Immunization with a Modified Vaccinia Virus Expressing Simian Immunodeficiency Virus (SIV) Gag-Pol Primes for an Anamnestic Gag-Specific Cytotoxic T-Lymphocyte Response and Is Associated with Reduction of Viremia after SIV Challenge (original) (raw)
Abstract
The immunogenicity and protective efficacy of a modified vaccinia virus Ankara (MVA) recombinant expressing the simian immunodeficiency virus (SIV) Gag-Pol proteins (MVA-gag-pol) was explored in rhesus monkeys expressing the major histocompatibility complex (MHC) class I allele, MamuA*01. Macaques received four sequential intramuscular immunizations with the MVA-gag-pol recombinant virus or nonrecombinant MVA as a control. Gag-specific cytotoxic T-lymphocyte (CTL) responses were detected in all MVA-_gag-pol_-immunized macaques by both functional assays and flow cytometric analyses of CD8+ T cells that bound a specific MHC complex class I-peptide tetramer, with levels peaking after the second immunization. Following challenge with uncloned SIVsmE660, all macaques became infected; however, viral load set points were lower in MVA-_gag-pol_-immunized macaques than in the MVA-immunized control macaques. MVA-_gag-pol_-immunized macaques exhibited a rapid and substantial anamnestic CTL response specific for the p11C, C-M Gag epitope. The level at which CTL stabilized after resolution of primary viremia correlated inversely with plasma viral load set point (P = 0.03). Most importantly, the magnitude of reduction in viremia in the vaccinees was predicted by the magnitude of the vaccine-elicited CTL response prior to SIV challenge.
Evidence for a critical role of cytotoxic T lymphocytes (CTLs) in the containment of human immunodeficiency virus (HIV) infection (16, 23, 36, 39) has led to a consensus among those attempting to develop an AIDS vaccine that such a vaccine should generate CTL in addition to broadly neutralizing antibodies (26). An additional hurdle for an AIDS vaccine is the long-term maintenance of levels of CTL and antibody that will be necessary for protection (26). Both effector CTL and neutralizing antibodies induced by vaccination tend to be transient. Therefore, it may not be feasible to maintain immune responses essential for preventing infection. The importance of the magnitude of the vaccine-elicited memory and postinfection anamnestic immune responses thus become a critical issue in developing an AIDS vaccine.
At present, viable vaccine strategies that might effectively stimulate CTL include viral vectors, peptides, and DNA immunization (26). Viral vectors under investigation include adenovirus (10, 48), alphaviruses such as Semliki Forest virus (8, 35) and Venezuelan equine encephalitis virus (11), poliovirus replicons (23), and various poxviruses (12, 21, 29, 30, 34, 43). Among these approaches, use of the poxviruses is a particularly promising vaccine strategy to express viral proteins. Studies with conventional New York Board of Health vaccinia virus demonstrated that priming with a vaccinia virus recombinant expressing simian immunodeficiency virus (SIV) envelope and/or core proteins, followed by boosting the antibody response with recombinant envelope protein, provided protection against a homologous SIV challenge with a biologically cloned strain of limited pathogenicity (SIVmne/E11S) (20). However this approach provided only partial protection against a more pathogenic and heterogeneous SIV challenge (SIVmne) (44) and blunting of viremia in macaques challenged with the highly pathogenic SIVmac251 (2). Since there are side effects associated with using conventional vaccinia viruses that become potentially life threatening when used in immunocompromised individuals (34), the use of attenuated poxviruses is an attractive alternative (20, 34, 43).
A number of attenuated poxvirus strains have been developed as vaccine vectors: the avipoxviruses (43), canarypox virus, fowlpox virus, and the attenuated vaccinia virus derivatives, NYVAC (43), and modified vaccinia virus Ankara (MVA) (13, 32, 34, 43). The attenuated poxviruses appear to be safe in immunosuppressed animals (30), although their bases for attenuation differ. Thus, the avipoxviruses are genetically quite distinct from vaccinia viruses and do not complete an entire replication cycle in mammalian cells. NYVAC is a genetically engineered derivative of the Copenhagen strain with deletion of host range genes (43). MVA is a spontaneously derived attenuated variant of the Ankara strain that has multiple deletions in host range genes and genes involved in suppressing vaccinia virus-elicited immune responses (29, 30, 34). Perhaps due to these latter deletions, MVA appears to be as immunogenic as wild-type vaccinia virus strains, despite limited replication in mammalian cells (5, 9, 13, 32). In addition, MVA has an excellent safety record in humans, having been used without incident as a smallpox vaccine in approximately 100,000 individuals (30). Each of these attenuated poxvirus vectors has been evaluated in primate models and has shown some degree of efficacy (1, 4, 7, 14, 19, 22, 24, 37, 49, 50, 54).
In previous studies, we explored the use of MVA as a viral vector to express SIV antigens and have evaluated its efficacy in the SIVsm-macaque model (19). The SIV-macaque model is a highly relevant system in which to evaluate the efficacy of partially protective vaccines since it provides valid disease endpoints. In addition, the level at which plasma viremia stabilizes after primary infection (or viral set point) is a highly significant prognostic surrogate for the rate of disease progression in both HIV infection (31) and SIV infection (19, 52, 55). While the SIV-HIV (SHIV) chimeras provide a system to evaluate the role of envelope-specific immune response in vaccine protection, there is no particular advantage to the use of SHIV in evaluating Gag-Pol-specific immunity. Indeed, the pathogenesis of SIV infection of macaques more closely models the pathogenesis of human AIDS (18) than the rapid CD4+ T-cell depletion observed with the pathogenic SHIVs (21, 28, 51).
Prior immunization with MVA expressing the SIVsmH4 Gag-Pol and Env followed by boosting with whole inactivated SIV particles, administered without adjuvant, resulted in significant modulation of viremia and disease progression in macaques subsequently challenged with pathogenic SIVsmE660 (19). Two of these MVA-SIV vaccinees have maintained low viremia and normal CD4+ T-lymphocyte numbers throughout the 4 years since challenge, analogous to HIV type 1-infected clinical long-term nonprogressor humans (12, 42). The recent development of technology for measuring effector CTL by flow cytometric analyses of CD8+ T lymphocytes that bind specific peptide epitope-major histocompatibility complex (MHC) class I tetrameric complexes (3) has revolutionized the ability to quantitate CTL responses in SIV-infected macaques (24, 25). This technology is particularly useful for evaluation of CTL responses in recombinant vaccinia virus-immunized macaques since the background cytolysis that is seen in functional CTL assays with the use of vaccinia virus expression of viral proteins in target cells can be avoided. In a previous study we used tetramer technology to evaluate the ability of an MVA recombinant expressing SIV Gag-Pol to elicit CTL (50). In the present study, we evaluated immunogenicity of this recombinant through two subsequent boosts and evaluated the efficacy following intravenous challenge with pathogenic, uncloned, SIVsmE660.
MATERIALS AND METHODS
Selection of Mamu-A*01+ rhesus macaques.
Rhesus macaques expressing the Mamu-A*01 MHC class I molecule were selected by Mamu-A*01-specific reverse transcriptase-mediated PCR of total RNA isolated by RNeasy (Qiagen, Chatsworth, Calif.) purification from 5 × 106 peripheral blood mononuclear cells (PBMC) as described previously (50). Verification was achieved by direct sequencing of PCR products (QIAquick PCR purification kit; Qiagen) by automated sequencing on the ABI 377 sequencer. The ability of herpesvirus papio-transformed B-lymphoblastoid cell lines from these macaques to act as targets in p11C-specific functional cytotoxicity assays was used to confirm the expression of the Mamu-A*01 allele. Monkeys were maintained in accordance with the guidelines of the Animal Care and Use Committee of the National Institutes of Health (NIH) and Guide for the Care and Use of Laboratory Animals (38).
Immunization and SIV infection.
Macaques were immunized with 108 PFU of either MVA (n = 2) or MVA-gag-pol (n = 4) vaccinia virus at 0, 13, 35, and 52 weeks. The construction of the MVA-gag-pol recombinant vaccinia virus has been described previously (50). One of the control MVA macaques inadvertently received the MVA-gag-pol virus at the third immunization and was therefore eliminated from the study. Four additional Mamu-A*01 macaques received a single immunization of the nonrecombinant MVA at the 52-week time point.
The macaques were challenged intravenously with 50 50% macaque infectious doses of the pathogenic SIVsmE660 isolate (18, 19) 4 weeks after the final immunization. Animals were subsequently monitored for Mamu-A*01–p11C, C-M tetramer binding on EDTA anticoagulated samples collected at the time of challenge and 3, 7, 10, 14, 17, 21, 28, 35, and 49 days after SIV challenge. Virus isolation from PBMC was attempted at 14 and 28 days postchallenge to evaluate whether these animals were infected with SIV. Lymph node biopsies were collected at 7 and 14 days after SIV challenge, and in situ hybridization for SIV-specific RNA was performed. Viral load was monitored on EDTA-anticoagulated blood collected at 0, 3, 7, 10, 14, 21, 28, and 35 days after SIV challenge by quantitative real-time reverse transcriptase-mediated PCR assay, using procedures and primers described previously (53). The nominal threshold sensitivity of the assay was 500 eq/ml.
Functional cytotoxicity assays.
PBMC from Mamu-A*01+ rhesus monkeys were cultured with p11C (EGCTPYDINQML) (10 μg/ml) at a density of 5 × 106 cells/ml. On day 3 of culture, the medium was supplemented with recombinant human interleukin-2 (20 U/ml; provided by Hoffmann-La Roche), and the cultures were maintained a further 4 days. PBMC were then centrifuged over Ficoll-Hypaque and assessed as effector cells in a standard 51Cr release assay using U-bottomed microtiter plates containing 104 target cells with various concentrations of effector cells. All wells were assayed in duplicate. Autologous B-lymphoblastoid cell lines were used as targets and were incubated with 1 μg of p11C, C-M (CTPYDINQM) or the control peptide p11B (ALSEGCTPYDIN) per ml during overnight 51Cr labeling. Plates were incubated in a humidified incubator at 37°C for 4 h. Specific release was calculated as [(experimental release − spontaneous release)/(maximum release − spontaneous release)] × 100. Spontaneous release was <20% of maximal release with detergent (2% Triton X-100; Sigma) in all assays.
Staining and phenotypic analysis of p11C-specific CD8+ T lymphocytes.
The monoclonal antibodies (MAbs) used in this study were directly coupled to fluorescein isothiocyanate (FITC), phycoerythrin (PE), phycoerythrin-Texas red (ECD), or allophycocyanin (APC). The following MAbs were used: anti-CD8α (Leu2a)-FITC and anti-CD62L (Leu8)-PE (Becton Dickinson, San Jose, Calif.), anti-CD8αβ (2ST8-5H7)-ECD, anti-CD11a (25.3.1)-PE, anti-CD45RA (2H4)-PE (Beckman-Coulter, Inc.), and anti-CD95 (DX2)-PE (Caltag, Burlingame, Calif.). MAb FN18, which recognizes rhesus CD3, a gift from D. M. Neville, Jr., NIH, Bethesda, Md., was directly coupled to APC. The three reagents Alexa 488-coupled tetrameric Mamu-A*01/p11C, C-M complex, anti-CD8αβ-ECD, and anti-rhesus CD3-APC were used either with anti-CD11a-PE, anti-CD45RA-PE, anti-CD62L-PE, or anti-CD95-PE to perform four-color flow cytometric analyses. Since nearly all of the tetrameric Mamu-A*01–p11C, C-M complex-binding T cells express the CD8αβ molecules, all analyses were performed by gating on CD8αβ+ CD3+ cells. The PE-coupled tetrameric Mamu-A*01–p11C, C-M complex was used with anti-CD8α-FITC in conjunction with anti-CD8αβ-ECD and anti-rhesus CD3-APC. The tetramer staining of CD8αβ+ cells was performed on gated CD3+ cells since the CD8αβ-specific MAb used in this study binds occasionally to natural killer cells of rhesus monkeys. Alexa 488 or PE-coupled tetrameric Mamu-A*01–p11C, C-M complex (0.5 μg) was used in conjunction with the directly labeled MAbs to stain either 100 μl of fresh whole blood or 5 × 105 lymphocytes isolated by density centrifugation over Ficoll diatrizoate following in vitro culture. Samples were analyzed on a Coulter EPICS Elite ESP as described previously (50). Data presentation was performed using WinMDI software version 2.7 (Joseph Trotter, La Jolla, Calif.) and Microsoft PowerPoint 97 software (Microsoft, Redmond, Calif.).
Statistical analyses.
The Wilcoxon rank sum test was used for comparison of plasma SIV RNA levels between groups. The Spearman rank correlation test was used for associations between viral RNA levels and the percentage of tetramer+ CD8+ T cells, both before and after SIV challenge. StatXact version 4.0.1 (CYTEL Software Corp., Cambridge, Mass.) was used to calculate the P values by exact methods that take into account the small numbers of observations.
RESULTS
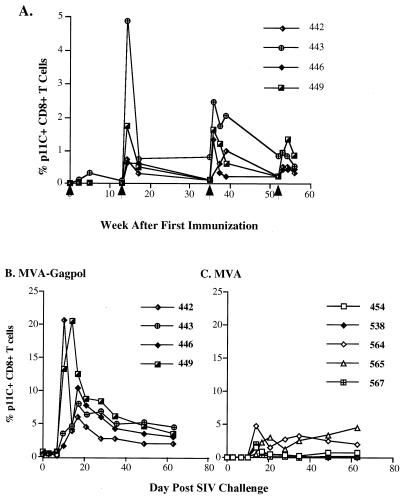
This study evaluated the individual contribution of Gag-Pol antigens and Gag-specific CTL to protection in the SIV-macaque model using rhesus monkeys that expressed the Mamu-A*01 MHC class I molecule. As described previously, the MVA-gag-pol recombinant induced a potent SIV Gag-specific CTL response, as measured by both functional assays and binding of Mamu-A*01 MHC class I–p11C tetrameric complex to CD8+ T lymphocytes (50). All macaques immunized with the MVA-gag-pol recombinant developed circulating Gag-specific CD8+ CTL after the second immunization (50), as summarized in Fig. 1A. Two additional boosts with the MVA-gag-pol recombinant virus were administered, and functional CTL and binding of the tetrameric complex to CD8+ lymphocytes were monitored sequentially throughout the remainder of the immunization protocol (Table 1). As shown in Fig. 1A, the most significant boost in tetramer+ CD8+ T cells was observed after the second immunization, with a less robust response following the third immunization. The third immunization did not boost tetramer levels to the levels achieved after the second immunization, with the exception of macaque 446. Regardless of the peak level achieved after the third immunization, the percentage of CD8+ T cells that bound the tetramer had waned to low levels by the time of the fourth immunization. The fourth boost had very little effect (Table 1 and Fig. 1A), and tetramer+ CD8+ T cells ranged from 0.3 to 0.8% by the time of SIV challenge 4 weeks later. The response to immunization of the four macaques varied significantly; two macaques achieved tetramer levels similar to those reported during SIV infection, whereas responses in two macaques were relatively weak despite repeated boosting (Table 1).
FIG. 1.
(A) Tetramer+ CD8+ T cells in whole blood of MVA-_gag-pol_-immunized rhesus monkeys after immunizations. Arrows show the weeks at which the animals were immunized. (B and C) Tetramer+ CD8+ T cells in whole blood after SIV challenge of MVA-_gag-pol_-immunized and MVA-immunized animals, respectively.
TABLE 1.
PBL of MVA-_gag-pol_-vaccinated Mamu-A*01+ rhesus macaques demonstrate tetramer+ CD8+ T cells and functional CTL
| Immunization | Monkey | Assay | Day postimmunization | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 3rd | 4th | |||||||||
| 0 | 7 | 17 | 28 | 0 | 7 | 14 | 28 | |||
| MVA-Gag-Pol | 442 | Fresha | 0.1 | NTd | 0.6 | 0.8 | 0.2 | 0.5 | 0.5 | 0.3 |
| Peptide stimulatedb | 20.8 | NT | 58.3 | 50.4 | 39.9 | 41.2 | 45.9 | 46.9 | ||
| % Specific lysisc | 48 | NT | 61 | 58 | 65 | 69 | 74 | 59 | ||
| 443 | Fresh | 0.8 | 2.4 | 1.7 | 2.0 | 0.8 | 0.9 | 0.8 | 0.5 | |
| Peptide stimulated | 4.8 | 23 | 61 | 39 | 68 | 58 | 69 | 77 | ||
| % Specific lysis | 19 | 44 | 57 | 56 | 67 | 72 | 54 | 67 | ||
| 446 | Fresh | 0.1 | 1.3 | 0.3 | 0.2 | 0.2 | 0.4 | 0.4 | 0.4 | |
| Peptide stimulated | 43 | 57 | 41 | 37 | 44 | 51 | 53 | 59 | ||
| % Specific lysis | 55 | 58 | 56 | 60 | 62 | 75 | 53 | 57 | ||
| 449 | Fresh | 0.1 | 1.6 | 1.0 | 0.6 | 0.2 | 0.9 | 1.3 | 0.8 | |
| Peptide stimulated | 13 | 16 | 25 | 23 | 24 | 39 | 59 | 52 | ||
| % Specific lysis | 36 | 37 | 57 | 32 | 48 | 68 | 52 | 53 | ||
| Control MVA | 454 | Fresh | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Peptide stimulated | 0.1 | 0.1 | 0.2 | 0.5 | 0.5 | 0.5 | 0.3 | 0.2 | ||
| % Specific lysis | NT | 0 | 2 | 0 | 0 | 0 | 0 | 0 | ||
| 538 | Fresh | 0.0 | 0.0 | 0.0 | 0.0 | |||||
| Peptide stimulated | 0.8 | 1.1 | 1.2 | 0.5 | ||||||
| % Specific lysis | 0 | 0 | 0 | 0 | ||||||
| 564 | Fresh | 0.0 | NT | NT | 0.0 | |||||
| Peptide stimulated | 1.2 | NT | NT | 0.3 | ||||||
| % Specific lysis | 1 | NT | NT | 0 | ||||||
| 565 | Fresh | 0.0 | NT | NT | 0.0 | |||||
| Peptide stimulated | 1.1 | NT | NT | 0.4 | ||||||
| % Specific lysis | 1 | NT | NT | 0 | ||||||
| 567 | Fresh | 0.0 | NT | NT | 0.0 | |||||
| Peptide stimulated | 1.2 | NT | NT | 0.3 | ||||||
| % Specific lysis | 2 | NT | NT | 0 |
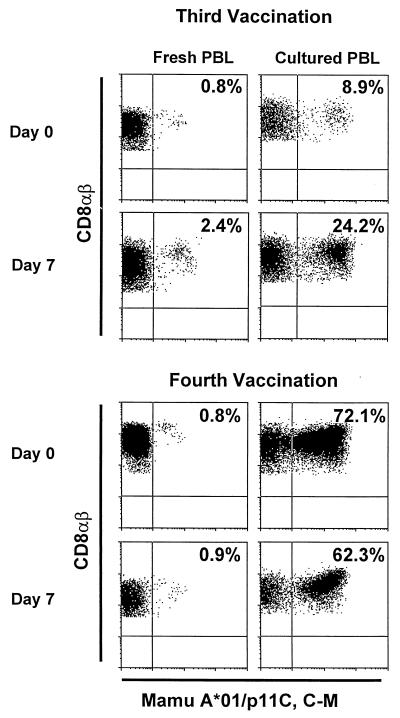
Figure 2 illustrates tetramer staining of CD8+ T cells from one representative MVA-_gag-pol_-immunized animal. Both fresh whole blood peripheral blood lymphocytes (PBL) and Ficoll-isolated PBL cultured for 7 days with the Gag epitope peptide p11C were analyzed by flow cytometry for tetramer+ CD8+ T cells. An increase in tetramer-binding cells was seen in both fresh and cultured PBL after the third immunization. However, after the fourth immunization, little difference was observed in the levels of tetramer-binding cells. The cultured cells were also assessed for functional cytotoxic activity in chromium release assays. As shown in Table 1, only PBL from MVA-_gag-pol_-immunized animals showed p11C, C-M-specific lytic activity. Thus p11C, C-M-specific CTL were observed by both tetramer binding and functional assays in PBL of the MVA-_gag-pol_-immunized but not the MVA-immunized macaques.
FIG. 2.
Tetrameric Mamu-A*01–p11C, C-M complex bound to CD8+ T cells in peripheral blood of a vaccinated rhesus macaque (443) after immunizations with MVA-gag-pol. Flow cytometry histograms illustrating tetramer binding to gated CD8αβ+ CD3+ T lymphocytes are shown for both fresh PBL and p11C-stimulated PBL for days 0 and 7 after the third and fourth immunizations. The values indicate the percentage of CD8αβ+ T lymphocytes that bound tetramer.
Anamnestic CTL responses after SIV challenge.
Four weeks after the fourth immunization, the monkeys were challenged intravenously with 50 50% macaque infectious doses of the pathogenic, uncloned SIVsmE660 virus (18). Five Mamu-A*01+ rhesus macaques immunized with nonrecombinant MVA were challenged in parallel. One of these animals received nonrecombinant MVA on the same schedule as the SIV-immunized macaques. Four additional Mamu-A*01+ macaques were added to the study prior to the last immunization to permit valid comparisons of viral load parameters following viral challenge. All nine macaques became infected, as evidenced by rescue of infectious virus from PBMC and lymph node mononuclear cells at 2, 4, and 8 weeks after SIV challenge (data not shown).
As shown in Fig. 1B and C, an increase in the number of tetramer+ CD8+ T cells was observed following SIV challenge in all macaques. However, the magnitude of the peak percentage of tetramer+ CD8+ T cells was much greater in animals immunized with MVA-gag-pol (Fig. 1B), as compared by the Wilcoxon rank sum test to those immunized with MVA (Fig. 1C) (P = 0.016). The kinetics of appearance of tetramer+ cells was also more rapid in the group immunized with MVA-gag-pol (10 days) than in those that received MVA (14 days). The magnitude of the anamnestic response also varied between the SIV-immunized macaques, with two macaques (442 and 449) achieving levels of 20% of circulating CD8+ T cells specific for the SIV Gag p11C, C-M epitope. These two macaques also exhibited the highest primary plasma viremia (442 and 449; Fig. 1B and 3), suggesting that the magnitude of the anamnestic p11C, C-M-specific CTL response was driven by antigen load. The marked expansion of p11C, C-M-specific CD8 T cells during primary viremia was relatively transient, with the levels stabilizing at much lower levels (2 to 5%) by 49 to 62 days postchallenge (Fig. 1B). In general, the levels observed in the MVA-_gag-pol_-vaccinated macaques were higher than those observed in those immunized with the MVA control (Fig. 1C).
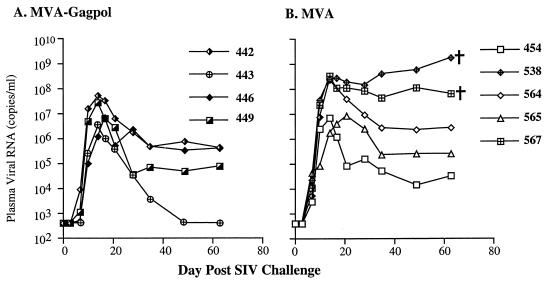
FIG. 3.
Sequential plasma viral load measurements in Mamu-A*01+ rhesus macaques immunized with MVA-gag-pol (A) and MVA (B) after intravenous challenge with SIVsmE660.
The phenotypic properties of the CTL detected before and after challenge were also compared by four-color flow cytometric analysis. The tetramer+ CD8+ T cells detected both before and after SIV challenge had similar phenotypes. These cells expressed high levels of the activation/adhesion molecules, CD11a and CD95, and low levels of naive lymphocyte-associated molecules, CD45RA and CD62L (data not shown).
Reduced virus load in MVA-gag-pol immunized macaques.
Sequential plasma SIV RNA levels in all macaques were assessed by a real-time assay (53). As can be seen in Fig. 3, a wide range in viral load was observed in both immunization groups. The macaque with the lowest viral load was within the MVA-_gag-pol_-immunized group, whereas the three macaques with the highest viremia segregated to the MVA control-vaccinated group (Fig. 3B). Two control-vaccinated macaques (538 and 567) exhibited no apparent control of viremia. These macaques developed only a transient CTL response, as assessed by tetramer+ CD8+ T cells (Fig. 1C), and failed to mount a detectable antibody response to SIV antigens (data not shown). Consistent with this failure in immune control of virus replication, these animals demonstrated weight loss, persistent diarrhea, as well as neurological and respiratory signs of disease and were euthanized at 9 and 10 weeks postchallenge. The other three macaques in the control group have remained healthy although two of these animals have significant lymphadenopathy.
In contrast, one of the MVA-_gag-pol_-immunized macaques (443) demonstrated impressive control of viremia, with plasma viral RNA below the detection limits of the assay (Fig. 3A). This animal has maintained normal peripheral blood CD4+ T cells and lymph node biopsies obtained at 13 weeks postchallenge have no evidence of virus expression by in situ hybridization for SIV RNA. In addition, consistent with extremely low viral load, attempts to isolate virus from PBMC collected after 8 weeks postchallenge have been unsuccessful. Viremia in another macaque in the MVA-_gag-pol_-immunized group stabilized at moderately low levels (approximately 50,000 copies/ml). This macaque has also maintained normal numbers of CD4+ T cells in peripheral blood. The viremia in the remaining two MVA-_gag-pol_-immunized macaques was more substantial and clearly overlapped with the range observed among the control-vaccinated group of macaques. Overall, a 50-fold reduction in mean plasma viral load (50) was observed in the MVA-_gag-pol_-immunized group compared to the control-immunized group at day 49, a time that corresponds to the plasma viral load set point. However, due to the small numbers of animals studied and substantial biological variability within the groups, this difference was not statistically significant (P = 0.11).
The response to immunization is predictive of viral load set point.
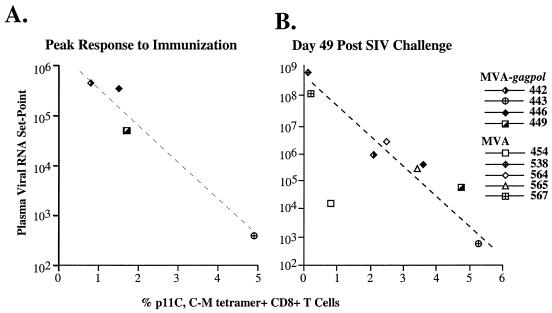
To evaluate the association between the frequency of the Gag-specific CTL response and control of viremia, we evaluated whether the T-cell response to immunization was predictive of relative protection observed in the experimental vaccinated macaques after SIV challenge. Data derived from restimulated cultures (Table 1) did not yield any meaningful associations with viral load in the vaccinated macaques. However, examination of the percent tetramer+ CD8+ T cells in fresh blood revealed considerable variation in response to immunization. As shown in Fig. 1A, one macaque (443) had a significantly more robust response to immunization, another (449) had an intermediate response, and two other macaques (442 and 446) were relatively low responders. Evaluation of the peak CTL response elicited by immunization revealed an inverse correlation with the plasma viral load set point following challenge, as depicted graphically in Fig. 4A. Macaque 443, with the highest frequency of CTL responses during the immunization period, exhibited the lowest viral load set point (<500 copies/ml at day 49 and 63). The macaques (442 and 446) with the weakest immune response to the vaccination exhibited the highest viral load set point. Macaque 449, with a CTL response of intermediate frequency, was intermediate in terms of viral load following challenge. This trend did not reach statistical significance when analyzed by the Spearman rank correlation due to the small number of animals evaluated (P = 0.08). A similar relationship was observed using peak tetramer levels after the second immunization or mean tetramer levels throughout the immunization period for this comparison (data not shown).
FIG. 4.
Correlations between viral load set point and the percent of tetramer+ CD8+ T cells in fresh blood samples detected before and after SIV challenge. (A) Scatter plot of the inverse correlation between viral load set point (day 49 after SIV challenge) shown in a log scale on the y axis and the overall peak percentage of tetramer+ CD8+ T cells induced by vaccination on the x axis in macaques immunized with MVA-gag-pol. (B) Inverse correlation between viral load set point (y axis) and percent tetramer+ CD8+ T cells (x axis) at the same time point after SIV challenge (day 49) (P = 0.03). Macaques vaccinated with MVA-gag-pol are indicated by their identification numbers (442, 443, 446, and 449) and symbols in common with panel A. Macaques vaccinated with the MVA control are shown as open diamonds. This correlation was statistically significant (P = 0.030; _r_2 = −0.72) by the Spearman rank correlation test.
In summary, the rank order of CTL frequency achieved prior to SIV challenge in the MVA-_gag-pol_-immunized group was predictive of the rank order of CTL frequency at viral set point. These data suggest that the strength of the immunologic response to immunization was a major influence on the viral set point after SIV challenge. Since previous studies have demonstrated that the viral set point is an excellent prognostic indicator (55), response to immunization may also be predictive of protection from AIDS.
Correlation of Gag-specific CTL frequency and viral load at set point.
The correlation between frequency of Gag-specific CTL and viral load in both groups was also assessed at sequential time points after SIV challenge by the Spearman rank correlation. The percentage of tetramer+ CD8+ peripheral T cells did not correlate with relative plasma virus level during primary viremia. Thus, animals with higher anamnestic peak CTL frequencies did not necessarily exhibit better containment of viremia. However, by day 49 after SIV challenge (a time corresponding to viral set point), a statistically significant inverse correlation was observed between the frequency of tetramer+ CD8+ T cells and plasma viral load (P = 0.030; _r_2 = −0.72) (Fig. 4B). Animals with a higher percentage of circulating tetramer+ CD8+ T cells had lower viral load set points than those with a lower percentage of tetramer+ CD8+ T cells. As expected, this correlation was observed regardless of immunization status of the macaques. There was considerable overlap between the two groups, with control macaques predominating at the top of the slope and MVA-_gag-pol_-immunized macaques predominating at the lower end of the slope. Overall, similar to observations in humans infected with HIV (39), these data indicate that the level at which the Gag-specific CTL plateau after primary viremia predicts the degree to which viremia is contained in SIV-infected macaques. These data suggest a functional role for p11C+ CTL in the down-regulation of plasma viremia that occurs following primary viremia. Alternatively, the p11C+ CTL response may be predictive of the overall strength of the CTL response to other Gag or Pol epitopes in these macaques.
DISCUSSION
In this study, Mamu-A*01+ rhesus macaques were immunized with MVA expressing SIV Gag-Pol to assess (i) the ability of this vaccine strategy to elicit SIV-specific CTL and (ii) the role of such CTL responses in containing SIV viremia. These animals were immunized solely with the MVA recombinant and received no subunit antigen boosting. Thus, any protection observed in this study was likely due to cell-mediated immune responses to SIV Gag-Pol antigens. The MVA-gag-pol recombinant proved to be highly effective in eliciting SIV-specific CTL. Despite waning in the CTL responses through the period of vaccination, all immunized macaques responded with robust anamnestic CTL responses after SIV challenge. Immunization with MVA-gag-pol resulted in a reduction in the plasma viral load set point compared to control vaccinated macaques, although the difference in viral load between the two groups was not statistically significant. Two control macaques developed high persistent viremia characteristic of rapid progressors, a phenomenon commonly observed with this pathogenic SIV challenge in naive macaques. In contrast, all of the SIV-vaccinated macaques showed some degree of containment of viremia. Indeed, the rapidly progressive disease has not been observed in any of 22 other macaques immunized with MVA-SIV recombinants (19, 41).
A similar but statistically significant reduction in plasma viremia was observed in a parallel study evaluating the relative efficacy of MVA recombinants expressing Gag-Pol, Env or a combination of Gag-Pol and Env (40, 41). Therefore, in the absence of neutralizing antibodies, Gag-Pol-specific CTL induced by prior immunization have a protective effect following SIV challenge in terms of suppressing viremia. Such a reduction of viremia has also been observed in macaques immunized with conventional vaccinia virus expressing SIV Gag-Pol, although Gag-specific CTL responses were not assessed (46). A similar correlation has also been observed previously between Nef-specific CTL responses induced by a vaccinia virus-SIV recombinant and suppression of viral replication in macaques (15). Cumulatively, these data suggest that induction of virus-specific CTL responses, regardless of the antigen, provide partial protection following challenge in primate models of AIDS. However, a combination of antigens, as in our parallel study (41) and in studies with conventional vaccinia virus (46), appear to offer more solid protection than a single antigen.
Although immunization with MVA-gag-pol resulted in reduction in the viral set point following SIV challenge, there was overlap in the ranges between the MVA-_gag-pol_- and MVA nonrecombinant-immunized macaques. This overlap is due in part to biological variation in intrinsic susceptibility of outbred macaques to SIV infection (27). This tremendous variability in response to SIV infection is evident among the control macaques, with plasma viral loads varying by as much as 4 orders of magnitude (Fig. 3). Thus, two of the control macaques consistently exhibited relatively low levels of plasma viremia suggestive of a higher intrinsic resistance to SIV infection than the remaining controls. Interestingly, the one outlier animal in the correlation between plasma viral load and tetramer percentage at set point shown in Fig. 4B was one of these control macaques (454). The lower level of viral replication in this macaque may have been responsible for a less robust CTL response.
Regardless of this variable, the relative response to SIV antigens during immunization appeared to have a major influence on viral load set point in the MVA-_gag-pol_-immunized animals. The effect of immunization is best demonstrated in the MVA-_gag-pol_-vaccinated macaque with the most robust CTL response. This particular macaque maintained the highest CTL frequency before and after SIV challenge of all study animals and was able to control viremia to levels below the threshold of detection of the plasma viral RNA assay. Follow-up of plasma viremia and clinical outcome will be necessary to determine whether this control of viral replication translates into a long-term clinical benefit. However, previous studies have demonstrated that the plasma viral load set point is an excellent prognostic indicator in SIV-infected macaques (19, 42, 55). Indeed, historical data from our laboratory have demonstrated that 9 of 10 macaques with low post-acute-phase set points remained healthy, some for as long as 5 years postchallenge (data not shown). The macaque with an intermediate Gag-specific CTL response to immunization showed a less effective control of viremia, but still to levels significantly less than the majority of the control MVA-immunized macaques. The two macaques with the least robust vaccine-elicited CTL responses demonstrated viral loads indistinguishable from those of the control MVA-vaccinated animals. Additionally, the frequency of the p11C-specific CTL following the primary phase of infection was highly predictive of the plasma viral load set point. Macaques with low levels of Gag-specific CTL by 7 weeks postchallenge exhibited massive viremia and were euthanized due to progressive SIV-induced disease. Macaques in which Gag-specific CTL stabilized at higher levels exhibited the lowest viral load set points. Critically, the rank order of CTL frequency elicited by immunization with MVA-gag-pol was highly predictive of the rank order of CTL frequency at viral set point. While the number of animals evaluated in this study was too low to allow definitive conclusions, these data suggest that much of the spectrum in viral load observed in immunized macaques is due to the relative effectiveness of immunization.
The tetramer data in the present study contributed a quantitative aspect to CTL assessment that has been lacking in previous vaccine studies in the SIV-macaque model. In this study, specific lysis, as determined by functional assays, was indistinguishable among immunized macaques. Perhaps because the percentage of tetramer+ CD8+ T cells can be determined from fresh cells, these values may be more predictive of the relative strength of CTL responses than functional assays performed on in vitro-stimulated PBMC. Since the animals evaluated in this study expressed other MHC class I alleles in addition to Mamu-A*01, they are likely to have mounted CTL responses to other undefined Gag-Pol epitopes that were not assessed. Therefore, the p11C-specific CTL responses may be indicative of the CTL responses to other Gag-Pol epitopes induced by the MVA-gag-pol vaccine in these animals. The p11C-specific CTL response appears to be a useful surrogate marker to indicate more general effectiveness of immunization in Mamu-A*01+ rhesus macaques. Comparative analyses of the strength of CTL responses elicited by different vaccine regimens should now be feasible using the tetramer technology. Based on the diminishing responses after the first two immunizations with the MVA-gag-pol recombinant, immune responses generated to MVA probably limit the usefulness of repeated immunization with this vector. Therefore, combination of immunization strategies such as poxvirus recombinants with DNA immunization (22, 49) are a promising avenue for boosting CTL responses.
In summary, the present study demonstrates that recombinant MVA-SIV as a sole immunogen generates a robust CTL response in macaques that mediates protection from high levels of viremia following SIV challenge. Despite waning of effector CTL prior to challenge, the MVA-_gag-pol_-immunized macaques responded with vigorous anamnestic CTL responses after SIV challenge. Critically, the macaque with the best immunologic response to vaccination demonstrated the most effective suppression of viremia after SIV challenge. The effectiveness of recombinant MVA in generating SIV-specific CTL in the macaque model suggests that MVA-based vaccines warrant evaluation for preventing HIV infection and AIDS in humans.
ACKNOWLEDGMENTS
We thank J. D. Lifson (SAIC-NCI, Frederick, Md.) for performing analysis of plasma viral RNA levels, N. Cooper (LVD, NIAID) for preparation of MVA stocks for animals studies, C. R. Brown (LMM, NIAID) for performing SIV-specific in situ hybridization, S. Whitted and R. Goeken (LMM, NIAID) for technical assistance, and R. Byrum (Bioqual, Inc.) for conducting the animal studies.
This study was supported in part by NIH grants AI-85343 and AI-26507.
REFERENCES
- 1.Abimuki A G, Robert-Guroff M, Benson J, Tartaglia J, Paoletti E, Gallo R C, Markham P D, Franchini G. Long-term survival of SIVmac251-infected macaques previously immunized with NYVAC-SIV vaccines. J Acquired Immune Defic Syndr Hum Retrovirol. 1997;15:S78–S85. [Google Scholar]
- 2.Ahmad S, Lohman B, Marthas M, Giavedoni L, el-Amad Z, Haigwood N L, Scandella C J, Gardner M B, Luciw P A, Yilma T. Reduced virus load in rhesus macaques immunized with recombinant gp160 and challenged with simian immunodeficiency virus. AIDS Res Hum Retroviruses. 1994;10:195–204. doi: 10.1089/aid.1994.10.195. [DOI] [PubMed] [Google Scholar]
- 3.Altman J D, Moss P A H, Goulder P J R, Barouch D H, McHeyzer-Williams M G, Bell J I, McMichael A J, Davis M M. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996;274:94–96. [PubMed] [Google Scholar]
- 4.Andersson S, Makitalo B, Thorstensson R, Franchini G, Tartaglia J, Limbach K, Paoletti E, Putkonen P, Biberfeld G. Immunogenicity and protective efficacy of a human immunodeficiency virus type 2 recombinant canarypox (ALVAC) vaccine candidate in cynomolgus monkeys. J Infect Dis. 1996;174:977–985. doi: 10.1093/infdis/174.5.977. [DOI] [PubMed] [Google Scholar]
- 5.Antoine G, Scheiflinger F, Dorner F, Falkner F G. The complete genomic sequence of the modified vaccinia Ankara strain: comparison with other orthopoxviruses. Virology. 1998;244:365–396. doi: 10.1006/viro.1998.9123. [DOI] [PubMed] [Google Scholar]
- 6.Belyakov I M, Wyatt L S, Ahlers J D, Earl P, Pendleton C D, Kelsall B L, Strober W, Moss B, Berzofsky J A. Induction of a mucosal cytotoxic T-lymphocyte response by intrarectal immunization with a replication-deficient recombinant vaccinia virus expressing human immunodeficiency virus 89.6 envelope protein. J Virol. 1998;72:8264–8272. doi: 10.1128/jvi.72.10.8264-8272.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benson J, Chougnet C, Robert-Guroff M, Montefiori D, Markham P, Shearer G, Gallo R C, Cranage M, Paoletti E, Limbach K, Venzon D, Tartaglia J, Franchini G. Recombinant vaccine-induced protection against the highly pathogenic simian immunodeficiency virus SIVmac251: dependence on route of challenge exposure. J Virol. 1998;72:4170–4182. doi: 10.1128/jvi.72.5.4170-4182.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berglund P, Quesada-Rolander M, Putkonen P, Biberfeld G, Thorstensson R, Liljestrom P. Outcome of immunization of cynomolgus monkeys with recombinant Semliki Forest virus encoding human immunodeficiency virus type 1 envelope protein and challenge with a high dose of SHIV-4 virus. AIDS Res Hum Retroviruses. 1997;13:1487–1495. doi: 10.1089/aid.1997.13.1487. [DOI] [PubMed] [Google Scholar]
- 9.Blanchard T J, Alcami A, Panayota A, Smith G L. Modified vaccinia virus Ankara undergoes limited replication in human cells and lack several immunomodulatory proteins: implications for use as a human vaccine. J Gen Virol. 1998;79:1159–1167. doi: 10.1099/0022-1317-79-5-1159. [DOI] [PubMed] [Google Scholar]
- 10.Buge S L, Richardson E, Alipanah S, Markham P, Cheng S, Kalyan N, Miller C J, Lubeck M, Udem S, Eldridge J, Robert-Guroff M. An adenovirus simian immunodeficiency virus env vaccine elicits humoral, cellular, and mucosal immune responses in rhesus macaques and decreases viral burden following vaginal challenge. J Virol. 1997;71:8531–8541. doi: 10.1128/jvi.71.11.8531-8541.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caley I J, Betts M R, Irlbeck D M, Davis N L, Swanstrom R, Frelinger J A, Johnston R E. Humoral, mucosal, and cellular immunity in response to a human immunodeficiency virus type 1 immunogen expressed by a Venezuelan equine encephalitis virus vaccine vector. J Virol. 1997;71:3031–3038. doi: 10.1128/jvi.71.4.3031-3038.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cao Y, Qin L, Zhang L, Safrit J, Ho D. Virologic and immunologic characterization of long-term survivors of human immunodeficiency virus type 1 infection. N Engl J Med. 1995;332:201–208. doi: 10.1056/NEJM199501263320401. [DOI] [PubMed] [Google Scholar]
- 13.Carroll M, Moss B. Host range and cytopathogenicity of the highly attenuated MVA strain of vaccinia virus: propagation and generation of recombinant viruses in nonhuman mammalian cell line. Virology. 1997;244:365–396. doi: 10.1006/viro.1997.8845. [DOI] [PubMed] [Google Scholar]
- 14.Franchini G, Robert-Guroff M, Tartaglia J, Aggarwal A, Abimiku A, Benson J, Markham P, Limbach K, Hurteau G, Fullen J, et al. Highly attenuated HIV type 2 recombinant poxviruses, but not HIV-2 recombinant Salmonella vaccines, induce long-lasting protection in rhesus macaques. AIDS Res Hum Retroviruses. 1995;11:909–920. doi: 10.1089/aid.1995.11.909. [DOI] [PubMed] [Google Scholar]
- 15.Gallimore A, Cranage M, Cook N, Almond N, Bootman J, Rud E, Silvera P, Dennis M, Corcoran T, Stott J, McMichael A, Gotch F. Early suppression of SIV replication by CD8+ nef-specific cytotoxic T cells in vaccinated macaques. Nat Med. 1995;1:1167–1173. doi: 10.1038/nm1195-1167. [DOI] [PubMed] [Google Scholar]
- 16.Goh W C, Markee J, Akridge R E, Meldorf M, Musey L, Karchmer T, Krone M, Collier A, Corey L, Emerman M, McElrath M J. Protection against human immunodeficiency virus type 1 infection in persons with repeated exposure: evidence for T cell immunity in the absence of inherited CCR5 coreceptor defects. J Infect Dis. 1999;179:548–557. doi: 10.1086/314632. [DOI] [PubMed] [Google Scholar]
- 17.Hanke T, Blanchard T J, Schneider J, Ogg G S, Tan R, Becker M, Gilbert S C, Hill A V S, Smith G L, McMichael A. Immunogenicity of intravenous and intramuscular administrations of modified vaccinia virus Ankara-based multi-CTL epitope vaccine for human immunodeficiency virus type 1 in mice. J Gen Virol. 1998;79:83–90. doi: 10.1099/0022-1317-79-1-83. [DOI] [PubMed] [Google Scholar]
- 18.Hirsch V M, Johnson P R. Pathogenic diversity of simian immunodeficiency viruses. Virus Res. 1994;32:183–203. doi: 10.1016/0168-1702(94)90041-8. [DOI] [PubMed] [Google Scholar]
- 19.Hirsch V M, Fuerst T R, Sutter G, Carroll M W, Yang L C, Goldstein S, Piatak M, Elkins W R, Alvord W G, Montefiori D C, Moss B, Lifson J D. Patterns of viral replication correlate with outcome in simian immunodeficiency virus (SIV)-infected macaques: effect of prior immunization with a trivalent SIV vaccine in modified vaccinia virus Ankara. J Virol. 1996;70:3741–3752. doi: 10.1128/jvi.70.6.3741-3752.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu S L, Abrams K, Barber G N, Moran P, Zarling J M, Langlois A J, Kuller L, Morton W R, Benveniste R E. Protection of macaques against SIV infection by recombinant subunit vaccines using SIV env glycoprotein gp 160. Science. 1992;255:456–459. doi: 10.1126/science.1531159. [DOI] [PubMed] [Google Scholar]
- 21.Joag S V, Li Z, Wang C Y, Jia F L, Foresman L, Adany I, Pinson D M, Stephens E B, Narayan O. Chimeric SHIV that causes CD4(+) T cell loss and AIDS in rhesus macaques. J Med Primatol. 1998;27:59–64. doi: 10.1111/j.1600-0684.1998.tb00227.x. [DOI] [PubMed] [Google Scholar]
- 22.Kent S J, Zhao A, Best S J, Chandler J D, Boyle D B, Ramshaw I A. Enhanced T-cell immunogenicity and protective efficacy of a human immunodeficiency virus type 1 vaccine regimen consisting of consecutive priming with DNA and boosting with recombinant fowlpox virus. J Virol. 1998;72:10180–10188. doi: 10.1128/jvi.72.12.10180-10188.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koup R A, Safrit J T, Cao Y Z, Andrews C A, McLeod G, Borkowsky W, Farthing C, Ho D D. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J Virol. 1994;68:4650–4655. doi: 10.1128/jvi.68.7.4650-4655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuroda M J, Schmitz J E, Barouch D H, Craiu A, Allen T M, Sette A, Watkins D I, Forman M A, Letvin N L. Analysis of Gag-specific cytotoxic T lymphocytes in simian immunodeficiency virus-infected rhesus monkeys by cell staining with a tetrameric major histocompatibility complex class I-peptide complex. J Exp Med. 1998;187:1373–1381. doi: 10.1084/jem.187.9.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuroda M J, Schmitz J E, Charini W A, Nickerson C E, Lifton M A, Lord C I, Forman M A, Letvin N L. Emergence of CTL coincides with clearance of virus during primary simian immunodeficiency virus infection in rhesus monkeys. J Immunol. 1999;162:5127–5133. [PubMed] [Google Scholar]
- 26.Letvin N L. Progress in the development of an HIV-1 vaccine. Science. 1998;280:1875–1880. doi: 10.1126/science.280.5371.1875. [DOI] [PubMed] [Google Scholar]
- 27.Lifson J D, Nowak M A, Goldstein S, Rossio J L, Kinter A, Vasquez G, Wiltrout T A, Brown C, Schneider D, Wahl L, Lloyd A L, Williams J, Elkins W R, Fauci A S, Hirsch V M. The extent of early virus replication is a critical determinant of the natural history of AIDS virus infection. J Virol. 1997;71:9508–9514. doi: 10.1128/jvi.71.12.9508-9514.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu Y C, Pauza C D, Lu X S, Montefiori D C, Miller C J. Rhesus macaques that become systemically infected with pathogenic SHIV 89.6-PD after intravenous, rectal, or vaginal inoculation and fail to make an antiviral antibody response rapidly develop AIDS. J Acquired Immune Defic Syndr. 1998;19:6–18. doi: 10.1097/00042560-199809010-00002. [DOI] [PubMed] [Google Scholar]
- 29.Mayr A, Hochstein-Mintzel V, Stickl H. Abstammung, Eigenschaften und Verwendung des attenuierten Vaccinia-stammes MVA. Infection. 1975;3:6–14. [Google Scholar]
- 30.Mayr A, Stickl H, Muller H K, Danner K, Singer H. The smallpox vaccination strain MVA: marker, genetic structure, experience gained with the parenteral vaccination and behavior in organisms with a debilitated defence mechanism. Zentbl Bakteriol Hyg B. 1978;167:375–390. [PubMed] [Google Scholar]
- 31.Mellors, J. W., C. R. Rinaldo, Jr., P. Gupta, R. M. White, J. A. Todd, and L. A. Kingsley. 1996. Prognosis in HIV-1 infection predicted by the quantity of virus in plasma. Science **272:**1167–1170. [DOI] [PubMed]
- 32.Meyer H, Sutter G, Mayr A. Mapping of deletions in the genome of the highly attenuated vaccinia virus MVA and their influence on virulence. J Gen Virol. 1992;72:1031–1038. doi: 10.1099/0022-1317-72-5-1031. [DOI] [PubMed] [Google Scholar]
- 33.Morrow C D, Novak M J, Ansardi D C, Porter D C, Moldoveanu Z. Recombinant viruses as vectors for mucosal immunity. Curr Top Microbiol Immunol. 1999;236:255–273. doi: 10.1007/978-3-642-59951-4_13. [DOI] [PubMed] [Google Scholar]
- 34.Moss B, Carroll M W, Wyatt L S, Bennink J R, Hirsch V M, Goldstein S, Elkins W R, Lifson J D, Piatak M, Restifo N P, Owerwijk W, Chamberlain R, Rosenberg S A, Sutter G. Host range restricted, non-replicating vaccinia virus vectors as vaccine candidates. Adv Exp Med Biol. 1996;397:7–13. doi: 10.1007/978-1-4899-1382-1_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mossman S P, Bex F, Berglund P, Arthos J, ONeil S P, Riley D, Maul D H, Bruck C, Momin P, Burny A, Fultz P N, Mullins J I, Liljestrom P, Hoover E A. Protection against lethal simian immunodeficiency virus SIVsmmPBj14 disease by a recombinant Semliki Forest virus gp160 vaccine and by a gp120 subunit vaccine. J Virol. 1996;70:1953–1960. doi: 10.1128/jvi.70.3.1953-1960.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Musey L, Hughes J, Schacker T, Shea T, Corey L, McElrath M J. Cytotoxic-T-cell responses, viral load, and disease progression in early human immunodeficiency virus type 1 infection. N Engl J Med. 1997;337:1267–1274. doi: 10.1056/NEJM199710303371803. [DOI] [PubMed] [Google Scholar]
- 37.Myagkikh M, Alipanah S, Markham P D, Tartaglia J, Paoletti E, Gallo R C, Franchini G, Robert-Guroff M. Multiple immunizations with attenuated poxvirus HIV type 2 recombinants and subunit boosts required for protection of rhesus macaques. AIDS Res Hum Retroviruses. 1996;12:985–992. doi: 10.1089/aid.1996.12.985. [DOI] [PubMed] [Google Scholar]
- 38.National Institutes of Health. Guide for the care and use of laboratory animals, rev. ed. Department of Health and Human Services publication no. (NIH) 85-23. Bethesda, Md: National Institutes of Health; 1985. [Google Scholar]
- 39.Ogg G S, Jin X, Bonhoeffer S, Dunbar P R, Nowak M A, Monard S, Segal J P, Cao Y Z, Rowland-Jones S L, Cerundolo V, Hurley A, Markowitz M, Ho D D, Nixon D F, McMichael A J. Quantitation of HIV-1-specific cytotoxic T lymphocytes and plasma load of viral RNA. Science. 1998;279:2103–2106. doi: 10.1126/science.279.5359.2103. [DOI] [PubMed] [Google Scholar]
- 40.Ourmanov I, Bilska M, Hirsch V M, Montefiori D C. Recombinant modified vaccinia virus Ankara expressing the surface gp120 of simian immunodeficiency virus (SIV) primes for a rapid neutralizing antibody response to SIV infection in macaques. J Virol. 2000;74:2960–2965. doi: 10.1128/jvi.74.6.2960-2965.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ourmanov I, Brown C R, Moss B, Carroll M, Wyatt L, Pletneva L, Goldstein S, Venzon D, Hirsch V M. Comparative efficacy of recombinant modified vaccinia virus Ankara expressing simian immunodeficiency virus (SIV) Gag-Pol and/or Env in macaques challenged with pathogenic SIV. J Virol. 2000;74:2740–2751. doi: 10.1128/jvi.74.6.2740-2751.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Panteleo G, Menzo S, Vaccarezza M, Graziosi C, Cohen O J, Demarest J F, Montefiori D C, Orenstein J M, Fox C, Schrager L K, Fauci A S. Studies in subjects with long-term nonprogressive human immunodeficiency virus infection. N Engl J Med. 1995;332:209–216. doi: 10.1056/NEJM199501263320402. [DOI] [PubMed] [Google Scholar]
- 43.Paoletti E. Applications of pox virus vectors to vaccination: an update. Proc Natl Acad Sci USA. 1996;93:11349–11353. doi: 10.1073/pnas.93.21.11349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Polacino P, Stallard V, Klaniecki J E, Montefiori D C, Langlois A J, Richardson B A, Overbaugh J, Morton W R, Benveniste R E, Hu S L. Limited breadth of the protective immunity elicited by simian immunodeficiency virus SIVmne gp160 vaccines in a combination immunization regimen. J Virol. 1999;73:618–630. doi: 10.1128/jvi.73.1.618-630.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Polacino P, Stallard V, Montefiori D C, Brown C R, Richardson B A, Morton W R, Benveniste R E, Hu S L. Protection of macaques against intrarectal infection by a combination immunization regimen with recombinant simian immunodeficiency virus SIVmne gp160 vaccines. J Virol. 1999;73:3134–3146. doi: 10.1128/jvi.73.4.3134-3146.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Polacino P S, Stallard V, Klaniecki J E, Pennathur S, Montefiori D C, Langlois A J, Richardson B A, Morton W R, Benveniste R E, Hu S L. Role of immune responses against the envelope and the core antigens of simian immunodeficiency virus SIVmne in protection against homologous cloned and uncloned virus challenge in macaques. J Virol. 1999;73:8201–8215. doi: 10.1128/jvi.73.10.8201-8215.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Redfield R R, Wright D C, James W D, Jones T S, Brown C, Burke D S. Disseminated vaccinia in a military recruit with human immunodeficiency virus (HIV) disease. N Engl J Med. 1987;316:673–676. doi: 10.1056/NEJM198703123161106. [DOI] [PubMed] [Google Scholar]
- 48.Robert-Guroff M, Kaur H, Patterson L J, Leno M, Conley A J, McKenna P M, Markham P D, Richardson E, Aldrich K, Arora K, Murty L, Carter L, Zolla-Pazner S, Sinangil F. Vaccine protection against a heterologous, non-syncytium-inducing, primary human immunodeficiency virus. J Virol. 1998;72:10275–10280. doi: 10.1128/jvi.72.12.10275-10280.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Robinson H L, Montefiori D C, Johnson R P, Manson K H, Kalish M L, Lifson J D, Rizvi T A, Lu S, Hu S L, Mazzara G P, Panicali D L, Herndon J G, Glickman R, Candido M A, Lydy S L, Wyand M S, McClure H M. Neutralizing antibody-independent containment of immunodeficiency virus challenges by DNA priming and recombinant pox virus booster immunization. Nat Med. 1999;5:526–534. doi: 10.1038/8406. [DOI] [PubMed] [Google Scholar]
- 50.Seth A, Ourmanov I, Kuroda M, Schmitz J E, Carroll M W, Wyatt L S, Moss B, Forman M A, Hirsch V M, Letvin N L. Recombinant modified vaccinia virus Ankara-simian immunodeficiency virus gag pol elicits cytotoxic T lymphocytes in rhesus monkeys detected by major histocompatibility complex class I/peptide tetramer. Proc Natl Acad Sci USA. 1998;95:10112–10116. doi: 10.1073/pnas.95.17.10112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shibata R, Maldarelli F, Siemon C, Matano T, Parta M, Miller G, Fredrickson T, Martin M A. Infection and pathogenicity of chimeric simian-human immunodeficiency viruses in macaques: determinants of high virus loads and CD4 cell killing. J Infect Dis. 1997;176:362–373. doi: 10.1086/514053. [DOI] [PubMed] [Google Scholar]
- 52.Staprans S I, Dailey P J, Rosenthal A, Horton C, Grant R M, Lerche N, Feinberg M B. Simian immunodeficiency virus disease course is predicted by the extent of virus replication during primary infection. J Virol. 1999;73:4829–4839. doi: 10.1128/jvi.73.6.4829-4839.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Suryanarayana K, Wiltrout T A, Vasques G M, Hirsch V M, Lifson J D. Plasma SIV RNA viral load determination by real-time quantification of product generation in reverse transcriptase-polymerase chain reaction. AIDS Res Hum Retroviruses. 1998;14:183–189. doi: 10.1089/aid.1998.14.183. [DOI] [PubMed] [Google Scholar]
- 54.Sutter G, Moss B. Nonreplicating vaccinia vector efficiently express recombinant genes. Proc Natl Acad Sci USA. 1992;89:10847–10851. doi: 10.1073/pnas.89.22.10847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Watson A, Ranchalis J, Travis B, McClure J, Sutton W, Johnson P R, Hu S L, Haigwood N L. Plasma viremia in macaques infected with simian immunodeficiency virus: plasma viral load early in infection predicts survival. J Virol. 1997;71:284–290. doi: 10.1128/jvi.71.1.284-290.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]