Differential Susceptibility to Human Immunodeficiency Virus Type 1 Infection of Myeloid and Plasmacytoid Dendritic Cells (original) (raw)
Abstract
Human immunodeficiency virus type 1 (HIV-1) infection of dendritic cells (DCs) plays an important role in HIV-1 transmission and pathogenesis. Here, we studied the susceptibility of ex vivo-isolated CD11c+ myeloid DCs (MDCs) and CD123+ plasmacytoid DCs (PDCs) to HIV-1 infection and the function of these cells early after infection. Both DC subsets were susceptible to CCR5- and CXCR4-using HIV-1 isolates (BaL and IIIB, respectively). However, MDCs were more susceptible to HIV-1BaL infection than donor-matched PDCs. In addition, HIV-1BaL infected MDCs more efficiently than HIV-1IIIB, whereas PDCs were equally susceptible to both isolates. While exposure to HIV-1 alone resulted in only weak maturation of DCs, Toll-like receptor 7/8 ligation induced full maturation in both infected and uninfected DCs. Maturation did not increase HIV-1 replication in infected DCs, and infected DCs retained their ability to produce tumor necrosis factor alpha after stimulation. Both HIV-1 isolates induced alpha interferon production exclusively in PDCs, irrespective of productive infection. In conclusion, PDCs and MDCs were susceptible to HIV-1 infection, but neither displayed functional defects as a consequence of infection. The difference in susceptibility of PDCs and MDCs to HIV-1 may have implications for HIV-1 transmission and DC-mediated transfer of HIV-1 to T cells.
Dendritic cells (DCs) are specialized leukocytes that bridge the innate and adaptive immune systems and act as antigen-presenting cells with the unique capacity to initiate primary T-cell responses. DCs migrate from the blood to reside in the periphery, where they capture antigens. Upon antigen encounter, DCs mature and migrate to regional draining lymph nodes, where they present the antigen to T cells (2). Two distinct subtypes of DCs have been identified in the human immune system, CD11c+ myeloid DCs (MDCs) and CD11c− CD123+ plasmacytoid DCs (PDCs). MDCs and PDCs both express high levels of major histocompatibility complex class II and lack expression of the lineage markers CD3, CD14, CD19, and CD56. MDCs are the more prevalent DCs and are found throughout the body, mainly in the skin (Langerhans cells) and mucosal tissues. PDCs are more sparsely distributed and are normally found only in blood, lymph nodes, and thymus but are recruited to sites of inflammation under pathological conditions (6, 12, 21, 22). Both DC subsets have antigen-presenting capacity but differ in their expression of Toll-like receptors (TLRs) and some important functional aspects. In general, MDCs are characterized by their ability to secrete high levels of interleukin-12 (IL-12), whereas PDCs produce high levels of alpha interferon (IFN-α) in response to TLR-induced activation and maturation (1).
Several studies have shown that the numbers of both MDCs and PDCs are reduced in blood during human immunodeficiency virus type 1 (HIV-1) infection (3, 8, 10, 13, 19). Although DCs in blood may not serve as a major reservoir for HIV-1 (9, 35), both PDCs and MDCs have been shown to be susceptible to HIV-1 infection ex vivo (15, 37, 38, 43). Due to the low frequencies of DCs in vivo and limitations on the numbers of these cells that can be recovered, the biology of DCs in the context of HIV-1 infection has mostly been studied using DCs generated from progenitors in vitro (4, 5, 7, 30, 40). Peripheral monocytes cultured in the presence of granulocyte-macrophage colony-stimulating factor (GM-CSF) and IL-4 develop into monocyte-derived DCs (MDDCs) that resemble MDCs with respect to cell surface markers and their response to most stimuli. However, MDCs and MDDCs are phenotypically and functionally distinct DCs with respect to their migratory capacity and ability to secrete cytokines like IL-12p70 (23, 32). Furthermore, these two types of DCs differ in their ability to stimulate cytokine production in T cells (23, 32). In addition, no in vitro model for PDCs is available.
Here, we have studied early productive HIV-1 infection in primary human blood-derived DCs. We sought to investigate the susceptibility of MDCs and PDCs to different isolates of HIV-1 and the functional properties of the DCs early after infection. To do this, high numbers of donor-matched MDCs and PDCs were isolated from elutriated monocytes and DCs were exposed to highly concentrated and purified HIV-1 isolates. We used intracellular p24 staining to distinguish HIV-1-infected (p24+) from uninfected (p24−) DCs and to compare the phenotype and function of infected DCs to those of exposed but not infected DCs. We report that both DC subsets were susceptible to HIV-1 infection. However, we found a differential susceptibility to HIV-1 infection between the two subsets of DCs. MDCs were more susceptible to HIV-1BaL infection than donor-matched PDCs. In addition, MDCs were preferentially infected by CCR5-using isolates rather than CXCR4-using isolates, while there was no difference in PDCs. We studied the phenotype and cytokine profile of the HIV-1-infected DCs and found no functional defects upon TLR7/8 stimulation. HIV-1 infection did not prevent maturation and tumor necrosis factor alpha (TNF-α) production in p24+ MDCs and PDCs. In addition, PDCs produced IFN-α upon HIV-1 exposure regardless of whether the infection was productive or not. Taken together, we have found a difference in susceptibility to HIV-1 infection between PDCs and MDCs, which could have important implications for our understanding of HIV-1 transmission and pathogenesis.
MATERIALS AND METHODS
Dendritic cell isolation.
This study was approved by the National Institutes of Health (NIH) Institutional Review Board. Our sorting procedures for direct isolation of subsets of DCs from blood have been described previously (28). Briefly, peripheral blood mononuclear cells (PBMCs) were collected from healthy HIV-1-seronegative blood donors by automated leukapheresis. Enriched populations of lymphocytes and monocytes were obtained by counterflow centrifugal elutriation. MDCs and PDCs were isolated from elutriated monocytes using magnetic bead isolation including dendritic cell isolation kits (Miltenyi Biotec, Auburn, CA) followed by sequential separation on AutoMacs (Miltenyi Biotec). The BDCA-4 and the CD1c isolation kits were used for isolation of PDCs and MDCs, respectively. To maintain viability, the PDCs and MDCs were cultured in complete medium (RPMI 1640 medium; BioWhittaker, Inc., Walkersville, MD) supplemented with 2 mmol/liter l-glutamine, 1% streptomycin and penicillin, and 10% fetal bovine serum (Invitrogen, Carlsbad, CA) in the presence of the recombinant human cytokine IL-3 (1 ng/ml; R&D Systems, Minneapolis, MN) or GM-CSF (2 ng/ml; PeproTech Inc, Rocky Hill, NJ).
HIV-1 virus growth and preparation.
The CCR5-using HIV-1BaL isolate and the CXCR4-using HIV-1IIIB isolate (NIH AIDS Research and Reference Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases, NIH, Bethesda, MD) were grown on phytohemagglutinin (PHA) (Sigma, St. Louis, MO)- and IL-2 (Roche, Indianapolis, IN)-stimulated PBMCs. p24 levels in the culture supernatants were monitored by enzyme-linked immunosorbent assay (ELISA), and viruses were harvested at a peak time point. To minimize the presence of bystander activation factors that could influence DC growth, the viruses were concentrated by ultracentrifugation (30,000 rpm, 70 min, 4°C [Sorvall Surespin 630; Kendro, Asheville, NC]). The virus pellet was resuspended in fresh complete medium to obtain a highly concentrated virus stock.
Characterization of HIV-1 stocks.
Viral titers were determined by p24 ELISA (Perkin-Elmer, Boston, MA) according to the manufacturer's protocol. The HIV-1BaL stock had an HIV-1 p24 content of 10 μg/ml, and the HIV-1IIIB stock had an HIV-1 p24 content of 5 μg/ml. Virus 50% tissue culture infective doses (TCID50) were determined by a sensitive 14-day endpoint titration assay using PHA- and IL-2-stimulated PBMCs, as previously described (34). The HIV-1BaL stock had 2.6 × 106 TCID50/ml, and the HIV-1IIIB stock had 1.75 × 106 TCID50/ml.
HIV-1 infection and stimulation of dendritic cells.
DC populations were cultured at 1 × 106 cells/ml, with no less than 0.25 × 106 DCs per tube, for 12 h at 37°C in polystyrene round-bottom tubes in complete medium supplemented with GM-CSF or IL-3. HIV-1BaL/IIIB (final concentration, 1 μg p24/ml) or mock was added to the DCs, and the cells were cultured for the indicated time periods. Exposure of sorted CD4+ T cells to 1 μg p24/ml of HIV-1 for 72 h resulted in 1.65 to 1.99% p24+ cells using HIV-1BaL and 0.91 to 15.2% p24+ cells after exposure to HIV-1IIIB. Use of SEB (0.2 μg/ml; Sigma, St. Louis, MO)-stimulated CD4+ T cells resulted in 5.04 to 11.1% of p24+ cells after exposure to HIV-1BaL, and 30.9 to 40.9% CD4+ T cells were p24+ after exposure to HIV-1IIIB. To prevent productive infection, azidothymidine (AZT) (10 μM; Sigma) was added to the cultures together with the virus. In some experiments, the DCs were stimulated with 1 μg/ml of the TLR7/8 ligand R-848 (4-amino-2-ethoxymethyl-α,α-dimethyl-1H-imidazoquinoline-1-ethanol; GLSynthesis Inc., Worcester, MA), as previously described (28).
Quantitative real-time PCR.
HIV-1 p17 Gag (full reverse transcript) and human albumin DNA were quantified by real-time PCR with an ABI7900HT (Applied Biosystems, Foster City, CA) as previously described (11). Briefly, the cells were lysed in proteinase K buffer (0.1 mg/ml in Tris-Cl, pH 8.0) at 100,000 cells per 10 μl buffer by incubation at 56°C for 1 h and then at 95°C for 10 min. The lysate was cleared by centrifugation at 14,000 × g for 2 min. Five microliters of the lysate was used in a reaction volume of 25 μl. Primers for the reactions were gag FWD (GGTGCGAGAGCGTCAGTATTAAG), gag REV (AGCTCCCTGCTTGCCCATA), Albumin Fwd (TGCATGAGAAAACGCCAGTAA), and Albumin Rev (ATGGTCGCCTGTTCACCAA) (BioSource International) used at 500 nM. Probes were gag probe (6-carboxyfluorescein-AAAATTCGGTTAAGGCCAGGGGGAAAGAA-BHQ1) and Albumin probe (6-carboxyfluorescein-TGACAGAGTCACCAAATGCTGCACAGAA-BHQ1) (BioSource International, Camarillo) used at 200 nM. The other reagents were used at the following concentrations: deoxynucleoside triphosphates, 200 mM; MgCl2, 3.5 mM; and Blue 636 reference dye, 1.25 mM with Platinum Taq (0.625 U) in the buffer supplied. The conditions were 95°C for 15 s and 60°C for 1 min for 50 cycles after a 2-min activation at 95°C. Quantitation was carried out by running standard curves for p17 Gag and albumin controls.
Phenotypic characterization and quantification of HIV-1 protein in dendritic cells.
Cells were harvested, washed in phosphate-buffered saline with 0.5% bovine serum albumin (ICN Biomedicals, Aurora, OH), and surface stained for 20 min at 4°C with different combinations of anti-CD11c, anti-CD123, anti-CD3, anti-CD14, anti-CD20, anti-CD40, anti-CD80, anti-CD83, anti-CD86, and anti-HLA-DR antibodies (BD Biosciences, San Diego, CA). Cells were washed, fixed, and permeabilized for 10 min using a 2× fixation-permeabilization solution (Becton Dickinson Immunocytometry Systems, San Jose, CA). The frequency of HIV-1 infection in DCs was determined by intracellular staining for HIV-1 p24. Cells were washed twice and stained intracellularly with anti-p24 antibody (clone KC57; Beckman Coulter Corporation, Fullerton, CA). Cell viability was evaluated by propidium iodide (Sigma) staining. Stainings for the chemokine receptors CCR5, CXCR4, and CCR7 were done by incubation with the respective antibody (BD Biosciences) at 37°C for 20 min followed by addition of any other antibodies and incubation at 4°C. The cells were analyzed with a FACSCalibur flow cytometer (Becton Dickinson).
Intracellular cytokine staining in dendritic cells.
The frequency of cytokine-producing DCs was determined by intracellular staining for TNF-α (clone mAb11; BD Biosciences). Brefeldin A (10 μg/ml) (Sigma) was added to the cell cultures 6 to 12 h prior to intracellular staining. Expression was assessed by a FACSCalibur.
Measurement of cytokine release.
Cytokine release from the DCs was studied by culturing the DCs at 1 × 106 cells/ml at 37°C in the presence or absence of HIV-1 for 72 h with or without R-848 during the final 24 h. All supernatants were analyzed in duplicate by IFN-α ELISA (detection limit, 12.5 pg/ml; Biosource International) performed according to the manufacturer's instructions.
Statistical analyses.
Statistical analyses were performed using Wilcoxon's paired t test. Correlations were analyzed with the Spearman rank test.
RESULTS
Productive HIV-1 infection in MDCs and PDCs.
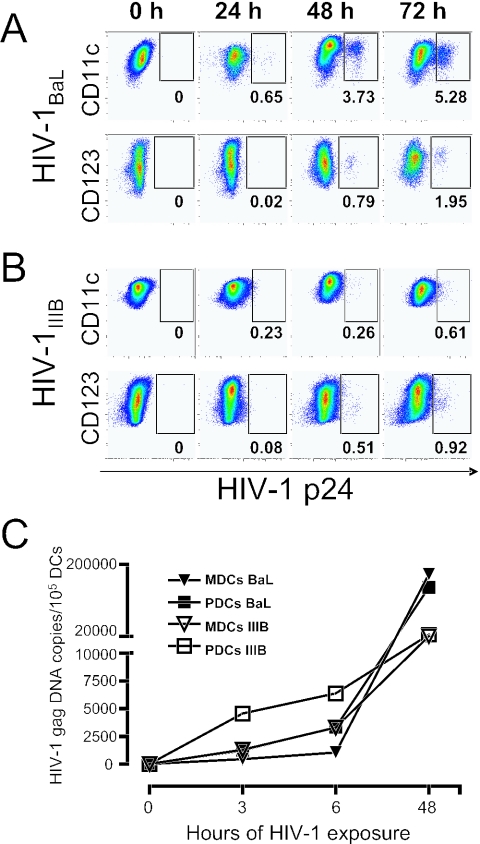
Recently, we established a direct isolation procedure to purify relatively high numbers of human CD123+ PDCs and CD11c+ MDCs from elutriated monocytes (28). On average, the recovery of isolated DCs was 1.1 × 106 PDCs and 2.2 × 106 MDCs per 108 elutriated monocytes. The subsets of DCs were highly enriched (on average, >75% PDCs and >85% MDCs), as determined by the lack of the lineage markers (CD3, CD20, CD14, and CD56) and expression of HLA-DR and CD123 or CD11c. The contaminating cells were CD14+ monocytes (data not shown). Overnight culture in IL-3 or GM-CSF of the freshly sorted PDCs and MDCs, respectively, led to development of the characteristic DC morphology (especially MDCs) and immature DC phenotype with low expression of CD40, CD80, CD83, and CD86. Blood-derived MDCs and PDCs are susceptible to HIV-1 infection ex vivo, as demonstrated by others (15, 37, 38, 43), using long-term culture of DCs and sensitive PCR techniques. However, extending the time of culture after HIV-1 exposure is problematic, since DCs (especially PDCs) are sensitive to long-term culture and begin to die. Therefore, we exposed DCs to virus isolates with a high multiplicity of infection to generate high and fast infection without inducing cell death. We have previously developed such a protocol for infection of MDDCs (40). To optimize the conditions to study early HIV-1 infection of primary DCs, immature sorted MDCs and PDCs were exposed to CCR5-using HIV-1BaL (Fig. 1A) or CXCR4-using HIV-1IIIB (Fig. 1B) for 0, 24, 48, or 72 h. The frequency of HIV-1 infection at the indicated time points was determined by intracellular HIV-1 p24 staining (Fig. 1A and B) or quantitative real-time PCR for HIV-1 Gag (Fig. 1C). A small but distinct HIV-1 p24+ population could be detected in the MDCs exposed to HIV-1BaL after only 24 h. After 48 h, p24+ MDCs and PDCs were detected in the cultures exposed to HIV-1BaL and HIV-1IIIB. The number of p24+ DCs continued to increase over the first 72 h of HIV-1 exposure (Fig. 1A and B). HIV-1IIIB appeared to propagate slower in both MDCs and PDCs than HIV-1BaL. Seventy-two hours of HIV-1 exposure did not increase cell death in the DCs compared to the medium control (data not shown). At later time points (>4 days), increased cell death was evident in the HIV-1-exposed DC cultures. Overall, PDCs were found to be more sensitive to long-term culture than MDCs, regardless of virus exposure. Decreasing the amount of virus and/or the time (<72 h) of HIV-1 exposure resulted in no or lower frequencies of p24+ DCs. For all further experiments, 72 h of HIV-1 exposure without washing off the virus was therefore chosen to study the effects of early HIV-1 infection in DCs.
FIG. 1.
Rapid infection and propagation of HIV-1BaL and HIV-1IIIB in MDCs and PDCs. Sorted CD11c+ MDCs and CD123+ PDCs were exposed to HIV-1BaL (A) or HIV-1IIIB (B) and harvested after 0, 24, 48, and 72 h for assessment of the level of HIV-1 infection by intracellular staining for HIV-1 p24 by flow cytometry. Any contaminating monocytes were excluded in the analysis by gating on the cells that expressed CD11c (MDCs) or CD123 (PDCs) but not CD14. (C) Sorted CD11c+ MDCs and CD123+ PDCs were exposed to HIV-1BaL or HIV-1IIIB for 0, 3, 6, and 48 h and subsequently harvested. DNA was extracted, and the amount of full-length HIV-1 Gag DNA was measured relative to the amount of albumin DNA by quantitative real-time PCR. All samples were analyzed in duplicate. One representative experiment of three is shown.
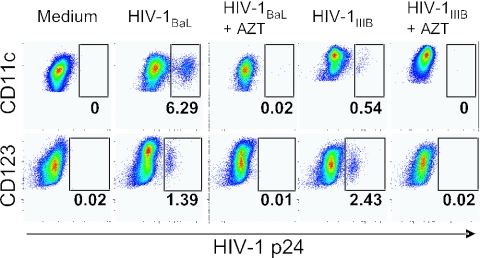
We questioned whether the p24+ DCs were productively infected by HIV-1, since DCs do not divide. Also, immature DCs efficiently take up antigen, raising the possibility that the p24 staining was the result of virus uptake and not productive infection. While p24+ DCs could be detected only by intracellular flow cytometry after 24 to 48 h of exposure, full-length HIV-1 Gag DNA, a product of complete reverse transcription, was detected already after 3 h and increased with time of exposure (Fig. 1C). This was true for both DC subsets irrespective of which isolate was used. These data show that DCs were productively infected and not capturing virions, as most of the viral RNA had undergone complete reverse transcription. In addition, no p24+ cells were detected if the DCs had been cultured with AZT during the infection, indicating that the p24+ cells were the result of productive infection and not continuous uptake of virions (Fig. 2). This was also observed in either DC subset cultured with HIV-1BaL or HIV-1IIIB.
FIG. 2.
Productive HIV-1BaL and HIV-1IIIB infection in MDCs and PDCs. Sorted CD11c+ MDCs (A) and CD123+ PDCs (B) were exposed to HIV-1BaL or HIV-1IIIB in the presence or absence of AZT for 72 h. The level of HIV-1 infection was determined by intracellular p24 staining and flow cytometry. One representative experiment of six is shown.
Differential susceptibility to HIV-1 infection in MDCs and PDCs.
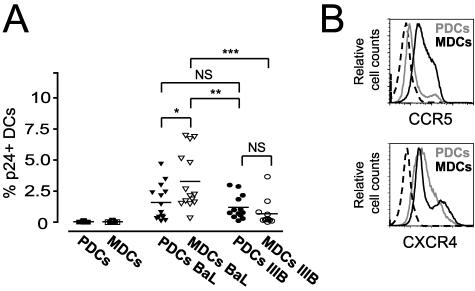
To further investigate if there was a difference in the susceptibility to HIV-1 infection in the two DC subsets, MDCs and PDCs from 14 individual donors were isolated and exposed to HIV-1. After 72 h of HIV-1BaL exposure, 0.1 to 4.68% (mean, 1.59%) of p24+ DCs were detected in the PDCs, and 0.12 to 2.99% (mean, 1.19%) were detected for HIV-1IIIB. For MDCs, the average frequency of p24+ DCs was 3.28% (range, 0.32 to 6.96%) for HIV-1BaL and 0.68% (range, 0.1 to 3.66%) for HIV-1IIIB (Fig. 3A). We found that MDCs showed a significantly higher susceptibility to the R5-using HIV-1BaL virus than to the X4-using HIV-1IIIB virus (P = 0.0005). However, the susceptibility to the two HIV-1 isolates was not significantly different in PDCs. MDCs showed a significantly higher susceptibility to HIV-1BaL than PDCs (P = 0.013), while the difference in susceptibility to HIV-1IIIB infection was not significant between MDCs and PDCs (Fig. 3A).
FIG. 3.
MDCs are more susceptible to CCR5-using HIV-1 isolates than CXCR4-using HIV-1 isolates. Sorted CD11c+ MDCs and CD123+ PDCs from 14 donors were exposed to CCR5-using HIV-1BaL or CXCR4-using HIV-1IIIB for 72 h, and the level of infection was determined by intracellular p24 staining and flow cytometry. (A) The graph shows the individual donors and the average percentage of p24+ DCs detected after 72 h of HIV-1 exposure. Significant differences compared between the groups were assessed by Wilcoxon's paired t test and considered statistically significant at P < 0.05. (B) The expression level of the chemokine receptors, also used as coreceptors by HIV-1, CCR5, and CXCR4 on MDCs (black) and PDCs (grey) and the corresponding isotype controls (dashed), at the start of infection (0 h) was determined by flow cytometry. One representative donor of six is shown. *P = 0.013; **P = 0.003. ***P = 0.0005. NS, not significant.
To evaluate whether the differential susceptibility to HIV-1 isolates by the DC subsets as well as in individual donors was due to differences in coreceptor expression, we analyzed the expression of the chemokine receptors CCR5 and CXCR4 on MDCs and PDCs prior to infection. MDCs consistently had a higher expression of CCR5 than PDCs (Fig. 3B), which likely accounts to their high susceptibility to HIV-1BaL. In contrast, PDCs often exhibited a higher and more uniform expression of CXCR4 than did donor-matched MDCs. MDCs frequently consisted of distinct CXCR4low and CXCR4high populations (Fig. 3B). However, there was no significant correlation between the CCR5 and CXCR4 expression on DCs prior to virus infection and the frequency of p24+ DCs found in the cultures after 72 h (data not shown).
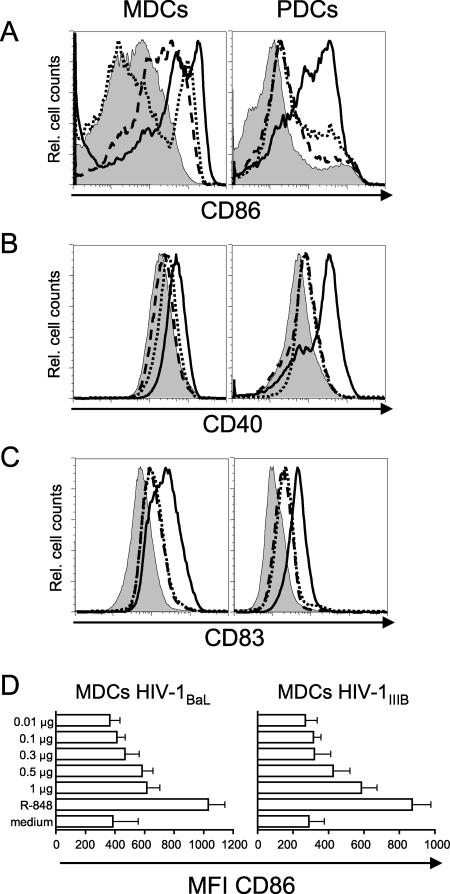
HIV-1 exposure does not induce full maturation of MDCs and PDCs.
DCs located at sites in vivo where initial transmission of HIV-1 is most likely to occur, such as mucosal surfaces and blood, express an immature phenotype. Immature DCs are therefore most relevant to examine for HIV-1 susceptibility in vitro. Although CD4+ T cells are the main producers of HIV-1 in vivo and in vitro, they require activation to induce and maintain HIV-1 replication. In contrast, isolated Langerhans cells and MDDCs have been found to propagate HIV-1 without additional activation (25, 26, 40). Here, we found that MDCs and PDCs replicate HIV-1 without intentional prior activation or DC maturation (Fig. 1 to 3). Next, we analyzed whether exposure to HIV-1 induced maturation of the DCs. After 72 h of culture in medium alone, the expression of CD40 was relatively high on both MDCs and PDCs, and MDCs expressed intermediate levels of CD83 (Fig. 4). We found that 72 h of HIV-1 exposure resulted in upregulation of CD86, CD40, and CD83 on both MDCs and PDCs (Fig. 4). HIV-1 exposure was most effective in increasing the expression of CD86 compared to CD40 and CD83. Also, MDCs appeared to respond more to HIV-1 exposure than did PDCs in terms of CD86 upregulation (Fig. 4A). However, PDCs upregulated CD83 more in response to virus exposure than did MDCs (Fig. 4C). CCR7 expression was not affected by HIV-1 exposure on either DC subset (data not shown). HIV-1IIIB exposure tended to induce higher expression of these molecules compared to HIV-1BaL. To assure that the DCs could respond correctly to stimuli and undergo maturation, we added the TLR ligand R-848 (an imidazoquino-like compound that signals through TLR7 and TLR8) during the final 24 h of culture. R-848 stimulation has previously been shown to induce maturation of MDCs and PDCs as measured by upregulation of the costimulatory molecules CD40, CD80, CD83, and CD86 and secretion of cytokines (17, 28). Stimulation with R-848 resulted in complete upregulation, always exceeding that seen after HIV-1 exposure alone, of all the costimulatory molecules on both MDCs and PDC, in the presence or absence of the HIV-1 isolates (Fig. 4 and data not shown). Therefore, the maturation induced after HIV-1 exposure alone was only partial, since the addition of R-848 to the DC cultures resulted in much greater upregulation of CD86, CD40, and CD83. To investigate whether the maturation induced by HIV-1 itself was a function of virus inoculum, we exposed MDCs to decreasing doses of HIV-1BaL or HIV-1IIIB (Fig. 4D) for 72 h. We found that decreasing the dose of HIV-1 from 1 μg to 0.5, 0.3, 0.1, or 0.01 μg p24/ml resulted in reduced or no induction of maturation, as measured by upregulation of CD86 expression (Fig. 4D).
FIG. 4.
HIV-1 exposure does not induce full maturation in MDCs and PDCs. Sorted CD11c+ MDCs and CD123+ PDCs were exposed to HIV-1 for 72 h with or without R-848 stimulation during the final 24 h. The expression of CD86 (A), CD40 (B), and CD83 (C) on MDCs and PDCs was determined by flow cytometry. Histograms show medium control (filled), HIV-1BaL (dashed), HIV-1IIIB (dotted), and R-848 (black). One representative experiment of nine is shown. In panel D, sorted CD11c+ MDCs were exposed to decreasing doses of HIV-1BaL or HIV-1IIIB for 72 h. R-848 stimulation was used as a positive control for DC maturation. The bar graphs show the average mean fluorescence intensity (MFI) of CD86 expression on the MDCs from four to six individual donors ± standard deviations.
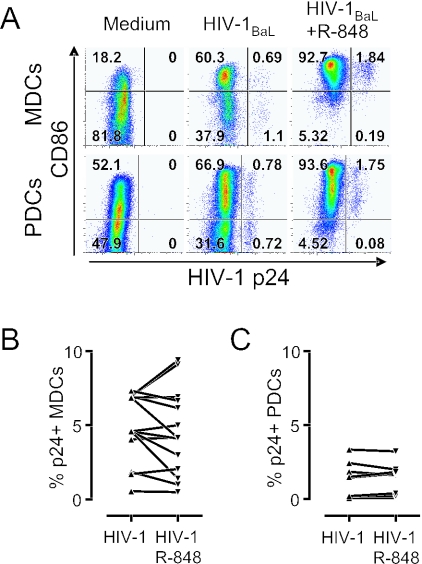
We next investigated whether the HIV-1-infected p24+ DCs had a different phenotype compared to the p24− DCs in the same culture and if they could further mature in response to R-848 stimulation. We found that HIV-1 induced a small and comparable upregulation of CD86 on both the p24− and p24+ MDCs and PDCs. Furthermore, the CD86 expression was upregulated to a similar magnitude on both the infected and uninfected DCs after R-848 stimulation (Fig. 5A). To investigate the effect of stimulation and activation of DCs on their ability to promote HIV-1 replication, we compared the frequency of p24+ MDCs (Fig. 5B) and PDCs (Fig. 5C) in HIV-1-exposed DCs that did or did not receive R-848 stimulation during the final 24 h of culture. We found that R-848 stimulation and subsequent maturation of HIV-1-infected DCs did not result in a significant difference in HIV-1 replication (P = 0.326 and P = 1.063 for MDCs and PDCs, respectively). The average frequency of infection in MDCs was 4.89% without stimulation and 4.54% with R-848 stimulation. For PDCs, the mean percentage of p24+ DCs was 1.39% and 1.38% in the absence and presence of R-848 stimulation, respectively (Fig. 5B and C). These data indicate that HIV-1 exposure rather than infection per se induces partial maturation in DCs and that both infected and uninfected DCs remain susceptible to further maturation by TLR ligation without increased HIV-1 replication.
FIG. 5.
HIV-1-infected MDCs and PDCs are susceptible to R-848 stimulation without induction of viral replication. Sorted CD11c+ MDCs and CD123+ PDCs were exposed to HIV-1BaL for 72 h with or without R-848 stimulation for the final 24 h. The expression of CD86 was plotted against HIV-1 p24 to compare the level of maturation in uninfected p24− DCs and HIV-1-infected p24+ MDCs and PDCs before and after R-848 stimulation (A). One representative experiment of six is shown. R-848 stimulation of HIV-1BaL-exposed MDCs (B) and PDCs (C) did not significantly affect the frequency of p24+ DCs.
HIV-1-infected MDCs and PDCs produce TNF-α in response to R-848.
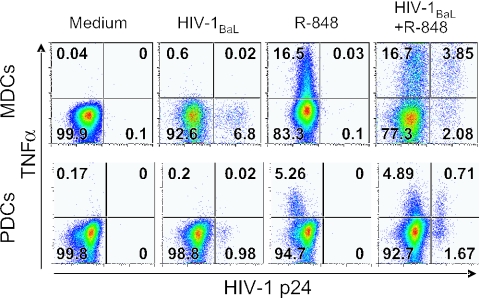
To determine if HIV-1-infected MDCs and PDCs remained functionally capable of producing cytokines, we analyzed the capacity of the p24+ DCs to respond to R-848 stimulation by induction of TNF-α production. Sorted CD11c+ MDCs and CD123+ PDCs were exposed to HIV-1BaL for 72 h with or without R-848 stimulation during the final 12 h. We found that HIV-1BaL alone did not induce TNF-α production when analyzed after 72 h of HIV-1 exposure. However, both p24+ and p24− DCs had the capacity to produce TNF-α in response R-848 stimulation (Fig. 6). Also, the frequency of HIV-1-infected p24+ DCs that produced TNF-α was higher than that of the p24− DCs; on average, 35% ± 9.6% and 24% ± 8.9% of the p24+ MDCs and PDCs produced TNF-α, respectively, compared to 18% ± 5.9% and 13% ± 5.1% of the p24− MDCs and PDCs, on average. In addition, the TNF-α+ DCs were more frequently infected than the TNF-α− DCs; 27% ± 8.1% and 8.0% ± 2.3% (mean values) of the TNF-α+ MDCs and PDCs were p24+, respectively, compared to 15% ± 5.4% and 2.9% ± 0.4% (mean values) of the TNF-α− MDCs and PDCs (Fig. 6). Overall, more MDCs produced TNF-α in response to R-848 stimulation than PDCs. These results suggest that HIV-1 infection does not prevent the ability of primary DCs to produce cytokine in response to TLR ligation.
FIG. 6.
HIV-1-infected DCs produce TNF-α in response to R-848 stimulation. Sorted CD11c+ MDCs and CD123+ PDCs were exposed to HIV-1BaL for 72 h with or without R-848 stimulation during the final 12 h. The frequency of p24+ and TNF-α+ MDCs (upper panel) and PDCs (lower panel) was analyzed by flow cytometry. Quadrants were set to separate p24− and p24+ DCs as well as TNF-α+ and TNF-α− DCs. One representative experiment of eight (MDCs) or six (PDCs) is shown.
Production of IFN-α by PDCs does not require productive HIV-1 infection.
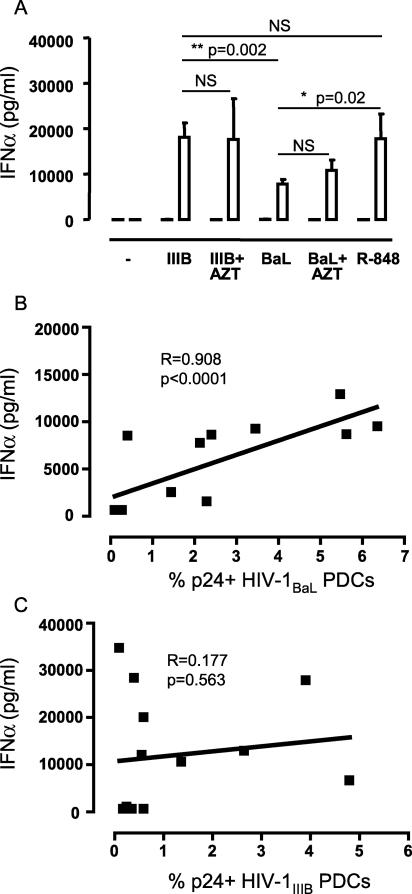
PDCs have been reported to produce large amounts of IFN-α upon stimulation with viruses such as HIV-1, HSV, influenza virus, and Sendai virus (14, 16, 17, 20, 33, 43). Here, we also found significant amounts of IFN-α in the PDC culture supernatants in response to HIV-1 exposure for 72 h (Fig. 7A). Both HIV-1BaL and HIV-1IIIB induced significant levels of IFN-α in PDCs compared to the medium control and to MDCs. The levels of IFN-α secreted in response to HIV-1IIIB were significantly higher (P = 0.002) than those seen after exposure to HIV-1BaL and comparable to the amounts of IFN-α secreted in response to R-848 stimulation (Fig. 7A). The high levels of IFN-α induced by viral exposure were not further augmented when the HIV-1IIIB-exposed PDCs were stimulated with R-848 (data not shown), which may suggest that the cells had reached their maximum ability to produce IFN-α. We found IFN-α in the culture supernatant of PDCs already after 6 h of HIV-1 exposure. The levels increased up to 24 to 48 h of HIV-1 exposure and then plateaued (data not shown). MDCs did not produce detectable levels of IFN-α after either HIV-1 exposure or R-848 stimulation (Fig. 7A). It has been reported that inactivated HIV-1 can induce IFN-α production in PDCs (17, 43). Here, IFN-α production was also observed in supernatants of HIV-1-exposed PDC cultured in the presence of AZT (Fig. 7A). This confirms that induction of IFN-α production requires only interaction of PDCs with HIV-1 virions or viral components but not productive HIV-1 infection.
FIG. 7.
IFN-α production by PDCs does not require productive infection by HIV-1. (A) Sorted CD11c+ MDCs and CD123+ PDCs were exposed to HIV-1BaL or HIV-1IIIB in the presence or absence of AZT. The levels of IFN-α in the cell culture supernatants were determined by ELISA. The levels were compared with the levels secreted by DCs stimulated by R-848. The graph shows the average IFN-α secretion ± standard error of the mean for six donors in MDCs (black bars) and PDCs (white bars). Significant differences compared between the groups were assessed by Wilcoxon's paired t test and considered statistically significant at P < 0.05 or nonsignificant (NS) at _P_ > 0.05. The amount of secreted IFN-α was measured in PDCs from 13 donors and plotted against the frequency of p24+ PDCs detected after 72 h of (B) HIV-1BaL or (C) HIV-1IIIB exposure. Correlation was assessed using the Spearman rank test and considered statistically significant if P was <0.05.
As IFN-α is an antiviral cytokine, its production could potentially interfere with the ability of PDCs to propagate virus and may cause lower infection rates in these cells. We looked at the correlation between the levels of IFN-α produced and frequency of p24+ cells in the PDC cultures after HIV-1BaL or HIV-1IIIB exposure. There was a positive correlation between the amounts of IFN-α secreted and the frequency of HIV-1BaL infection in PDCs (R = 0.908; P < 0.001 [Fig. 7B]), while no such correlation was found for HIV-1IIIB and IFN-α (Fig. 7C). Without determining the source of the secreted IFN-α by intracellular staining, it is difficult to interpret this correlation. However, it could indicate that the IFN-α did not have a direct negative effect on HIV-1 replication in these DCs but rather was associated with increased frequencies of infection.
DISCUSSION
The numbers of MDCs and PDCs are reduced in blood during HIV-1 infection (3, 8, 10, 13, 19, 36). Given that DCs are the only antigen-presenting cells with the capacity to stimulate naïve T cells, they are crucial to successful therapeutic vaccination with the induction of primary immune responses. It is therefore important to understand how HIV-1 infection affects DC numbers and function. This study characterized the infectibility and function of DCs after exposure to HIV-1. Previously, the role and function of DCs in HIV-1 infection have mainly been studied using in vitro-generated DCs (4, 5, 7, 30, 40). However, comparative studies on autologous MDDCs and primary MDCs have shown that these myeloid cell populations do not respond in an identical manner with respect to maturation, cytokine production, and T-cell-stimulatory activity (23, 32, 39). In addition, there is no in vitro system available for generating PDCs. Studies using pure preparations of isolated primary DCs to examine the effects of HIV-1 infection have been limited (17, 24, 37, 41, 43). In this study, we isolated MDCs and PDCs from elutriated monocytes and thereby obtained relatively high numbers of DCs. Throughout the study, we used donor-matched MDCs and PDCs isolated from healthy donors. We could confirm that human PDCs and MDCs are susceptible to HIV-1 infection (15, 37, 43). The presence of detectable p24+ DCs allowed us to extend prior findings with detailed studies of the effects of early HIV-1 infection on DC maturation and cytokine production.
Previous reports have described that blood-derived DCs are susceptible to HIV-1 in vitro by detecting proviral HIV-1 DNA in the DCs or monitoring the cell culture supernatant for reverse transcriptase activity or p24 content after 9 to 21 days of culture (15, 37, 43). Here, we showed productive HIV-1 infection in MDCs and PDCs by applying intracellular p24 staining and flow cytometry. CD4+ T cells need stimulation to induce significant viral replication, while most reports point out that HIV-1 infection of DCs does not require prior activation of the cells (4, 25, 37, 40, 43). Here, we found that MDCs and PDCs replicated HIV-1 and expressed significant amounts of p24 intracellularly without prior activation. Viral replication started early after HIV-1 exposure, and viral DNA transcripts could be detected as early as 3 h after exposure. HIV-1 p24+ cells appeared after 24 h of viral exposure, and the numbers increased over time. No viral replication was detected when the DCs were cultured in the presence of AZT. We found that MDCs were more effectively infected by HIV-1BaL than donor-matched PDCs. MDCs were less susceptible to infection with HIV-1IIIB than HIV-1BaL. However, no significant difference was found in PDCs, although the expression of CCR5 was low and CXCR4 was dim/high on these cells. These differences in susceptibility to HIV-1 in PDCs and MDCs could potentially play a role in HIV-1 transmission and pathogenesis, as DCs are proposed to be important in the spread of virus to T cells. Although it is established that DCs have the capacity to bind and transfer HIV-1 to T cells in the absence of infection of the DCs (18, 27, 42), transfer via productive infection of DCs may predominate in vivo. Turville and coworkers have proposed a two-phase model (42) where early viral transfer from DCs to T cells is not dependent on infection of the DCs. However, at later time points, productive infection of the DCs is required for virus transfer to T cells to occur (42). In that model, those authors proposed that the second phase of transfer (via productive infection of DCs) may predominate in vivo, as there is selective transmission and persistence of R5-using isolates in early HIV-1 infection. Viral transfer in the absence of infection should be nonselective for X4- and R5-using viruses, while productive infection of DCs should depend on the preferential infection of DCs with one or the other type of HIV-1 isolate. Still, the relative contribution of these different pathways in vivo remains to be determined and may not be the same in all DC subsets. As MDCs are more prevalent in mucosal tissues, they may contribute to the dominance of R5 virus isolates found in infected individuals. PDCs may primarily encounter virus that reaches lymphatic tissues and play a role in DC-mediated transfer of HIV-1 to T cells during antigen presentation at that site.
The reduction of DCs in blood in HIV-1-infected individuals can be an effect of infection and depletion of DCs. It may also be due to relocalization of DCs to secondary lymphoid tissues as a consequence of antigen uptake, migration, and/or maturation (17, 29, 31). Several studies have shown that MDDCs do not alter their expression of costimulatory molecules such as CD40, CD80, CD83, CD86, or major histocompatibility complex class II after HIV-1 exposure (5, 7, 30, 40). Still, little is known about the maturation pattern after HIV-1 exposure of DCs directly isolated from blood. It has recently been shown that PDCs, but not MDCs, mature in response to HIV-1 exposure. However, the cytokines secreted by PDCs after 16 h of HIV-1 exposure induce maturation in bystander MDCs (17). Important differences between that study and the current study are the dose of HIV-1 used to expose the DCs and the length of virus exposure. We exposed MDCs and PDCs to 1 μg p24/ml of HIV-1 for 72 h, which resulted in a p24+ population of DCs, without induction of cell death above background levels. The presence of detectable p24+ DCs allowed us to extend prior findings with detailed studies of the effects of early HIV-1 infection on DC maturation and cytokine production. However, the dose of 1 μg p24/ml also resulted in partial maturation of the MDCs and PDCs after 72 h of HIV-1 exposure. In the study performed by Fonteneau and colleagues, a lower dose, 0.3 μg p24/ml, was used to expose the DCs for 16 h, which resulted in upregulation of CD83 and CCR7 on the PDCs but not on the MDCs. Use of a similar dose of HIV-1 in our experimental system resulted in no or minimal maturation of the MDCs. Thus, the effect of HIV-1 exposure on DC maturation appears to be a consequence of virus dose. Importantly, the maturation induced after HIV-1 exposure was only partial, since the addition of R-848 to the DC cultures resulted in further upregulation of these molecules.
HIV-1-infected DCs were also able to upregulate costimulatory molecules and produce TNF-α after TLR7/8 stimulation, which may again suggest that HIV-1 infection does not induce major functional defects in DCs. Collectively, these results indicate that there is no major functional impairment in infected primary DCs early after HIV-1 infection, with respect to the parameters investigated here. In addition, we demonstrated that R-848 stimulation induced DC maturation without increased HIV-1 replication. This could indicate that the use of adjuvants that stimulate DCs via defined TLRs may be a way to augment HIV-1-specific immune responses without enhancing HIV-1 replication in DCs. However, we cannot exclude that TLR stimulation and maturation of DCs would lead to more efficient activation of CD4+ T cells and replication of HIV-1.
We previously reported a differential lack of IL-12p70 production but not TNF-α in HIV-1-infected p24+ MDDCs (40). Here, we extended these findings by showing that HIV-1-infected primary MDCs and PDCs also produce TNF-α after stimulation. We have previously shown that R-848 stimulation induces IL-12p70 production in MDCs (28). However, it has also been shown that blood-derived MDCs produce significantly less IL-12p70 than MDDCs in response to stimuli (23). This could be one explanation as to why we failed to detect intracellular IL-12p70 in R-848-stimulated MDCs and PDCs (data not shown). These results show that certain functional capabilities of primary MDCs and PDCs are retained despite HIV-1 infection and that HIV-1 alone does not fully activate DCs.
Our data offer an increased understanding of the effects of early HIV-1 infection on the functional properties of blood-derived MDCs and PDCs. In conclusion, MDCs and PDCs are susceptible to HIV-1 infection but display no functional defects upon stimulation. Given the central role of DCs in generating primary immune responses, these data may help to address the issue of how to mount proper anti-HIV-1 immune responses in infected individuals.
Acknowledgments
This work was supported by NIH intramural research funds and the Swedish Research Council, the Swedish Foundation for Strategic Research, Swedish Physicians Against AIDS Research Foundations, Swedish Cancer Society, and Swedish International Development Cooperation Agency/Department for Research Cooperation.
We thank Johan K. Sandberg and Markus Moll for critical reading of the manuscript.
REFERENCES
- 1.Akira, S., K. Takeda, and T. Kaisho. 2001. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat. Immunol. 2**:**675-680. [DOI] [PubMed] [Google Scholar]
- 2.Banchereau, J., and R. M. Steinman. 1998. Dendritic cells and the control of immunity. Nature 392**:**245-252. [DOI] [PubMed] [Google Scholar]
- 3.Barron, M. A., N. Blyveis, B. E. Palmer, S. MaWhinney, and C. C. Wilson. 2003. Influence of plasma viremia on defects in number and immunophenotype of blood dendritic cell subsets in human immunodeficiency virus 1-infected individuals. J. Infect. Dis. 187**:**26-37. [DOI] [PubMed] [Google Scholar]
- 4.Canque, B., Y. Bakri, S. Camus, M. Yagello, A. Benjouad, and J. C. Gluckman. 1999. The susceptibility to X4 and R5 human immunodeficiency virus-1 strains of dendritic cells derived in vitro from CD34(+) hematopoietic progenitor cells is primarily determined by their maturation stage. Blood 93**:**3866-3875. [PubMed] [Google Scholar]
- 5.Canque, B., M. Rosenzwajg, S. Camus, M. Yagello, M. L. Bonnet, M. Guigon, and J. C. Gluckman. 1996. The effect of in vitro human immunodeficiency virus infection on dendritic-cell differentiation and function. Blood 88**:**4215-4228. [PubMed] [Google Scholar]
- 6.Cella, M., D. Jarrossay, F. Facchetti, O. Alebardi, H. Nakajima, A. Lanzavecchia, and M. Colonna. 1999. Plasmacytoid monocytes migrate to inflamed lymph nodes and produce large amounts of type I interferon. Nat. Med. 5**:**919-923. [DOI] [PubMed] [Google Scholar]
- 7.Charbonnier, A. S., B. Verrier, C. Jacquet, C. Massacrier, M. M. Fiers, F. Mallet, C. Dezutter-Dambuyant, and D. Schmitt. 1996. In vitro HIV1 infection of CD34+ progenitor-derived dendritic/Langerhans cells at different stages of their differentiation in the presence of GM-CSF/TNF alpha. Res. Virol. 147**:**89-95. [DOI] [PubMed] [Google Scholar]
- 8.Chehimi, J., D. E. Campbell, L. Azzoni, D. Bacheller, E. Papasavvas, G. Jerandi, K. Mounzer, J. Kostman, G. Trinchieri, and L. J. Montaner. 2002. Persistent decreases in blood plasmacytoid dendritic cell number and function despite effective highly active antiretroviral therapy and increased blood myeloid dendritic cells in HIV-infected individuals. J. Immunol. 168**:**4796-4801. [DOI] [PubMed] [Google Scholar]
- 9.Donaghy, H., B. Gazzard, F. Gotch, and S. Patterson. 2003. Dysfunction and infection of freshly isolated blood myeloid and plasmacytoid dendritic cells in patients infected with HIV-1. Blood 101**:**4505-4511. [DOI] [PubMed] [Google Scholar]
- 10.Donaghy, H., A. Pozniak, B. Gazzard, N. Qazi, J. Gilmour, F. Gotch, and S. Patterson. 2001. Loss of blood CD11c(+) myeloid and CD11c(−) plasmacytoid dendritic cells in patients with HIV-1 infection correlates with HIV-1 RNA virus load. Blood 98**:**2574-2576. [DOI] [PubMed] [Google Scholar]
- 11.Douek, D. C., J. M. Brenchley, M. R. Betts, D. R. Ambrozak, B. J. Hill, Y. Okamoto, J. P. Casazza, J. Kuruppu, K. Kunstman, S. Wolinsky, Z. Grossman, M. Dybul, A. Oxenius, D. A. Price, M. Connors, and R. A. Koup. 2002. HIV preferentially infects HIV-specific CD4+ T cells. Nature 417**:**95-98. [DOI] [PubMed] [Google Scholar]
- 12.Farkas, L., K. Beiske, F. Lund-Johansen, P. Brandtzaeg, and F. L. Jahnsen. 2001. Plasmacytoid dendritic cells (natural interferon-alpha/beta-producing cells) accumulate in cutaneous lupus erythematosus lesions. Am. J. Pathol. 159**:**237-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feldman, S., D. Stein, S. Amrute, T. Denny, Z. Garcia, P. Kloser, Y. Sun, N. Megjugorac, and P. Fitzgerald-Bocarsly. 2001. Decreased interferon-alpha production in HIV-infected patients correlates with numerical and functional deficiencies in circulating type 2 dendritic cell precursors. Clin. Immunol. 101**:**201-210. [DOI] [PubMed] [Google Scholar]
- 14.Ferbas, J. J., J. F. Toso, A. J. Logar, J. S. Navratil, and C. R. Rinaldo, Jr. 1994. CD4+ blood dendritic cells are potent producers of IFN-alpha in response to in vitro HIV-1 infection. J. Immunol. 152**:**4649-4662. [PubMed] [Google Scholar]
- 15.Fong, L., M. Mengozzi, N. W. Abbey, B. G. Herndier, and E. G. Engleman. 2002. Productive infection of plasmacytoid dendritic cells with human immunodeficiency virus type 1 is triggered by CD40 ligation. J. Virol. 76**:**11033-11041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fonteneau, J. F., M. Gilliet, M. Larsson, I. Dasilva, C. Munz, Y. J. Liu, and N. Bhardwaj. 2003. Activation of influenza virus-specific CD4+ and CD8+ T cells: a new role for plasmacytoid dendritic cells in adaptive immunity. Blood 101**:**3520-3526. [DOI] [PubMed] [Google Scholar]
- 17.Fonteneau, J. F., M. Larsson, A. S. Beignon, K. McKenna, I. Dasilva, A. Amara, Y. J. Liu, J. D. Lifson, D. R. Littman, and N. Bhardwaj. 2004. Human immunodeficiency virus type 1 activates plasmacytoid dendritic cells and concomitantly induces the bystander maturation of myeloid dendritic cells. J. Virol. 78**:**5223-5232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geijtenbeek, T. B., D. S. Kwon, R. Torensma, S. J. van Vliet, G. C. van Duijnhoven, J. Middel, I. L. Cornelissen, H. S. Nottet, V. N. KewalRamani, D. R. Littman, C. G. Figdor, and Y. van Kooyk. 2000. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell 100**:**587-597. [DOI] [PubMed] [Google Scholar]
- 19.Grassi, F., A. Hosmalin, D. McIlroy, V. Calvez, P. Debre, and B. Autran. 1999. Depletion in blood CD11c-positive dendritic cells from HIV-infected patients. AIDS 13**:**759-766. [DOI] [PubMed] [Google Scholar]
- 20.Izaguirre, A., B. J. Barnes, S. Amrute, W. S. Yeow, N. Megjugorac, J. Dai, D. Feng, E. Chung, P. M. Pitha, and P. Fitzgerald-Bocarsly. 2003. Comparative analysis of IRF and IFN-alpha expression in human plasmacytoid and monocyte-derived dendritic cells. J. Leukoc. Biol. 74**:**1125-1138. [DOI] [PubMed] [Google Scholar]
- 21.Jahnsen, F. L., F. Lund-Johansen, J. F. Dunne, L. Farkas, R. Haye, and P. Brandtzaeg. 2000. Experimentally induced recruitment of plasmacytoid (CD123high) dendritic cells in human nasal allergy. J. Immunol. 165**:**4062-4068. [DOI] [PubMed] [Google Scholar]
- 22.Jahnsen, F. L., E. D. Moloney, T. Hogan, J. W. Upham, C. M. Burke, and P. G. Holt. 2001. Rapid dendritic cell recruitment to the bronchial mucosa of patients with atopic asthma in response to local allergen challenge. Thorax 56**:**823-826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jefford, M., M. Schnurr, T. Toy, K. A. Masterman, A. Shin, T. Beecroft, T. Y. Tai, K. Shortman, M. Shackleton, I. D. Davis, P. Parente, T. Luft, W. Chen, J. Cebon, and E. Maraskovsky. 2003. Functional comparison of DCs generated in vivo with Flt3 ligand or in vitro from blood monocytes: differential regulation of function by specific classes of physiologic stimuli. Blood 102**:**1753-1763. [DOI] [PubMed] [Google Scholar]
- 24.Kawamura, T., S. E. Bruce, A. Abraha, M. Sugaya, O. Hartley, R. E. Offord, E. J. Arts, P. A. Zimmerman, and A. Blauvelt. 2004. PSC-RANTES blocks R5 human immunodeficiency virus infection of Langerhans cells isolated from individuals with a variety of CCR5 diplotypes. J. Virol. 78**:**7602-7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kawamura, T., H. Gatanaga, D. L. Borris, M. Connors, H. Mitsuya, and A. Blauvelt. 2003. Decreased stimulation of CD4+ T cell proliferation and IL-2 production by highly enriched populations of HIV-infected dendritic cells. J. Immunol. 170**:**4260-4266. [DOI] [PubMed] [Google Scholar]
- 26.Kawamura, T., F. O. Gulden, M. Sugaya, D. T. McNamara, D. L. Borris, M. M. Lederman, J. M. Orenstein, P. A. Zimmerman, and A. Blauvelt. 2003. R5 HIV productively infects Langerhans cells, and infection levels are regulated by compound CCR5 polymorphisms. Proc. Natl. Acad. Sci. USA 100**:**8401-8406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kwon, D. S., G. Gregorio, N. Bitton, W. A. Hendrickson, and D. R. Littman. 2002. DC-SIGN-mediated internalization of HIV is required for trans-enhancement of T cell infection. Immunity 16**:**135-144. [DOI] [PubMed] [Google Scholar]
- 28.Lore, K., M. R. Betts, J. M. Brenchley, J. Kuruppu, S. Khojasteh, S. Perfetto, M. Roederer, R. A. Seder, and R. A. Koup. 2003. Toll-like receptor ligands modulate dendritic cells to augment cytomegalovirus- and HIV-1-specific T cell responses. J. Immunol. 171**:**4320-4328. [DOI] [PubMed] [Google Scholar]
- 29.Lore, K., A. Sonnerborg, C. Brostrom, L. E. Goh, L. Perrin, H. McDade, H. J. Stellbrink, B. Gazzard, R. Weber, L. A. Napolitano, Y. van Kooyk, and J. Andersson. 2002. Accumulation of DC-SIGN+CD40+ dendritic cells with reduced CD80 and CD86 expression in lymphoid tissue during acute HIV-1 infection. AIDS 16**:**683-692. [DOI] [PubMed] [Google Scholar]
- 30.Lore, K., A. Sonnerborg, J. Olsson, B. K. Patterson, T. E. Fehniger, L. Perbeck, and J. Andersson. 1999. HIV-1 exposed dendritic cells show increased pro-inflammatory cytokine production but reduced IL-1ra following lipopolysaccharide stimulation. AIDS 13**:**2013-2021. [DOI] [PubMed] [Google Scholar]
- 31.Lore, K., A. L. Spetz, T. E. Fehniger, A. Sonnerborg, A. L. Landay, and J. Andersson. 2001. Quantitative single cell methods that identify cytokine and chemokine expression in dendritic cells. J. Immunol. Methods 249**:**207-222. [DOI] [PubMed] [Google Scholar]
- 32.Luft, T., M. Jefford, P. Luetjens, T. Toy, H. Hochrein, K. A. Masterman, C. Maliszewski, K. Shortman, J. Cebon, and E. Maraskovsky. 2002. Functionally distinct dendritic cell (DC) populations induced by physiologic stimuli: prostaglandin E(2) regulates the migratory capacity of specific DC subsets. Blood 100**:**1362-1372. [DOI] [PubMed] [Google Scholar]
- 33.Lund, J., A. Sato, S. Akira, R. Medzhitov, and A. Iwasaki. 2003. Toll-like receptor 9-mediated recognition of Herpes simplex virus-2 by plasmacytoid dendritic cells. J. Exp. Med. 198**:**513-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mascola, J. R., M. K. Louder, S. R. Surman, T. C. Vancott, X. F. Yu, J. Bradac, K. R. Porter, K. E. Nelson, M. Girard, J. G. McNeil, F. E. McCutchan, D. L. Birx, and D. S. Burke. 1996. Human immunodeficiency virus type 1 neutralizing antibody serotyping using serum pools and an infectivity reduction assay. AIDS Res. Hum. Retrovir. 12**:**1319-1328. [DOI] [PubMed] [Google Scholar]
- 35.Otero, M., G. Nunnari, D. Leto, J. Sullivan, F. X. Wang, I. Frank, Y. Xu, C. Patel, G. Dornadula, J. Kulkosky, and R. J. Pomerantz. 2003. Peripheral blood dendritic cells are not a major reservoir for HIV type 1 in infected individuals on virally suppressive HAART. AIDS Res. Hum. Retrovir. 19**:**1097-1103. [DOI] [PubMed] [Google Scholar]
- 36.Pacanowski, J., S. Kahi, M. Baillet, P. Lebon, C. Deveau, C. Goujard, L. Meyer, E. Oksenhendler, M. Sinet, and A. Hosmalin. 2001. Reduced blood CD123+ (lymphoid) and CD11c+ (myeloid) dendritic cell numbers in primary HIV-1 infection. Blood 98**:**3016-3021. [DOI] [PubMed] [Google Scholar]
- 37.Patterson, S., A. Rae, N. Hockey, J. Gilmour, and F. Gotch. 2001. Plasmacytoid dendritic cells are highly susceptible to human immunodeficiency virus type 1 infection and release infectious virus. J. Virol. 75**:**6710-6713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Patterson, S., S. P. Robinson, N. R. English, and S. C. Knight. 1999. Subpopulations of peripheral blood dendritic cells show differential susceptibility to infection with a lymphotropic strain of HIV-1. Immunol. Lett. 66**:**111-116. [DOI] [PubMed] [Google Scholar]
- 39.Schnurr, M., T. Toy, P. Stoitzner, P. Cameron, A. Shin, T. Beecroft, I. D. Davis, J. Cebon, and E. Maraskovsky. 2003. ATP gradients inhibit the migratory capacity of specific human dendritic cell types: implications for P2Y11 receptor signaling. Blood 102**:**613-620. [DOI] [PubMed] [Google Scholar]
- 40.Smed-Sorensen, A., K. Lore, L. Walther-Jallow, J. Andersson, and A. L. Spetz. 2004. HIV-1-infected dendritic cells up-regulate cell surface markers but fail to produce IL-12 p70 in response to CD40 ligand stimulation. Blood 104**:**2810-2817. [DOI] [PubMed] [Google Scholar]
- 41.Sugaya, M., K. Lore, R. A. Koup, D. C. Douek, and A. Blauvelt. 2004. HIV-infected Langerhans cells preferentially transmit virus to proliferating autologous CD4+ memory T cells located within Langerhans cell-T cell clusters. J. Immunol. 172**:**2219-2224. [DOI] [PubMed] [Google Scholar]
- 42.Turville, S. G., J. J. Santos, I. Frank, P. U. Cameron, J. Wilkinson, M. Miranda-Saksena, J. Dable, H. Stossel, N. Romani, M. Piatak, Jr., J. D. Lifson, M. Pope, and A. L. Cunningham. 2004. Immunodeficiency virus uptake, turnover, and 2-phase transfer in human dendritic cells. Blood 103**:**2170-2179. [DOI] [PubMed] [Google Scholar]
- 43.Yonezawa, A., R. Morita, A. Takaori-Kondo, N. Kadowaki, T. Kitawaki, T. Hori, and T. Uchiyama. 2003. Natural alpha interferon-producing cells respond to human immunodeficiency virus type 1 with alpha interferon production and maturation into dendritic cells. J. Virol. 77**:**3777-3784. [DOI] [PMC free article] [PubMed] [Google Scholar]