cells with COP-I led to a significantly elevated level of Foxp3 expression in resulting CD4+CD25+ T cells (Fig. 2_A_, P < 0.05). CD4+CD25+ T cells converted from the CD4+CD25- T cell fractions by COP-I exhibited considerable inhibitory activities on T cell proliferation induced by anti-CD3/CD28 antibodies (Fig. 2_B_). The increased Foxp3 expression was confirmed in parallel by immunoblot analysis (Fig. 3). The results indicate that COP-I induced conversion of CD4+CD25- to CD4+CD25+ T cells of regulatory function. Furthermore, human T cell lines were generated from healthy individuals by repeated stimulation with COP-I and characterized for the reactivity, Foxp3 expression and the inhibitory rate. As shown in Table 1, T cell lines reactive to COP-I had high expression of Foxp3 that was roughly 10 times higher than that seen in control T cell lines or T cells stimulated one time with COP-I and displayed considerable inhibitory properties compared to control T cell lines generated by irrelevant peptide control. When compared to control T cell lines, the cytokine profile of COP-I reactive T cell lines was noticeably biased toward high production of IFN-γ (Table 1).
Fig. 2.
Conversion of CD4+CD25- T cells to CD4+CD25+ regulatory T cells. CD4+CD25- T cell preparations were purified from six healthy individuals and cultured with COP-I or the peptide control, respectively, in the presence of irradiated autologous PBMC as a source of antigen-presenting cells. Cells were collected on day 4 and analyzed for Foxp3 expression in refractionated CD4+CD25- and CD4+CD25+ T cell subsets by real-time PCR (A) and the inhibition on autologous T cell proliferation induced by anti-CD3/CD28 antibodies in inhibition assays (B). *, Significant statistical differences between T cells treated with COP-I and those treated with the peptide control (P < 0.05).
Fig. 3.
Immunoblot analysis of Foxp3 expression in CD4+CD25+ T cells after COP-I treatment. PBMC were treated with 40 μg/ml COP-I or control peptides for 4 days. The resulting CD4+CD25+ T cells were subsequently purified and lysed. Lysate was then subjected to 10% SDS/PAGE and followed by immunoblot analysis with an anti-human Foxp3 antibody. An antibody to human β-actin was used as a control.
Table 1. Foxp3 mRNA expression and cytokine productions in COP-I specific T cell lines and control T cell lines.
| Cytokine production, pg/ml | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| No. of T cell lines | Specificity | Foxp3 mRNA | SI | Inhibition, % | IFN-γ | TGF-β1 | TNF-α | IL-4 | IL-10 |
| 15 | COP-I | 26.2 ± 20.4 | 4.9 ± 1.9 | 45.5 ± 18.4 | 1,650.12 | 368.91 | 56.33 | 29.71 | 149.65 |
| 10 | Peptide control | 2.1 ± 3.3 | 5.2 ± 3.5 | −55.5 ± 12.6 | 56.45 | 385.91 | 64.65 | 32.17 | 122.34 |
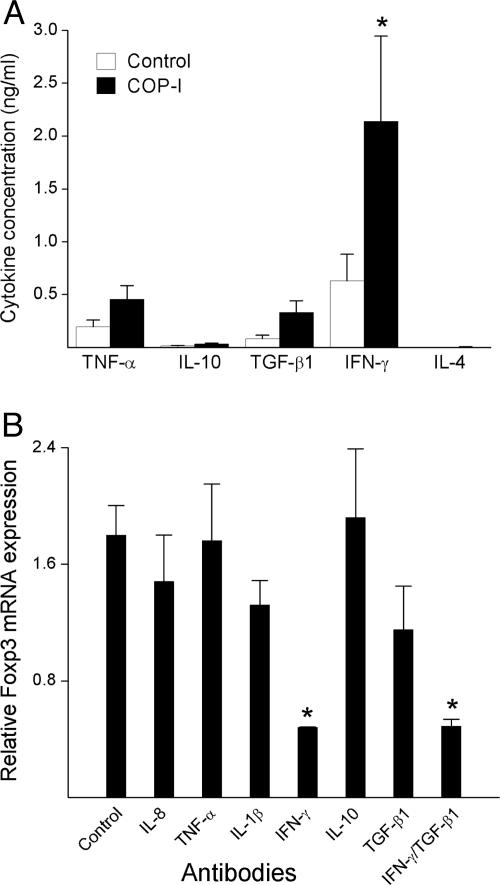
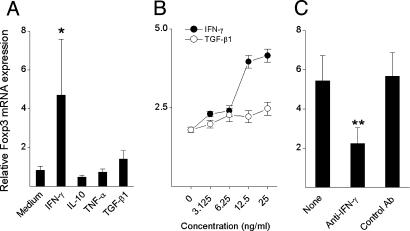
Induction of Foxp3 Expression and Regulatory Function in CD4+ T Cells by COP-I Is Mediated Through IFN-γ. We further evaluated whether the effect induced by COP-I was mediated through its ability to induce the production of certain cytokines. It was evident that T cell activation induced by COP-I resulted in the significantly increased production of IFN-γ, TGF-β1, and TNF-α but not IL-10 and IL-4 (Fig. 4_A_), which was in agreement with a number of the previous reports, including our own (5, 28, 29). A panel of six monoclonal antibodies to the selected cytokines that were predominantly produced in response to COP-I described here and in other reports (30) was analyzed for its potential blocking effect on the property of COP-I. As illustrated in Fig. 4_B_, only IFN-γ antibody significantly blocked the effect of COP-I on the induction of Foxp3 (73.3% inhibition, P < 0.05), whereas the observed effect of TGF-β1 antibody did not reach a statistically significant level (36.1% inhibition, P = 0.095). The results indicated that the effect of COP-I was mediated by IFN-γ and, to a lesser degree, by TGF-β1. TGF-β1in the presence of anti-CD3 antibody was recently described to have an effect on Foxp3 expression in T cells (31), whereas IFN-γ had not been known for a similar ability to induce Foxp3 expression. The role of IFN-γ in the induction of Foxp3 expression by COP-I was confirmed in further characterization with recombinant IFN-γ.As shown in Fig. 5, IFN-γ but not other cytokines (including TGF-β1) used at a similar concentration in the absence of a T cell stimulus induced the expression of Foxp3 in CD4+CD25- T cells.
Fig. 4.
Cytokine profile of PBMC in response to COP-I stimulation and antibody blocking experiments. (A) PBMC preparations derived from healthy individuals were cultured in the presence of COP-I or the peptide control at a concentration of 40 μg/ml. Culture supernatants were collected on day 4 and analyzed for the production of the indicated cytokines by ELISA. Data are presented as the mean cytokine concentrations of six individual samples. (B) In parallel experiments, the same PBMC preparations were cultured with 40 μg/ml COP-I in the presence of the indicated purified monoclonal antibodies used at concentrations of 50 ng/ml to 10 μg/ml as instructed, respectively. Cells were collected on day 4 and analyzed for Foxp3 expression by real-time PCR. *, Statistical differences (P < 0.05).
Fig. 5.
The effect of IFN-γ on the expression of Foxp3 in human PBMC. PBMC preparations were obtained from 10 healthy individuals and cultured in the presence or absence of the indicated recombinant cytokines at a concentration of 25 ng/ml for 72 h. (A) The resulting cells were analyzed for the expression of Foxp3 by real-time PCR. The data are presented as relative expression of Foxp3. CD4+CD25- T cells were purified from the same PBMC cultured in the presence of recombinant IFN-γ at the indicated concentrations for 72 h. (B) mRNA expression of Foxp3 in the resulting T cells was measured under the same experimental conditions. CD4+CD25- T cells were cultured in the presence of human recombinant IFN-γ (25 ng/ml) and a monoclonal antibody to human IFN-γ (10 μg/ml). An isotype-matched antibody was used at the same concentration as a control. (C) The resulting T cells were analyzed for mRNA expression of Foxp3 by real-time PCR. The results were reproducible in at least three independent experiments. *, Statistical difference between IFN-γ and medium control (P < 0.01); **, statistical difference between anti-IFN-γ and the controls (P < 0.05).
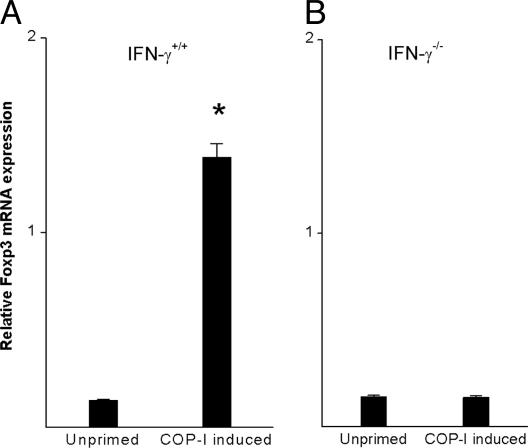
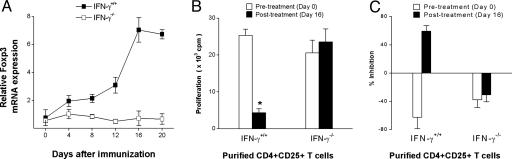
The role of IFN-γ in the induction of Foxp3 expression and regulatory function in T cells was further investigated in IFN-γ knockout mice. As shown in Fig. 6, in vitro treatment of CD4+CD25- T cells with COP-I resulted in increased expression of Foxp3 in wild-type mice but not IFN-γ knockout mice of the same C57/BL6 background. It was evident that although administration of COP-I induced an increase in CD4+CD25+ T cells in wild-type mice and IFN-γ knockout mice, Foxp3 expression was found in CD4+CD25+ T cells obtained from wild-type mice but not those of IFN-γ gene-knockout mice (Fig. 7_A_). CD4+CD25+ T cells derived from wild-type mice but not IFN-γ knockout mice exhibited reduced proliferation in response to stimulation induced by anti-CD3/CD28 monoclonal antibodies, indicating a trait of CD4+CD25+ regulatory T cells (Fig. 7_B_) and significant inhibitory activities at day 16 (Fig. 7_C_). Taken together, the results indicate that the activation of Foxp3 expression and the conversion of CD4+CD25- to CD4+CD25+ regulatory T cells by COP-I required IFN-γ as a mediator.
Fig. 6.
Induction of Foxp3 expression in T cells by in vitro treatment with COP-I in wild-type and IFN-γ-deficient mice. Splenocytes were derived from wild-type mice (A) and IFN-γ knockout mice (B) of the B6/C57 background. CD4+CD25- T cells were subsequently isolated by magnetic bead separation and cultured in the presence or absence of 40 μg/ml COP-I and irradiated splenocytes predepleted for T cells as a source of antigen-presenting cells. The resulting T cells were collected for Foxp3 mRNA expression on day 4. *, Statistical significance between the COP-I untreated group and the COP-I-induced group (P < 0.01).
Fig. 7.
Induction of CD4+CD25+ regulatory T cells in wild-type and IFN-γ knockout mice in response to administration of COP-I. Wild-type and IFN-γ knockout mice were administered COP-I at 5 mg per mouse, which represented an effective dosage demonstrated in previous studies (39). Mice were killed on the indicated days. Splenocytes were obtained and analyzed ex vivo for Foxp3 expression in purified CD4+CD25- and CD4+CD25+ T cell subsets by real-time PCR (A), the proliferative response to anti-CD3/CD28 antibodies (B), and the inhibition on syngeneic T cell proliferation induced by anti-CD3/CD28 antibodies in inhibition assays (C). *, Significant statistical differences between posttreated and pretreated CD4+CD25+ T cells (P < 0.05).
Discussion
In this study, we demonstrated that COP-I, an immunoregulatory agent of unknown mechanism, has a unique property in the conversion of CD4+CD25- T cells to CD4+CD25+ regulatory T cells. There is compelling evidence presented in this study, indicating that the induction of CD4+CD25+ regulatory T cells by COP-I is mediated through the activation of transcription factor Foxp3 in CD4+ T cells. The conclusions are based on the following findings. First, we demonstrated direct evidence that COP-I induced in vitro conversion of unprimed human CD4+CD25- T cells to CD4+CD25+ T cells that acquired high expression of Foxp3 and the inhibitory functions. These CD4+CD25+ regulatory T cells appeared to expand in response to COP-I from the CD4+ T cell pool of CD45RA and CD45RO phenotypes. Secondly, COP-I T cell lines generated by repeated stimulation cycles exhibited very high expression levels of Foxp3 and the inhibition rate when compared with single stimulation of CD4+CD25- T cells with COP-I and control T cell lines. This finding has not only strengthened the key conclusion mentioned above but also indicated that a large proportion of COP-I reactive T cell lines represent CD4+CD25+ regulatory T cells. Our experiments have confirmed correlation between the expression of Foxp3 and the inhibitory function in CD4+ T cells. Furthermore, standard treatment with COP-I in MS patients and administration of COP-I in mice resulted in significant increase of Foxp3 expression in T cells, providing the compelling in vivo evidence for the role of COP-I in the induction of Foxp3. It is conceivable, however, that not all CD4+CD25+ T cells induced by COP-I represent regulatory T cells. As discussed below, COP-I activates CD4+ effector secreting IFN-γ that renders conversion of a proportion of CD4+CD25+ T cells into regulatory T cells.
The findings described here are highly significant in the understanding of the mechanism of action of COP-I in relation to its treatment efficacy in MS. In this regard, COP-I may act, through its ability to induce CD4+CD25+ regulatory T cell response, to compensate a functional deficit in this important regulatory mechanism in MS (23). It is conceivable that this unique property of COP-I in the induction of CD4+CD25+ regulatory T cells may attribute, at least in part, to the treatment efficacy of COP-I in MS. The observation is also likely to offer a reasonable explanation for, or it may reconcile with, some previously described regulatory functions of COP-I of unknown mechanism, including so-called “bystander” inhibitory effect of COP-I on T cell activation, which is a frequently reported phenomenon (8). The antigen nonspecific inhibitory effect of CD4+CD25+ regulatory T cell response induced by COP-I is consistent with the spectrum of inhibition induced by COP-I that is often not limited to one antigen (i.e., MBP) and includes various other myelin antigens (16, 32). Furthermore, if the induction of CD4+CD25+ regulatory T cells is closely associated with the treatment effect of COP-I in MS, one potentially important aspect of the clinical significance of the study may involve the role of Foxp3 as a surrogate biomarker in measurement of treatment efficacy. Currently, the treatment efficacy of COP-I can only be measured ≈9 months after the treatment by using the standard clinical and magnetic resonance imaging techniques (33). However, it should be cautioned that this study does not completely exclude the role of other regulatory properties of COP-I in the treatment of MS, especially its ability to induce Th2 immunity through repeated administration (5). It is conceivable that these regulatory mechanisms induced by COP-I may work in concert to achieve sufficient immune regulation ultimately beneficial to the clinical course of MS.
Another important aspect of the study is related to defining the mechanism of action potentially responsible for the induction of Foxp3 expression by COP-I in CD4+ T cells, which leads to the conversion of CD4+CD25- T cells to CD4+CD25+ regulatory T cells. It was evident in the study that the observed effect of COP-I is largely mediated through IFN-γ. It is known from this and other reports that COP-I has the ability to stimulate the production of Th1 and Th2 cytokines, including IFN-γ (13, 28). The role of IFN-γ in the induction of Foxp3 expression and CD4+CD25+ regulatory T cell response is supported by the following experimental evidence: (i) Blocking of IFN-γ (but not other cytokines also induced by COP-I) by specific antibody resulted in significant inhibition of the effect of COP-I. (ii) Treatment of CD4+ T cells with recombinant IFN-γ led to increased Foxp3 expression. (iii) COP-I failed to induce Foxp3 expression in T cells of IFN-γ knockout mice in in vitro and in vivo settings. The role of IFN-γ appears different from that of TGF-β1 in the induction of Foxp3 and conversion of CD4+CD25- T cells to CD4+CD25+ regulatory T cells. TGF-β1 requires costimulation of T cells with an anti-CD3 antibody to induce Foxp3 expression (34). As described here and by other investigators (31, 34), in contrast to IFN-γ, TGF-β1 is insufficient when used alone to directly induce Foxp3 expression in T cells. Our observation that the induction of Foxp3 expression by COP-I is partially blocked by antibody to TGF-β1 supports the possibility that the involvement of TGF-β1 in the induction of Foxp3 expression requires a stimulatory signal provided by COP-I.
The finding that IFN-γ has the effect of mediating the induction of Foxp3 expression and CD4+CD25+ regulatory T cells is consistent with a recent report indicating that signal transducer and activator of transcription-1 (STAT1), a signaling molecule closely associated with the IFN-γ signaling pathway, is critical to the induction of CD4+CD25+ regulatory T cells (24). The authors demonstrated that STAT1-deficient mice expressing a transgenic T cell receptor against MBP spontaneously developed experimental autoimmune encephalomyelitis, which was attributable to a functional impairment of CD4+CD25+ regulatory T cells in STAT1-deficient mice (24). Furthermore, the study described here has also raised new questions regarding the role of IFN-γ in T cell regulation. This is another example adding to the recent debate on the functional role of Th1 and Th2 cytokines in autoimmune conditions, such as MS (35-38). The traditionally held Th1 paradigm is being challenged by mounting evidence that not all Th1 cytokines (e.g., IFN-γ and TNF-α) are necessarily the culprits for MS; in some aspects, they may be beneficial. In conclusion, it is clear from the present study that COP-I acts as an inducer for CD4+CD25+ regulatory T cell response through the activation of Foxp3 expression. This agent has a potent and selective property for the induction of Foxp3 expression and CD4+CD25+ regulatory T cells in human and animal experimental systems, making it an excellent tool for the study of CD4+CD25+ regulatory T cells in future investigations.
Supplementary Material
Supporting Information
Acknowledgments
This work was supported by Chinese Ministry of Science and Technology Grant 863, Projects 04DZ1920, 2002AA216121 and 202CCCD2000; Shanghai Commission of Science and Technology Grants 01JC14036, 20014319207, 04DZ14902, and 03XD14015; National Natural Science Foundation of China Grant 30430650; and National Institutes of Health Grants NS41289 and NS48860 (to J.Z.Z.).
Author contributions: J.Z.Z. designed research; J.H., N.L., X.Z., and B.Z. performed research; J.H., N.L., B.Z., and J.Z.Z. analyzed data; N.L. and B.Z. contributed new reagents/analytic tools; J.Z.Z. wrote the paper; and J.Z.Z. supervised the entire project.
Abbreviations: COP-I, copolymer-I; MBP, myelin basic protein; MS, multiple sclerosis; PBMC, peripheral blood mononuclear cells; Th, T helper.
References
- 1.Bornstein, M. B., Miller, A., Slagle, S., Weitzman, M., Crystal, H., Drexler, E., Keilson, M., Merriam, A., Wassertheil-Smoller, S. & Spada, V. (1987) N. Engl. J. Med. 317**,** 408-414. [DOI] [PubMed] [Google Scholar]
- 2.Gran, B., Tranquill, L. R., Chen, M., Bielekova, B., Zhou, W., Dhib-Jalbut, S. & Martin, R. (2000) Neurology 55**,** 1704-1714. [DOI] [PubMed] [Google Scholar]
- 3.Teitelbaum, D., Milo, R., Arnon, R. & Sela, M. (1992) Proc. Natl. Acad. Sci. USA 89**,** 137-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang, J. & Hutton, G. (2005) Annu. Rev. Med. 56**,** 237-302. [DOI] [PubMed] [Google Scholar]
- 5.Miller, A., Shapiro, S., Gershtein, R., Kinarty, A., Rawashdeh, H., Honigman, S. & Lahat, N. (1998) J. Neuroimmunol. 92**,** 113-121. [DOI] [PubMed] [Google Scholar]
- 6.Dhib-Jalbut, S., Chen, M., Said, A., Zhan, M., Johnson, K. P. & Martin, R. (2003) J. Neuroimmunol. 140**,** 163-171. [DOI] [PubMed] [Google Scholar]
- 7.Chen, M., Gran, B., Costello, K., Johnson, K., Martin, R. & Dhib-Jalbut, S. (2001) Mult. Scler. 7**,** 209-219. [DOI] [PubMed] [Google Scholar]
- 8.Aharoni, R., Teitelbaum, D., Sela, M. & Arnon, R. (1998) J. Neuroimmunol. 91**,** 135-146. [DOI] [PubMed] [Google Scholar]
- 9.Duda, P. W., Schmied, M. C., Cook, S. L., Krieger, J. I. & Hafler, D. A. (2000) J. Clin. Invest. 105**,** 967-976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neuhaus, O., Farina, C., Yassouridis, A., Wiendl, H., Then Bergh, F., Dose, T., Wekerle, H. & Hohlfeld, R. (2000) Proc. Natl. Acad. Sci. USA 97**,** 7452-7457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Musette, P., Benveniste, O., Lim, A., Bequet, D., Kourilsky, P., Dormont, D. & Gachelin, G. (1996) Res. Immunol. 147**,** 435-441. [DOI] [PubMed] [Google Scholar]
- 12.Brod, S. A., Nelson, L. D., Khan, M. & Wolinsky, J. S. (1997) Int. J. Neurosci. 90**,** 187-202. [DOI] [PubMed] [Google Scholar]
- 13.Ziemssen, T., Kumpfel, T., Klinkert, W. E., Neuhaus, O. & Hohlfeld, R. (2002) Brain 125**,** 2381-2391. [DOI] [PubMed] [Google Scholar]
- 14.Fridkis-Hareli, M., Teitelbaum, D., Gurevich, E., Pecht, I., Brautbar, C., Kwon, O. J., Brenner, T., Arnon, R. & Sela, M. (1994) Proc. Natl. Acad. Sci. USA 91**,** 4872-4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Racke, M. K., Martin, R., McFarland, H. & Fritz, R. B. (1992) J. Neuroimmunol. 37**,** 75-84. [DOI] [PubMed] [Google Scholar]
- 16.Teitelbaum, D., Fridkis-Hareli, M., Arnon, R. & Sela, M. (1996) J. Neuroimmunol. 64**,** 209-217. [DOI] [PubMed] [Google Scholar]
- 17.Fridkis-Hareli, M., Neveu, J. M., Robinson, R. A., Lane, W. S., Gauthier, L., Wucherpfennig, K. W., Sela, M. & Strominger, J. L. (1999) J. Immunol. 162**,** 4697-4704. [PubMed] [Google Scholar]
- 18.Neuhaus, O., Farina, C., Wekerle, H. & Hohlfeld, R. (2001) Neurology 56**,** 702-708. [DOI] [PubMed] [Google Scholar]
- 19.Hori, S., Nomura, T. & Sakaguchi, S. (2003) Science 299**,** 1057-1061.12522256 [Google Scholar]
- 20.Khattri, R., Cox, T., Yasayko, S. A. & Ramsdell, F. (2003) Nat. Immunol. 4**,** 337-342. [DOI] [PubMed] [Google Scholar]
- 21.Fontenot, J. D., Gavin, M. A. & Rudensky, A. Y. (2003) Nat. Immunol. 4**,** 330-336. [DOI] [PubMed] [Google Scholar]
- 22.Brunkow, M. E., Jeffery, E. W., Hjerrild, K. A., Paeper, B., Clark, L. B., Yasayko, S. A., Wilkinson, J. E., Galas, D., Ziegler, S. F. & Ramsdell, F. (2001) Nat. Genet. 27**,** 68-73. [DOI] [PubMed] [Google Scholar]
- 23.Viglietta, V., Baecher-Allan, C., Weiner, H. L. & Hafler, D. A. (2004) J. Exp. Med. 199**,** 971-979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nishibori, T., Tanabe, Y., Su, L. & David, M. (2004) J. Exp. Med. 199**,** 25-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kukreja, A., Cost, G., Marker, J., Zhang, C., Sun, Z., Lin-Su, K., Ten, S., Sanz, M., Exley, M., Wilson, B., Porcelli, S. & Maclaren, N. (2002) J. Clin. Invest. 109**,** 131-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walker, M. R., Kasprowicz, D. J., Gersuk, V. H., Benard, A., Van Landeghen, M., Buckner, J. H. & Ziegler, S. F. (2003) J. Clin. Invest. 112**,** 1437-1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schubert, L. A., Jeffery, E., Zhang, Y., Ramsdell, F. & Ziegler, S. F. (2001) J. Biol. Chem. 276**,** 37672-37679. [DOI] [PubMed] [Google Scholar]
- 28.Farina, C., Then Bergh, F., Albrecht, H., Meinl, E., Yassouridis, A., Neuhaus, O. & Hohlfeld, R. (2001) Brain 124**,** 705-719. [DOI] [PubMed] [Google Scholar]
- 29.Zang, Y., Hong, J., Robinson, R., Li, S., Rivera, V. M. & Zhang, J. Z. (2003) J. Neuroimmunol. 137**,** 144-153. [DOI] [PubMed] [Google Scholar]
- 30.Hong, J., Zang, Y. C., Hutton, G., Rivera, V. M. & Zhang, J. Z. (2004) J. Neuroimmunol. 152**,** 126-139. [DOI] [PubMed] [Google Scholar]
- 31.Chen, W., Jin, W., Hardegen, N., Lei, K. J., Li, L., Marinos, N., McGrady, G. & Wahl, S. M. (2003) J. Exp. Med. 198**,** 1875-1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ben-Nun, A., Mendel, I., Bakimer, R., Fridkis-Hareli, M., Teitelbaum, D., Arnon, R., Sela, M. & Kerlero de Rosbo, N. (1996) J. Neurol. 243**,** S14-22. [DOI] [PubMed] [Google Scholar]
- 33.Comi, G., Filippi, M. & Wolinsky, J. S. (2001) Ann. Neurol. 49**,** 290-297. [PubMed] [Google Scholar]
- 34.Fantini, M. C., Becker, C., Monteleone, G., Pallone, F., Galle, P. R. & Neurath, M. F. (2004) J. Immunol. 172**,** 5149-5153. [DOI] [PubMed] [Google Scholar]
- 35.Moreau, T., Coles, A., Wing, M., Isaacs, J., Hale, G., Waldmann, H. & Compston, A. (1996) Brain 119**,** 225-237. [DOI] [PubMed] [Google Scholar]
- 36.Waisman, A., Ruiz, P. J., Hirschberg, D. L., Gelman, A., Oksenberg, J. R., Brocke, S., Mor, F., Cohen, I. R. & Steinman, L. (1996) Nat. Med. 2**,** 899-905. [DOI] [PubMed] [Google Scholar]
- 37.Lafaille, J. J., Keere, F. V., Hsu, A. L., Baron, J. L., Haas, W., Raine, C. S. & Tonegawa, S. (1997) J. Exp. Med. 186**,** 307-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barth, H., Klein, K., Bortlein, A., Guseo, A., Berg, P. A., Wietholter, H. & Klein, R. (2002) J. Neuroimmunol. 133**,** 175-183. [DOI] [PubMed] [Google Scholar]
- 39.Lando, Z., Teitelbaum, D. & Arnon, R. (1979) J. Immunol. 123**,** 2156-2160. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information