Expression of CCR5 Increases during Monocyte Differentiation and Directly Mediates Macrophage Susceptibility to Infection by Human Immunodeficiency Virus Type 1 (original) (raw)
Abstract
The stage of differentiation and the lineage of CD4+ cells profoundly affect their susceptibility to infection by human immunodeficiency virus type 1 (HIV-1). While CD4+ T lymphocytes in patients are readily susceptible to HIV-1 infection, peripheral blood monocytes are relatively resistant during acute or early infection, even though monocytes also express CD4 and viral strains with macrophage (M)-tropic phenotypes predominate. CCR5, the main coreceptor for M-tropic viruses, clearly contributes to the ability of CD4+ T cells to be infected. To determine whether low levels of CCR5 expression account for the block in infection of monocytes, we examined primary monocyte lineage cells during differentiation. Culturing of blood monocytes for 5 days led to an increase in the mean number of CCR5-positive cells from <20% of monocytes to >80% of monocyte-derived macrophages (MDM). Levels of CCR5 expression per monocyte were generally lower than those on MDM, perhaps below a minimum threshold level necessary for efficient infection. Productive infection may be restricted to the small subset of monocytes that express relatively high levels of CCR5. Steady-state CCR5 mRNA levels also increased four- to fivefold during MDM differentiation. Infection of MDM by M-tropic HIV-1JRFL resulted in >10-fold-higher levels of p24, and MDM harbored >30-fold more HIV-1 DNA copies than monocytes. In the presence of the CCR5-specific monoclonal antibody (MAb) 2D7, virus production and cellular levels of HIV-1 DNA were decreased by >80% in MDM, indicating a block in viral entry. There was a direct association between levels of CCR5 and differentiation of monocytes to macrophages. Levels of CCR5 were related to monocyte resistance and macrophage susceptibility to infection because infection by the M-tropic strain HIV-1JRFL could be blocked by MAb 2D7. These results provide direct evidence that CCR5 functions as a coreceptor for HIV-1 infection of primary macrophages.
Tissue macrophages are a major target for human immunodeficiency virus type 1 (HIV-1) infection (19, 45). Chronically infected macrophages could provide a reservoir of HIV-1 that persists in patients whose CD4+ T-cell viral burdens have been drastically reduced by highly active antiretroviral combination therapies which include protease inhibitors (30). Monocyte-derived macrophages (MDM) are not killed by HIV-1 infection but produce virus for as long as several weeks in cultures. In contrast, activated CD4+ T cells are highly sensitive to the cytopathic effects of HIV-1. MDM have the potential to act as long-lived reservoirs for HIV-1 and to disseminate the virus to other tissues (30, 45, 46). Elucidation of the mechanism of macrophage infection is essential to the design of effective therapeutic strategies that not only block viable virus production by short-lived T cells but also prevent new infections of long-lived tissue macrophages.
The stage of differentiation and the lineage of CD4+ cells profoundly affect their susceptibility to infection by HIV-1. For example, memory CD4+ T cells, which express CD45RO and differentiate from naive CD4+ T cells in a postthymic antigen-dependent process, are infected to greater levels than naive CD45RA-expressing CD4+ T cells (36, 43). During acute stages of pediatric infection, as many as 1 to 10% of peripheral blood CD4+ T cells can harbor proviral forms of HIV-1 (3). In contrast, cells of the monocyte lineage in peripheral blood are rarely infected during acute or early infection (3, 25, 26, 37), even though monocytes express CD4 and viral strains with macrophage (M)-tropic phenotypes predominate (15, 31, 52). Differentiation of monocytes to macrophages in tissues such as brain or lung can result in susceptibility to infection. Monocytes in cultures must also undergo differentiation to macrophages to become maximally susceptible to productive infection by M-tropic viruses (20, 32, 45). Levels of CD4 expression in differentiated macrophages are lower than those in blood monocytes, indicating that monocyte resistance and macrophage susceptibility to infection involve some factor(s) other than absolute levels of CD4 (46).
The chemokine receptor CCR5 serves as a coreceptor for M-tropic HIV-1 entry into CD4-expressing T lymphocytes (4, 9, 11, 17, 18, 24) and is a critical factor in HIV-1 pathogenesis. Individuals deficient in CCR5 and peripheral blood mononuclear cells (PBMC) derived from these subjects show high levels of resistance to HIV-1 infection (16, 21, 24, 27, 34). CCR5 is differentially expressed on naive and memory T cells (8). Low levels of CCR5 on CD45RA T cells and high levels on CD45RO T cells appear to be associated with differential levels of infection in these CD4+ T-cell subsets. Infection of CD4+ T cells by M-tropic strains of HIV-1 is antagonized by the chemokine peptides RANTES, MIP-1α, and MIP-1β, which are natural ligands of CCR5 (5, 13, 17).
Although CCR5 clearly contributes to the susceptibility of CD4+ T lymphocytes to infection, the evidence that CCR5 participates in infection of CD4+ macrophages by M-tropic viruses is less convincing. For example, some blood monocytes and tissue macrophages express CCR5 (8, 50), and deletion of CCR5 renders MDM resistant to infection by viruses that require CCR5 (9), supporting a role for CCR5 in macrophage infection. However, the data are contradictory with regard to the ability of CCR5 ligands to inhibit infection of MDM, raising the possibility that macrophages are infected by a CCR5-independent mechanism or that cell type influences interactions among CCR5, its ligands, and the HIV-1 envelope glycoprotein (9, 35, 49).
Chemokine receptor-dependent HIV-1 infection can be inhibited not only by ligands for receptors but also by monoclonal antibodies (MAb) (49). We used 2D7, a MAb that recognizes an epitope in the second extracellular loop of CCR5 (49), to assess the role of CCR5 in HIV-1 infection of monocytes and macrophages. We found (i) that there is a direct association between levels of CCR5 and differentiation of monocytes to macrophages, (ii) that levels of CCR5 are related to macrophage susceptibility to infection, and (iii) that macrophage infection by M-tropic strain HIV-1JRFL can be blocked by 2D7. Our results provide direct evidence that CCR5 functions as a coreceptor for HIV-1 infection of macrophages.
MATERIALS AND METHODS
MDM cultures.
PBMC were obtained by Lymphoprep (Sigma) density gradient centrifugation of commercial leukocyte preparations from HIV-1-negative donors. Monocytes were enriched from the PBMC fraction by 42.55% Percoll density gradient centrifugation, adherence to plastic for 1 h in RPMI 1640 (Gibco-BRL) plus 20% human AB serum that was negative for human immunodeficiency virus and hepatitis B virus antibodies (Sigma), and extensive rinsing in RPMI 1640 without serum (14). Monocyte preparations in which 85 to 90% of the CD4-positive cells were also CD14 positive were cultured at approximately 5 × 105 cells per well in 24-well plates (Falcon 3047) containing differentiation medium (RPMI 1640 plus 20% human AB serum and supplemented with 1 ng of recombinant human granulocyte-macrophage colony-stimulating factor [Gibco-BRL] per ml, 100 U of penicillin per ml, and 100 μg of streptomycin per ml). Monocytes differentiated after 5 days in culture into macrophages (MDM); the macrophages were evaluated by morphological criteria, esterase staining, and flow cytometry analysis, which indicated <3% contamination with T cells. MDM preparations are routinely characterized for susceptibility to infection by M-tropic HIV-1JRFL and resistance to infection by T-cell-line-tropic HIV-1LAI or HIV-1MN.
Virus infection.
HIV-1JRFL, which uses the chemokine receptor CCR5 as a cofactor, was used in all experiments (9, 23). A stock of HIV-1JRFL was produced from infected PBMC supernatants, which were filtered through 0.45-μm-pore-size sterile membranes, and stored in aliquots at −80°C. The infectivity titer was quantified by a p24 antigen assay (Coulter) based upon a 50% tissue culture infective dose in a terminal dilution assay with MDM. The same stock of virus was used in all experiments. The HIV-1JRFL stock was also titrated on HOS-CD4.CCR5 cells (human osteosarcoma cells stably expressing CD4 and CCR5; NIH AIDS Research and Reference Reagent Program); the amount of virus used to infect primary monocytes or MDM was in the range of inoculation that maximally infected this cell line. Monocytes and MDM were infected with 15 50% tissue culture infective doses of virus in a medium volume of 0.25 ml at 37°C for 12 to 14 h. Excess virus was removed by three washes with phosphate-buffered saline. HIV-1 production was assessed by measuring the amount of soluble p24 antigen released into the supernatant by duplicate cultures. For experiments involving blocking of infection by antibodies, cells were treated for 30 min with various antibody concentrations prior to virus inoculation at 37°C. The stock CCR5-specific MAb 2D7 concentration was 392 μg/ml, and dilutions were made from this stock.
Analysis of chemokine receptor mRNA.
Total RNA was isolated from monocytes and MDM with RNeasy Kits (Qiagen), treated with DNase I (Gibco-BRL), and processed with the RNeasy RNA Clean-up protocol to remove residual deoxyribonucleotides prior to quantitation by spectrophotometry. Cyclophilin, which is expressed constitutively in human leukocytes and serves as a standard to normalize input template (6, 33), or CCR5 cDNAs were synthesized with specific downstream primers (6, 24), 0.2 or 1.0 μg of total RNA for cyclophilin or CCR5, respectively, by use of the Superscript Preamplification System (Gibco-BRL). Amplifications were performed in a 9600 GeneAmp PCR system thermocycler (Perkin-Elmer Cetus) by use of an initial denaturation cycle at 94°C for 5 min, 30 cycles of amplification (1 min at 94°C, 1 min at 60°C, and 1 min at 72°C for each cycle), and a final extension cycle at 72°C for 10 min. For each RNA preparation, cyclophilin and CCR5 were amplified in control reactions without reverse transcriptase to demonstrate the absence of DNA contamination. Products were separated by electrophoresis on ethidium bromide-stained 1% agarose gels. The image was stored digitally with a charge-coupled device camera system (29) and quantified with SIGMAGEL software (Jandel Scientific). Regression analysis was performed to determine the linear range in which the amount of product was proportional to the amount of template (39, 40). Regression coefficients for the linear range of cyclophilin amplification were always >0.97. Relative amounts of cyclophilin and CCR5 mRNAs were determined from double log graphs.
Analysis of HIV-1 DNA.
High-molecular-weight genomic DNA was isolated from monocytes or MDM at 5 and 10 days postinfection. Cell monolayers were washed in phosphate-buffered saline and lysed directly in wells containing 200 μl of K buffer (10 mM Tris-HCl [pH 8.3], 50 mM KCl, 2.5 mM MgCl2, 0.1 mg of gelatin per ml, 0.45% Nonidet P-40, 0.45% Tween 20, 60 μg of proteinase K per ml). Lysates were incubated at 50°C for 1 h and at 95°C for 5 min and then stored at 4°C.
Aliquots of cell lysates were amplified in 50-μl reaction mixtures containing 1× PCR Buffer II (Perkin-Elmer), 1.75 mM MgCl2, 0.2 mM each deoxyribonucleoside triphosphate, 1 U of Taq DNA polymerase (Perkin-Elmer), and 0.1 μM each env region primer (1). Reactions were carried out for an initial denaturation cycle at 94°C for 5 min, amplification at 94°C for 30 s, 60°C for 1 min, and 72°C for 2 min for 35 cycles, and a final extension cycle at 72°C for 10 min. These conditions yield products in the linear range of amplification, as determined by titration of 8E5 cell DNA (1). Intersample DNA amounts were standardized in reactions with primers specific for human β-actin (2). Products were analyzed by hybridization (2, 41). For calculation of cell numbers and estimation of numbers of HIV-1 DNA copies in infected cells, serial dilutions of DNA from 8E5 cells, which contain one complete proviral copy per cell, were amplified with β-actin and env primers, and the resulting products were hybridized for use as DNA standards (47).
Flow cytometry analysis.
PBMC and MDM were prepared for two-color flow cytometry as described previously (42), except that a final concentration of 2% human serum was added to all staining and wash buffers. MDM were harvested by scraping cells gently from culture dishes. Cells were stained with MAb for CCR5 (5C7 and 2D7) or CXCR4 (12G5), which were obtained from the NIH AIDS Research and Reference Reagent Program, and a fluorescein isothiocyanate-conjugated goat anti-mouse MAb. Isotype-matched control MAb for immunoglobulin G1 (IgG1) and IgG2a and phycoerythrin-conjugated MAb directed against CD4 (Leu-3a), CD14 (Leu-M3), or CD3 (Leu-4) were purchased from Becton Dickinson. Cell fluorescence was measured with a FACScan (Becton Dickinson) flow cytometer that was routinely standardized by use of Autocomp according to manufacturer’s protocols. The percentage of cells that were positive was determined by the cumulative subtraction method (38). Histograms of staining by the isotype-matched control antibody and the specific antibody were superimposed, and the channel number at the point of intersection of the two histograms was determined. The percentage of positive cells was determined by subtraction of the integrated area above that channel marker for the negative control antibody from that for the specific antibody.
Statistical analysis.
Statistical significance for differences in mean levels of specific mRNAs was assessed by one-way analysis of variance and Dunnett’s test (SigmaStat). For comparison of chemokine receptor fluorescence-activated cell sorter (FACS) analyses, differences in p24 production, and viral DNA amplification in monocytes and MDM, statistical significance was determined with paired Student’s t tests.
RESULTS
Susceptibility of monocytes and MDM to HIV-1 infection.
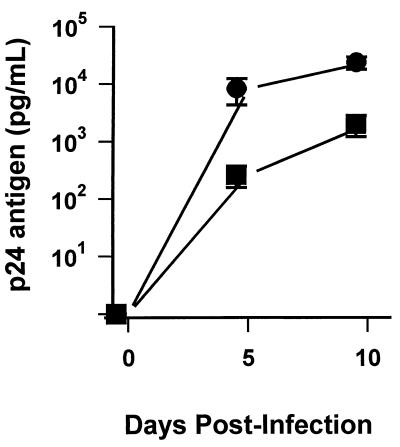
To evaluate the differential susceptibility of blood monocytes and MDM to HIV-1 infection, monocytes from six independent donors, as well as macrophages derived from the monocytes, were inoculated with HIV-1JRFL. Levels of p24 antigen in culture supernatants were assayed at 5 and 10 days postinfection (Fig. 1). At 5 days postinfection, mean levels of p24 produced by monocytes were 243 (±129) pg per ml. In contrast, infected MDM produced mean p24 levels of 9,288 (±3,824) pg per ml. By 10 days postinfection, after multiple rounds of virus replication had occurred, infected monocytes produced 2,019 (±982) pg of p24 per ml, while MDM produced 24,130 (±5,945) pg of p24 per ml (P < 0.05). Monocytes were permissive for low levels of virus replication, although differentiation for 5 days prior to infection enhanced virus production by as much as 100-fold. Absolute amounts of p24 production by infected monocytes or MDM varied depending upon individual donors. To account for this variation, p24 levels produced by MDM relative to levels produced by monocytes were calculated for each individual. By this calculation, mean levels of virus produced when MDM were infected were about 13-fold higher than those produced when monocytes were infected (P < 0.05).
FIG. 1.
HIV-1 infection of monocytes and MDM. Freshly isolated peripheral blood monocytes and MDM cultured in duplicate for 5 days were infected with HIV-1JRFL. p24 antigen levels in culture supernatants were determined for monocytes (squares) and MDM (circles) 5 and 10 days after infection. Values are the mean ± standard error of the mean for cells obtained from six uninfected donors.
To determine whether cultured monocytes might harbor viral DNA that was not expressed as p24 in culture supernatants, HIV-1 env region sequences were amplified from monocyte and MDM DNAs that were isolated 5 days postinfection. DNA quantities were standardized by amplification with β-actin primers so that similar cell equivalents of monocyte and MDM DNAs were analyzed. At 5 days postinfection, 20- to 30-fold differences in HIV-1 DNA copies were found between monocytes and MDM. The levels of infected cells in either the monocyte or the MDM populations were generally related to differences in virus production by monocytes and MDM. Results indicated that monocytes were less susceptible than differentiated macrophages to virus infection by an M-tropic virus that uses CCR5 as a coreceptor.
HIV-1 coreceptor mRNA levels in blood monocytes and MDM.
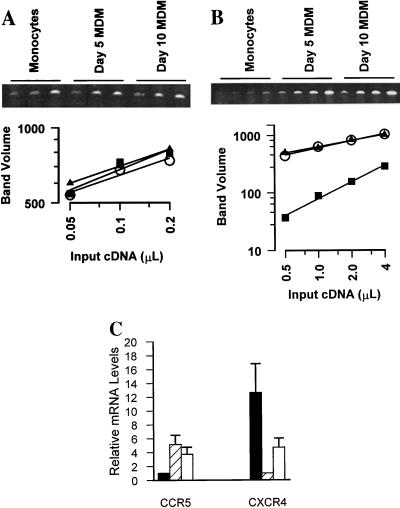
One mechanism that could account for the different susceptibilities of monocytes and MDM to HIV-1 infection is the level of CCR5 expression. To determine whether CCR5 was modulated during the differentiation of monocytes to macrophages, steady-state levels of chemokine receptor mRNAs in monocytes and MDM from each individual were assessed. RNA amounts were first standardized by cyclophilin amplification (Fig. 2A), and then levels of CCR5 mRNA from differentiated MDM were related to CCR5 mRNA levels in monocytes for one individual (Fig. 2B). Monocytes expressed CCR5 mRNA at levels that were about eightfold lower than the levels expressed by MDM after 5 days in culture (Fig. 2B). Steady-state levels of CCR5 expression by differentiated macrophages were stable between 5 and 10 days in culture.
FIG. 2.
Differentiation of monocytes to macrophages modulates coreceptor mRNA. Total RNA was extracted from blood monocytes or MDM after differentiation for 5 to 10 days. Relative levels of mRNAs were evaluated after synthesis of specific cDNA and amplification by a PCR titration method. Amplification of RNA carried out without reverse transcriptase never produced a product. (A) Twofold serial dilutions of cyclophilin cDNA were amplified (ethidium-stained agarose gel) and quantified (squares, monocytes; triangles, day-5 MDM; circles, day-10 MDM) to demonstrate that equal amounts of total RNA were introduced into each cDNA reaction mixture. (B) Twofold serial dilutions of CCR5 cDNA were amplified from monocytes and MDM from one individual (symbols are as in panel A). (C) CCR5 and CXCR4 mRNAs from five individuals were titrated and quantified. Mean (± standard error of the mean) levels of CCR5 in MDM after culturing for 5 days (hatched bar) or 10 days (open bar) are reported relative to levels in monocytes (solid bar). CXCR4 levels in monocytes (solid bar) and MDM cultured for 10 days (open bar) are reported relative to expression by MDM after culturing for 5 days (hatched bar).
To determine the range of changes in CCR5 expression among individuals, monocytes and MDM from five independent donors were evaluated. The mean increase in CCR5 mRNA levels was calculated for the group of individuals (Fig. 2C). Mean levels of CCR5 mRNA were about fivefold higher in differentiated macrophages than in peripheral blood monocytes (P < 0.05). Between 5 and 10 days in culture, CCR5 mRNA levels in MDM decreased but remained significantly higher than levels of CCR5 mRNA expressed by monocytes (P < 0.05). Conversely, mean steady state levels of CXCR4 mRNA were 12-fold higher in monocytes than in MDM cultured for 5 days (P < 0.05) (Fig. 2C).
To verify that enrichment of monocytes by Percoll and adherence did not affect CCR5 or CXCR4 expression and to rule out a contribution by residual T cells to levels of chemokine receptor expression, monocytes were selected by an alternative method with immunomagnetic beads and an anti-CD14 MAb prior to RNA extraction. This method results in monocyte preparations that contain fewer than 1% T cells (2, 44). We found that steady-state levels of CCR5 and CXCR4 mRNAs in monocytes enriched by immunoselection were indistinguishable from expression by monocytes enriched by adherence (data not shown). In addition, immunoselected monocytes regulated CCR5 and CXCR4 levels during differentiation to the same degrees as monocytes isolated by adherence. Consequently, levels of CCR5 and CXCR4 mRNAs were unperturbed by monocyte enrichment from peripheral blood and reflected expression by monocytes rather than residual T cells which might be present. CCR5 mRNA expression was low in monocytes and increased during differentiation, while high levels of CXCR4 expression by monocytes decreased during the course of differentiation into macrophages.
Cell surface protein expression of HIV-1 receptors differs between blood monocytes and MDM.
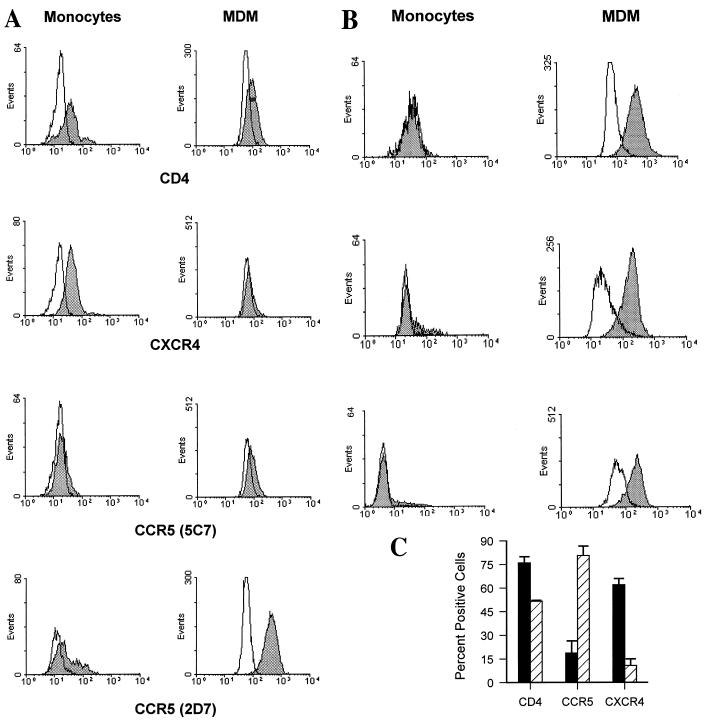
To determine whether cell surface protein levels of chemokine receptors were also modulated during differentiation, monocytes and MDM from one individual were analyzed for CD14, CD4, CCR5, and CXCR4 levels by two-color flow cytometry. CD4 or CXCR4 was detected on approximately 60 or 80% of CD14-expressing monocytes, respectively (Fig. 3A, left panels). In contrast, only about 11 to 38% of CD14-positive peripheral blood monocytes also expressed CCR5, depending on the anti-CCR5 MAb used in the analysis. MAb 5C7, which recognizes an epitope in the N-terminal domain, consistently detected a lower percentage of positive cells than an MAb (2D7) that recognizes an epitope in the second extracellular loop (49).
FIG. 3.
Surface expression of HIV-1 receptors on monocytes and MDM. Freshly isolated PBMC and MDM cultured for 5 days were stained with MAb specific for CD4, CXCR4 (MAb 12G5), or CCR5 (MAb 2D7 and 5C7) and analyzed by two-color flow cytometry. Cells were gated on CD14 for analysis, and percentages of positive cells were calculated as described in Materials and Methods. (A) Cells obtained from subject 1. Histograms of monocytes (left panels) and MDM (right panels) for specific antibody staining (shaded histograms) are shown relative to histograms for isotype-matched, nonspecific antibody staining (open histograms). Fluorescence intensity is displayed on the x axis. (B) Monocytes and MDM from three additional individuals were stained for CD14 and CCR5 with MAb 2D7 and reported as in panel A. (C) Mean (± standard error of the mean) percentages of positive cells for peripheral blood monocytes (solid bars) and MDM after 5 days of differentiation (hatched bars) from four individuals are shown.
Monocytes from the same individual were cultured in differentiation medium and, after 5 days, the resulting MDM were analyzed. Differentiation induced a modest decline in surface CD4 expression to about 50% of the CD14-positive MDM (Fig. 3A, right panel), while differentiation of monocytes to MDM had a dramatic impact on chemokine receptor levels. CXCR4 was reduced from expression on 80% of monocytes to essentially undetectable levels on MDM. In contrast, CCR5 was detected on virtually all CD14-positive MDM (>90%) when MAb 2D7 was used (Fig. 3A, right panel). N-terminal domain MAb 5C7 detected only a slight upregulation (10%) of surface CCR5 expression.
Significant individual variation in CCR5 expression, ranging from 0 to 38% positive, was revealed by analysis of monocytes from three additional individuals (Fig. 3B). However, MDM tended to display similar numbers of CCR5-positive cells following 5 days in culture (69 to 97%). The number of CCR5-positive MDM after 5 days in culture was independent of the levels displayed on a given individual’s monocytes.
To estimate differences in relative CCR5 expression per cell between monocytes and MDM, the ratios of peak fluorescence intensity of positively stained cells to that of control stained cells were calculated (38). The mean ratio of peak fluorescence intensity of positively stained cells to that of negatively stained cells in monocytes was 1.6, while that in MDM was 4.8, indicating greater per-cell CCR5 expression by MDM than by monocytes (Fig. 3B).
CD14-positive monocytes and MDM from a total of four different individuals were evaluated for CD4 and CCR5 expression, and mean values for the group were calculated (Fig. 3C). CD4 expression declined overall from 76% (±4%) of CD14-positive monocytes to 51% (±0.6%) of CD14-positive MDM, a small but significant reduction (P < 0.05) (Fig. 3C). During the same period of differentiation, mean surface expression of CCR5 (MAb 2D7) increased approximately fourfold, from 19% (range, 0 to 38%) of peripheral blood monocytes to 81% (range, 69 to 97%) of MDM (P < 0.01).
In contrast to the increased levels of surface CCR5 on differentiated macrophages, CXCR4 levels declined by sixfold. Approximately 62% (±4%) of monocytes but only about 10% (±1.5%) of MDM displayed levels of surface CXCR4 that were detectable by MAb 12G5 (P < 0.01). These results demonstrate that differentiation of monocytes into macrophages is accompanied by increased expression of CCR5 and downregulation of CXCR4. Changes in CCR5 or CXCR4 protein levels reflect steady-state expression of mRNAs for each of the chemokine receptors.
A CCR5-specific MAb blocks HIV-1 infection of MDM.
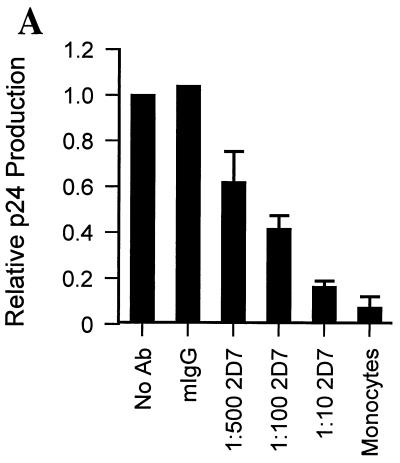
We wanted to demonstrate directly that increased expression of CCR5 on MDM was responsible for increased MDM susceptibility to HIV-1 infection. Although the CCR5 ligands MIP-1α, MIP-1β, and RANTES can block infection of PBMC by CCR5-dependent viruses, these chemokines can be inadequate to inhibit infection of MDM by viruses that depend upon CCR5 as a coreceptor (4, 12, 18, 28, 35; data not shown). Accordingly, we assessed the ability of the CCR5-specific MAb 2D7 to inhibit MDM infection by HIV-1JRFL.
Monocytes and MDM from the same four individuals whose cells were analyzed by FACS were incubated either in the absence or in the presence of various amounts of MAb 2D7 for 30 min prior to infection with HIV-1JRFL. To account for variation in the ability of MDM from different individuals to be infected and produce virus, supernatant p24 production in the presence of antibody is shown relative to p24 production in the absence of antibody for each individual (normalized to a value of 1; Fig. 4A). Mean p24 production by MDM was inhibited by MAb 2D7 in a concentration-dependent manner. Concentrations of 2D7 as low as 0.8 μg per ml of culture (1:500) reduced productive infection by 38% (±14%). The highest concentration of 2D7 (1:10, or 39 μg/ml of culture) produced a mean p24 decrease of 84% (±2.7%) compared to p24 production in MDM infected without prior antibody blocking (P < 0.001) (Fig. 4A). The effect of the CCR5-specific antibody on virus production was specific because treatment with 40 μg of control mouse IgG (mIgG) per ml prior to infection of MDM caused no suppression of p24 production (Fig. 4A).
FIG. 4.
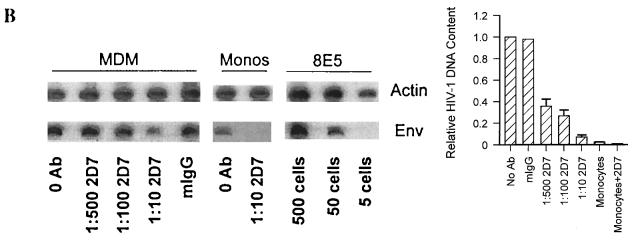
CCR5 MAb 2D7 blocks infection of MDM by HIV-1JRFL. (A) Monocytes and MDM obtained from four donors and analyzed for coreceptor expression in Fig. 3 were infected in the absence or presence of MAb (Ab) 2D7 or 40 μg of mIgG per ml. Dilutions of 1:500, 1:100, or 1:10 resulted in final MAb 2D7 concentrations of 0.8, 3.9, and 39 μg/ml, respectively. Mean levels of p24 produced by monocytes or MDM infected in the presence of antibody were calculated relative to levels of p24 produced by MDM infected in the absence of antibody. In the absence of antibody MDM produced a mean of 2,939 (±1,129) pg of p24 per ml (relative to a value of 1), which was reduced to 421 (±105) pg of p24 per ml (relative to a value of <0.2) in the presence of the largest amount of MAb 2D7 (1:10). The mean p24 level produced when monocytes from the same individuals were infected was 200 ± 102 pg/ml (relative to a value of <0.1). (B) Levels of HIV-1 DNA were determined for the same monocytes (Monos) and MDM as those infected in panel A. (Left panel) DNA was extracted, and the HIV-1 env region was amplified. env products amplified from monocytes were exposed four times longer than MDM-derived products to allow visualization. β-Actin amplification served as a control for amounts of input DNA. HIV-1 DNA standard curves were analyzed in parallel with β-actin and env amplifications of 8E5 cell DNA to allow estimation of numbers of infected cells. (Right panel) Levels of env product formation in infected cells from the four donors were quantified and are reported relative to those produced by PCR amplification of DNA from cells infected in the absence of antibody.
To demonstrate that MAb 2D7 blocked virus infection, cellular DNA from the same monocytes and MDM infected in the presence or absence of MAb 2D7 was extracted 5 days postinfection and amplified with β-actin and env primers. By comparison of amplification products from infected-cell DNA samples to standard curves of amplified 8E5 cell DNA, cell numbers, based on β-actin amplification, and numbers of HIV-1 DNA copies, based on env amplification, were estimated. Viral DNA in MDM declined with increasing levels of MAb 2D7 (Fig. 4B). In the absence of antibody against CCR5 or in the presence of an irrelevant antibody (mIgG), approximately 72,500 HIV-1 copies were present per 105 MDM (Fig. 4B, left panel). At the maximum amount of antibody, approximately 3,200 HIV-1 copies per 105 MDM were detected. Low levels of monocyte infection by HIV-1 were also reduced by 2D7 from about 2,100 copies per 105 MDM without antibody to fewer than 1,000 (below the level of detection) when monocytes were treated with 2D7 prior to infection (Fig. 4B, left panel).
To account for individual variations among monocytes obtained from four individuals, HIV-1 DNA quantities are expressed relative to those in cells infected in the absence of antibody pretreatment (Fig. 4B, right panel). The mean level of infection of MDM without antibody treatment was 63,000 HIV-1 copies per 105 cells (set at a relative value of 1); this value was reduced by more than 60% in the presence of as little as 800 ng (1:500 dilution) of MAb 2D7 per ml of culture. HIV-1 DNA levels were reduced by more than 90% when MDM were treated with the highest concentration of MAb 2D7 (1:10) prior to infection. These data indicate that MAb 2D7 inhibits productive infection and viral DNA formation by HIV-1JRFL in monocytes and MDM by blocking CCR5-dependent entry.
DISCUSSION
Our experiments were designed specifically to understand the role of CCR5 in infection of cells of the monocyte lineage by viruses that rely on CCR5 as a coreceptor. Monocytes and macrophages express CD4, the primary receptor for HIV-1 infection. However, monocytes in peripheral blood from patients who harbor M-tropic, non-syncytium-inducing viruses do not serve as significant reservoirs of HIV-1 infection (3, 25, 26, 37), and freshly isolated peripheral blood monocytes are poorly susceptible to productive infection by M tropic HIV-1 strains (45).
We provide direct evidence that the differentiation of monocytes to macrophages produces a significant increase in the number of cells expressing CCR5. The number of CCR5-expressing cells detected on monocytes and MDM by FACS was higher with MAb 2D7 than with MAb 5C7, presumably reflecting the specificities of the antibodies. MAb 2D7 recognizes an epitope in the second extracellular loop of CCR5, while MAb 5C7 recognizes an epitope in the N-terminal domain (49). In fact, extremely variant results for proportions of CCR5-positive monocytes, depending on the antibody used, have been reported (28a, 50, 51).
A direct relationship between levels of mRNA and surface expression of CCR5 or CXCR4 during differentiation indicates that chemokine receptors themselves are modulated and rules out the possibility that epitopes on receptors recognized by MAb 2D7 or 12G5 are merely altered or modulated. Immunomagnetic beads, which are used to select monocytes from peripheral blood, activate phagocytosis (2). However, the activation of phagocytosis was insufficient to affect CCR5 or CXCR4 levels on monocytes, supporting the conclusion that monocyte differentiation is essential for changes in chemokine receptor levels.
The patterns of CCR5 and CXCR4 expression on blood monocytes and macrophages are consistent with the physiological function(s) of the receptors. A natural ligand for CXCR4, SDF-1, has a role in early events in immune surveillance (7), so that elevated levels of CXCR4 on monocytes may initiate the differentiation of circulating monocytes into macrophages in vivo in response to SDF-1. In contrast, ligands for CCR5 (RANTES, MIP-1α, and MIP-1β) are produced as part of the inflammatory response in tissues and may involve later events in macrophage development (48). CCR5 expression is not restricted to macrophages that differentiate in cultures but is found as well on tissue macrophages (50). Reciprocal differences in patterns of expression of CCR5 and CXCR4 on monocytes and MDM indicate that CCR5 and CXCR4 can serve as differentiation markers for cells of the monocyte-macrophage lineage.
The critical question that we addressed is the role of CCR5 in infection of macrophages by M-tropic HIV-1. There are conflicting results regarding the ability of ligands for CCR5 to block infection of MDM by CCR5-dependent viruses (4, 12, 18, 28, 35), raising the possibility that virus entry into macrophages is independent of CCR5. However, blocking macrophage infection with MAb 2D7 is a significant result from our studies which provides direct evidence that virus infection of macrophages can be CCR5 receptor mediated. If infection occurred by some other mechanism, such as Fc receptors, irrelevant antibodies should have diminished infection. In fact, irrelevant antibodies were ineffective in diminishing MDM infection by CCR5-dependent virus.
The inability of CCR5 agonists to block macrophage infection while effectively inhibiting infection of T lymphocytes may reflect cell-specific differences in CCR5 or in the interactions among CCR5, its various ligands, and HIV-1 envelope glycoprotein. It is possible that CCR5 expressed by MDM has a faster turnover or a lower stability, although the demonstrated effectiveness of a single treatment with MAb 2D7 prior to infection indicates a relatively low rate of turnover of CCR5 on the surface of macrophages. Chemokine peptides could be less stable in MDM cultures either because of the human serum component or because of secretions by MDM into the media. Cell differences might also involve an altered affinity of ligands for CCR5 due to MDM-specific, CCR5-associated membrane or intracellular proteins that participate in signal transduction.
The proportion of peripheral blood monocytes expressing CCR5 varied among individuals to an extent similar to that observed for CCR5 expression on lymphocytes from individuals homozygous for wild-type alleles of CCR5 (50). Even though CCR5 levels on blood monocytes varied, differentiation into macrophages always involved significant increases in numbers of cells expressing CCR5. Macrophages from different donors expressed similar amounts of CCR5 independent of the proportion of CCR5-positive monocytes. However, macrophages differed in their ability to produce virus, suggesting that other cellular factors influence virus replication in macrophages (10).
Low-level infection of monocytes in our experiments could reflect the high titers of virus used or the long inoculation periods, during which CCR5 may be upregulated. Populations of monocytes included some CCR5-positive cells. Levels of CCR5 expression per monocyte were generally lower than those on MDM, perhaps below a minimum threshold level necessary for efficient infection. Productive infection may be restricted to the small subset of monocytes that express relatively high levels of CCR5. The fact that MAb 2D7 blocked infection of monocytes indicates that infection of these cells also can occur via CCR5. Our data demonstrate that 2D7 blocks HIV-1JRFL entry into both monocytes and MDM, indicating that CCR5 is the principal coreceptor used by HIV-1JRFL to gain access into these CD4-positive cells. However, entry into macrophages by some primary HIV-1 isolates may require cofactors other than CCR5 (10a; unpublished results).
Results from our experiments provide a molecular explanation for the infrequent infection of peripheral blood monocytes during acute or early infection by HIV-1, when high levels of M-tropic viruses can be found in peripheral blood lymphocytes and in plasma. Conversely, the presence of high levels of CXCR4 on monocytes provides a feasible molecular basis for the observation that blood monocytes can be infected in late-stage disease, when T-cell-line-tropic, CXCR4-requiring viruses predominate (22). Infection of tissue macrophages most likely occurs by association with T lymphocytes trafficking through tissues. If tissue macrophages can serve as a long-lived reservoir of viruses that are resistant to or sequestered from antiviral therapies, novel strategies are needed to protect macrophages from infection and to target the delivery of drugs to eliminate them as reservoirs of infection.
ACKNOWLEDGMENTS
The following reagents were obtained through the AIDS Research and Reference Reagent Program, AIDS Program, NIAID: CXCR4 monoclonal antibody (12G5) from James Hoxie, CCR5 MAb (2D7 and 5C7) from LeukoSite, Inc., and HIV-1JRFL from Irvin Chen.
This work was supported by PHS awards HD32259, HL58005, and AI28571. D.L.T. is an Elizabeth Glaser Pediatric AIDS Foundation Scholar and was supported by grant T32 CA09126.
REFERENCES
- 1.Aleixo L F, Goodenow M M, Sleasman J W. Zidovudine administered to women infected with human immunodeficiency virus type 1 and to their neonates reduces pediatric infection independent of an effect on levels of maternal virus. J Pediatr. 1997;130:906–914. doi: 10.1016/s0022-3476(97)70276-2. [DOI] [PubMed] [Google Scholar]
- 2.Aleixo L F, Goodenow M M, Sleasman J W. Molecular analysis of highly enriched populations of T-cell-depleted monocytes. Clin Diagn Lab Immunol. 1995;2:733–739. doi: 10.1128/cdli.2.6.733-739.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aleixo, L. F., and M. M. Goodenow. Unpublished data.
- 4.Alkhatib G, Combadiere C, Broder C C, Feng Y, Kennedy P E, Murphy P M, Berger E A. CC CKR5: a RANTES, MIP-1β receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 1996;272:1955–1958. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- 5.Arenzana-Seisdedos F, Virelizier J-L, Rousset D, Clark-Lewis I, Loetscher P, Moser B, Baggiolini M. HIV blocked by chemokine antagonist. Nature. 1996;383:400. doi: 10.1038/383400a0. [DOI] [PubMed] [Google Scholar]
- 6.Bergsma D, Eder C, Gross M, Kersten H, Sylvester D, Appelbaum E, Cusimano D, Livi G, McLaughlin M, Kasyan K, Porter T, Silverman C, Dunnington D, Hand A, Prichett W, Bossard M, Brandt M, Levy M. The cyclophilin multigene family of peptidyl-prolyl isomerases. Characterization of three separate human isoforms. J Biol Chem. 1991;266:23204–23214. [PubMed] [Google Scholar]
- 7.Bleul C C, Fuhlbrigge R C, Casasnovas J M, Aiuti A, Springer T A. A highly efficacious lymphocyte chemoattractant, stromal cell-derived factor 1 (SDF-1) J Exp Med. 1996;184:799–802. doi: 10.1084/jem.184.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bleul C C, Wu L, Hoxie J A, Springer T A, Mackay C R. The HIV coreceptors CXCR4 and CCR5 are differentially expressed and regulated on human T lymphocytes. Proc Natl Acad Sci USA. 1997;94:1925–1930. doi: 10.1073/pnas.94.5.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Broder C C, Collman R G. Chemokine receptors and HIV. J Leukocyte Biol. 1997;62:20–29. doi: 10.1002/jlb.62.1.20. [DOI] [PubMed] [Google Scholar]
- 10.Chang J, Naif H M, Li S, Sullivan J S, Randle C M, Cunningham A L. Twin studies demonstrate a host cell genetic effect on productive human immunodeficiency virus infection of human monocytes and macrophages in vitro. J Virol. 1996;70:7792–7803. doi: 10.1128/jvi.70.11.7792-7803.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10a.Cheng-Mayer C, Liu R, Landau N R, Stamatatos L. Macrophage tropism of human immunodeficiency virus type 1 and utilization of the CC-CKR5 coreceptor. J Virol. 1997;71:1657–1661. doi: 10.1128/jvi.71.2.1657-1661.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choe H, Farzan M, Sun Y, Sullivan N, Rollins B, Ponath P D, Wu L, Mackay C R, LaRosa G, Newman W, Gerard N, Gerard C, Sodroski J. The β-chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates. Cell. 1996;85:1135–1148. doi: 10.1016/s0092-8674(00)81313-6. [DOI] [PubMed] [Google Scholar]
- 12.Cocchi F, DeVico A L, Garzino-Demo A, Cara A, Gallo R C, Lusso P. Identification of RANTES, MIP-1α, and MIP-1β as the major HIV suppressive factors produced by CD8+ T cells. Science. 1995;270:1811–1815. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- 13.Cocchi F, DeVico A L, Garzino-Demo A, Arya S K, Gallo R C, Lusso P. The V3 domain of the HIV-1 gp120 envelope glycoprotein is critical for chemokine-mediated blockade of infection. Nat Med. 1996;2:1244–1247. doi: 10.1038/nm1196-1244. [DOI] [PubMed] [Google Scholar]
- 14.Collota S, Peri G, Villa A, Mantovani A. Rapid killing of actinomycin D-treated tumor cells by human mononuclear cells. J Immunol. 1984;132:936. [PubMed] [Google Scholar]
- 15.Connor R I, Sheridan K E, Ceradini D, Choe S, Landau N R. Change in coreceptor use correlates with disease progression in HIV-1-infected individuals. J Exp Med. 1997;185:621–628. doi: 10.1084/jem.185.4.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dean M, Carrington M, Winkler C, Huttley G A, Smith M W, Allikmets R, Goedert J J, Buchbinder S P, Vittinghof E, Gomperts E, Donfield S, Vlahov D, Kaslow R, Saah A, Rinaldo C, Detels R, O’Brien S J. Genetic restriction of HIV-1 infection and progression to AIDS by a deletion allele of the CKR5 structural gene. Science. 1996;273:1856–1862. doi: 10.1126/science.273.5283.1856. [DOI] [PubMed] [Google Scholar]
- 17.Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, Di Marzio P, Marmon S, Sutton R E, Hill C M, Davis C B, Peiper S C, Schall T J, Littman D, Landau N R. Identification of a major co-receptor for primary isolates of HIV-1. Nature. 1996;381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 18.Dragic T, Litwin V, Allaway G P, Martin S R, Huang Y, Nagashima K A, Cayanan C, Maddon P J, Koup R A, Moore J P, Paxton W A. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature. 1996;381:667–673. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- 19.Dunne A L, Siregar H, Mills J, Crowe S. HIV replication in chronically infected macrophages is not inhibited by the Tat inhibitors Ro-5-3335 and Ro-24-7429. J Leukocyte Biol. 1994;56:369–373. doi: 10.1002/jlb.56.3.369. [DOI] [PubMed] [Google Scholar]
- 20.Hollinger F B, Bremer J W, Myers L E, Gold J W, McQuay D L. Standardization of sensitive human immunodeficiency virus coculture procedures and establishment of a multicenter quality assurance program for the AIDS Clinical Trials Group. J Clin Microbiol. 1992;30:1787–1794. doi: 10.1128/jcm.30.7.1787-1794.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang Y, Paxton W A, Wolinsky S M, Neumann A U, Zhang L, He T, Kang S, Ceradini D, Jin Z, Yazdanbakhsh K, Kunstman K, Erickson D, Dragon E, Landau N R, Phair J, Ho D D, Koup R A. The role of a mutant CCR5 allele in HIV-1 transmission and disease progression. Nat Med. 1996;2:1240–1243. doi: 10.1038/nm1196-1240. [DOI] [PubMed] [Google Scholar]
- 22.Innocenti-Francillard P, Brengel K, Guillon C, Mallet F, Morand P, Gruters R, Seigneurin J-M. Blood monocytes infected in vivo by HIV-1 variants with a syncytium-inducing phenotype. AIDS Res Hum Retroviruses. 1994;10:683–690. doi: 10.1089/aid.1994.10.683. [DOI] [PubMed] [Google Scholar]
- 23.Koyanagi Y, O’Brien W A, Zhao J Q, Golde D W, Gasson J C, Chen I S. Cytokines alter production of HIV-1 from primary mononuclear phagocytes. Science. 1988;241:1673–1675. doi: 10.1126/science.241.4873.1673. [DOI] [PubMed] [Google Scholar]
- 24.Liu R, Paxton W A, Choe S, Ceradini D, Martin S R, Horuk R, MacDonald M E, Stuhlman H, Koup R A, Landau N R. Homozygous defect in HIV-1 coreceptor accounts for resistance of some multiply-exposed individuals to HIV-1 infection. Cell. 1996;86:367–377. doi: 10.1016/s0092-8674(00)80110-5. [DOI] [PubMed] [Google Scholar]
- 25.McElrath M J, Pruett J E, Cohn Z A. Mononuclear phagocytes of blood and bone marrow: comparative roles as viral reservoirs in human immunodeficiency virus type 1 infections. Proc Natl Acad Sci USA. 1989;86:675–679. doi: 10.1073/pnas.86.2.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McIlroy D, Autran B, Cheymier R, Wain-Hobson S, Claulel J-P, Oksenhendler E, Debre P, Hosmalin A. Infection frequency of dendritic cells and CD4+ T lymphocytes in spleens of human immunodeficiency virus-positive patients. J Virol. 1995;69:4737–4745. doi: 10.1128/jvi.69.8.4737-4745.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Michael N L, Chang G, Louie L G, Mascola J R, Dondero D, Birx D L, Sheppard H W. The role of viral phenotype and CCR-5 gene defects in HIV-1 transmission and disease progression. Nat Med. 1997;3:338–340. doi: 10.1038/nm0397-338. [DOI] [PubMed] [Google Scholar]
- 28.Moriuchi M, Moriuchi H, Combadiere C, Murphy P M, Fauci A S. CD8+ T-cell-derived factor(s), but not β-chemokines RANTES, MIP-1α, and MIP-1β, suppress HIV-1 replication in monocyte/macrophages. Proc Natl Acad Sci USA. 1996;93:15341–15345. doi: 10.1073/pnas.93.26.15341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28a.Naif H M, Li S, Alali M, Sloane A, Wu L, Kelly M, Lynch G, Lloyd A, Cunningham A L. CCR5 expression correlates with susceptibility of maturing monocytes to human immunodeficiency virus type 1 infection. J Virol. 1998;72:830–836. doi: 10.1128/jvi.72.1.830-836.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakayama H, Yokoi H, Fujita J. Quantification of mRNA by non-radioactive RT-PCR and CCD imaging system. Nucleic Acids Res. 1992;20:4939–4943. doi: 10.1093/nar/20.18.4939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perelson A S, Essunger P, Cao Y, Vesanen M, Hurley A, Saksela K, Markowitz M, Ho D D. Decay characteristics of HIV-1 infected compartments during combination therapy. Nature. 1997;387:189–191. doi: 10.1038/387188a0. [DOI] [PubMed] [Google Scholar]
- 31.Reinhardt P P, Reinhardt B, Lathey J L, Spector S A. Human cord blood mononuclear cells are preferentially infected by non-syncytium-inducing, macrophage-tropic human immunodeficiency virus type 1 isolates. J Clin Microbiol. 1995;33:292–297. doi: 10.1128/jcm.33.2.292-297.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rich E A, Chen I, Zack J A, Leonard M L, O’Brien W A. Increased susceptibility of differentiated mononuclear phagocytes to productive infection with human immunodeficiency virus-1 (HIV-1) J Clin Invest. 1992;89:176–183. doi: 10.1172/JCI115559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ryffel B, Woerly G, Greiner B, Haendler B, Mihatsch M J. Distribution of the cyclosporine binding protein cyclophilin in human tissues. Immunology. 1991;72:399–404. [PMC free article] [PubMed] [Google Scholar]
- 34.Samson M, Libert F, Doranz B J, Rucker J, Liesnard C, Farber C-M, Saragosti S, Lapoumeroulie C, Conaux J, Foceille C, Muyldermans G, Verhostede C, Burtonboy G, Georges M, Imai T, Rana S, Yi Y, Smyth R J, Collman R G, Doms R W, Vassart G, Parmentier M. Resistance to HIV-1 infection in caucasian individuals bearing mutant alleles of the CCR-5 chemokine receptor gene. Nature. 1996;382:722–725. doi: 10.1038/382722a0. [DOI] [PubMed] [Google Scholar]
- 35.Schmidtmayerova H, Sherry B, Burkinsky M. Chemokines and HIV replication. Nature. 1996;382:767. doi: 10.1038/382767a0. [DOI] [PubMed] [Google Scholar]
- 36.Schnittman S M, Lane H C, Greenhouse J, Justement J S, Baseler M, Fauci A S. Preferential infection of CD4+ memory T cells by human immunodeficiency virus type 1: evidence for a role in the selective T-cell functional defects observed in infected individuals. Proc Natl Acad Sci USA. 1990;87:6058–6062. doi: 10.1073/pnas.87.16.6058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schuitemaker H, Kootstra N A, Koppelman M, Bruisten M, Huisman H G, Tersmette M, Miedema F. Proliferation-dependent HIV-1 infection of monocytes occurs during differentiation into macrophages. J Clin Invest. 1992;89:1154–1160. doi: 10.1172/JCI115697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shapiro H M. Practical flow cytometry. 3rd ed. New York, N.Y: Wiley-Liss, Inc.; 1995. Cumulative (Overton) subtraction; p. 197. [Google Scholar]
- 39.Siebert P D. Quantitative RT-PCR. Palo Alto, Calif: Clontech Laboratories; 1993. [Google Scholar]
- 40.Singer-Sam J, Robinson M O, Bellve A R, Simon M I, Riggs A D. Measurement by quantitative PCR of changes in HPRT, PGK-1, PGK-2, APRT, MTase, and Zfy gene transcripts during mouse spermatogenesis. Nucleic Acids Res. 1990;18:1255–1259. doi: 10.1093/nar/18.5.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sleasman J W, Harville T O, White G B, George J F, Barrett D J, Goodenow M M. Arrested rearrangements of T cell receptor Vβ genes in thymocytes from children with X-linked severe combined immunodeficiency disease. J Immunol. 1994;153:442–448. [PubMed] [Google Scholar]
- 42.Sleasman J W, Henderson M, Barrett D J. Con A-induced suppressor cell function depends on the activation of the CD4+ CD45RA inducer T cell subpopulation. Cell Immunol. 1991;133:367–378. doi: 10.1016/0008-8749(91)90111-n. [DOI] [PubMed] [Google Scholar]
- 43.Sleasman J W, Aleixo L F, Morton A, Skoda-Smith S, Goodenow M M. CD4+ memory T cells are the predominant population of HIV-1 infected lymphocytes in neonates and children. AIDS. 1996;10:1477–1484. doi: 10.1097/00002030-199611000-00004. [DOI] [PubMed] [Google Scholar]
- 44.Sleasman J W, Leon B H, Aleixo L F, Rojas M, Goodenow M M. Immunomagnetic selection of purified monocyte and lymphocyte populations from peripheral blood mononuclear cells following cryopreservation. Clin Diagn Lab Immunol. 1997;4:653–658. doi: 10.1128/cdli.4.6.653-658.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sonza S, Maerz A, Deacon N, Meanger J, Mills J, Crowe S. Human immunodeficiency virus type 1 replication is blocked prior to reverse transcription and integration in freshly isolated peripheral blood monocytes. J Virol. 1996;70:3863–3869. doi: 10.1128/jvi.70.6.3863-3869.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sonza S A, Maerz A, Uren S, Violo A, Hunter S, Boyle W, Crowe S. Susceptibility of human monocytes to HIV type-1 infection in vitro is not dependent on their level of CD4 expression. AIDS Res Hum Retroviruses. 1995;11:769–776. doi: 10.1089/aid.1995.11.769. [DOI] [PubMed] [Google Scholar]
- 47.Spina C A, Guatelli J C, Richman D D. Establishment of a stable, inducible form of human immunodeficiency virus type 1 DNA in quiescent CD4 lymphocytes in vitro. J Virol. 1995;69:2977–2988. doi: 10.1128/jvi.69.5.2977-2988.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wong M, Silverman E D, Fish E N. Evidence for RANTES, MCP-1, and MIP-1β expression in Kawasaki disease. J Rheumatol. 1997;24:1179–1185. [PubMed] [Google Scholar]
- 49.Wu L, LaRosa G, Kassam N, Gordon C J, Heath H, Ruffing N, Chen H, Humblias J, Samson M, Parmentier M, Moore J P, MacKay C R. Interaction of chemokine receptor CCR5 with its ligands: multiple domains for HIV-1 gp120 binding and a single domain for chemokine binding. J Exp Med. 1997;186:1373–1381. doi: 10.1084/jem.186.8.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu L, Paxton W A, Kassam N, Ruffing N, Rottman J B, Sullivan N, Choe H, Sodroski J, Newman W, Koup R A, MacKay C R. CCR5 levels and expression pattern correlate with infectability by macrophage-tropic HIV-1 in vitro. J Exp Med. 1997;185:1681–1691. doi: 10.1084/jem.185.9.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zaitseva M, Blauvelt A, Lee S, Lapham C K, Klaus-Kovtun V, Mostowski H, Manischewitz J, Golding H. Expression and function of CCR5 and CXCR4 on human Langerhans cells and macrophages: implications for HIV primary infection. Nat Med. 1997;3:1369–1375. doi: 10.1038/nm1297-1369. [DOI] [PubMed] [Google Scholar]
- 52.Zhu T, Mo H, Wang N, Nam D S, Cao Y, Koup R A, Ho D D. Genotypic and phenotypic characterization of HIV-1 patients with primary infection. Science. 1993;261:1179–1181. doi: 10.1126/science.8356453. [DOI] [PubMed] [Google Scholar]