Mutations in AXIN2 Cause Familial Tooth Agenesis and Predispose to Colorectal Cancer (original) (raw)
Abstract
Wnt signaling regulates embryonic pattern formation and morphogenesis of most organs. Aberrations of regulation of Wnt signaling may lead to cancer. Here, we have used positional cloning to identify the causative mutation in a Finnish family in which severe permanent tooth agenesis (oligodontia) and colorectal neoplasia segregate with dominant inheritance. Eleven members of the family lacked at least eight permanent teeth, two of whom developed only three permanent teeth. Colorectal cancer or precancerous lesions of variable types were found in eight of the patients with oligodontia. We show that oligodontia and predisposition to cancer are caused by a nonsense mutation, Arg656Stop, in the Wnt-signaling regulator AXIN2. In addition, we identified a de novo frameshift mutation 1994-1995insG in AXIN2 in an unrelated young patient with severe tooth agenesis. Both mutations are expected to activate Wnt signaling. The results provide the first evidence of the importance of Wnt signaling for the development of dentition in humans and suggest that an intricate control of Wnt-signal activity is necessary for normal tooth development, since both inhibition and stimulation of Wnt signaling may lead to tooth agenesis. Our findings introduce a new gene for hereditary colorectal cancer and suggest that tooth agenesis may be an indicator of cancer susceptibility.
Morphogenesis and tissue regeneration are based on tight control of cell homeostasis. Wnt signaling has been implicated in regulation of diverse developmental events, as well as in aberrations of cell homeostasis that may lead to cancer (Huelsken and Birchmeier 2001; Alonso and Fuchs 2003; Lustig and Behrens 2003; Giles et al. 2003). Central to the mediation of Wnt signals is the regulation of β-catenin level and localization. When cells receive a Wnt signal, β-catenin is stabilized and binds transcription factors of the TCF family regulating expression of Wnt target genes. In the absence of Wnt signal, β-catenin is subjected to phosphorylation and subsequent degradation by action of a multiprotein complex (Seidensticker and Behrens 2000). The complex is organized by APC (product of the adenomatous polyposis coli gene) and Axin1 or its homolog Axin2, which serve as scaffolding components for the complex. Axin1 and Axin2 (also called “conductin” or “axil”) share a similar domain structure with binding sites for β-catenin, GSK3β, APC, and disheveled (Behrens et al. 1998; Yamamoto et al. 1998; Mai et al. 1999; Seidensticker and Behrens 2000). Axin1 is expressed uniformly during development, whereas Axin2 is expressed in a tissue- and stage-specific manner; for example, in somites, branchial arches, and limb buds (Jho et al. 2002; Aulehla et al. 2003). Axin2 is induced by Wnt signaling, suggesting that Axin2 expression serves as a negative-feedback regulator of Wnt signaling (Jho et al. 2002; Leung et al. 2002).
Mutations that facilitate the escape of β-catenin from the action of the degradation complex may lead to cancer due to increased transcription of Wnt target genes. Somatic mutations in β-catenin and molecules of the degradation complex have been found in cancer tissues, including skin, gastrointestinal, ovarian, and hepatocellular tumors (Huelsken and Birchmeier 2001; Giles et al. 2003; Lustig and Behrens 2003). Germline loss-of-function mutations in APC cause familial adenomatous polyposis (FAP [MIM 175100]), comprising ∼10% of hereditary colorectal cancer (Groden et al. 1991; Kinzler et al. 1991). It is interesting that familial colorectal polyposis is often accompanied by extracolonic neoplasia, especially cysts, osteomas, and odontomas (Gardner 1962).
Agenesis of one or more permanent teeth (hypodontia [MIM 106600]) is the most common congenital malformation in humans. More than 20% of the population lacks one or more third molars (wisdom teeth) congenitally, and >5% lack other permanent teeth, most commonly some of the second premolars or upper lateral incisors (Vastardis 2000; Arte 2001). Genetic factors causing these common types of hypodontia have remained unknown. The genetic background for more-severe tooth agenesis, oligodontia (MIM 604625), is better understood. Oligodontia, defined as congenital lack of six or more permanent teeth, third molars excluded, is relatively rare and is most often associated with some multiorgan syndrome. It has become obvious that both hypodontia and oligodontia are genetically very heterogeneous. It is plausible that mutations or common variants in genes in which loss-of-function mutations cause severe tooth agenesis may significantly contribute also to the background of the common hypodontia. For nonsyndromic oligodontia, dominant mutations in transcription factors MSX1 and PAX9 have been described (Vastardis et al. 1996; Stockton et al. 2000; Nieminen et al. 2001; Lammi et al. 2003). In homozygous _Msx1_- and _Pax9_-null mutant mice, tooth development is arrested at an early stage, whereas heterozygotes have normal dentition (Satokata and Maas 1994; Peters et al. 1998). There is experimental evidence indicating that Msx1 and Pax9 are required for the mediation of BMP and FGF signaling, respectively. Experiments in mice have also shown that Shh and Wnt signals are necessary for normal tooth development. It is thought that integrated networks of signaling pathways are the key regulators of tooth morphogenesis (Jernvall and Thesleff 2000; Thesleff 2003).
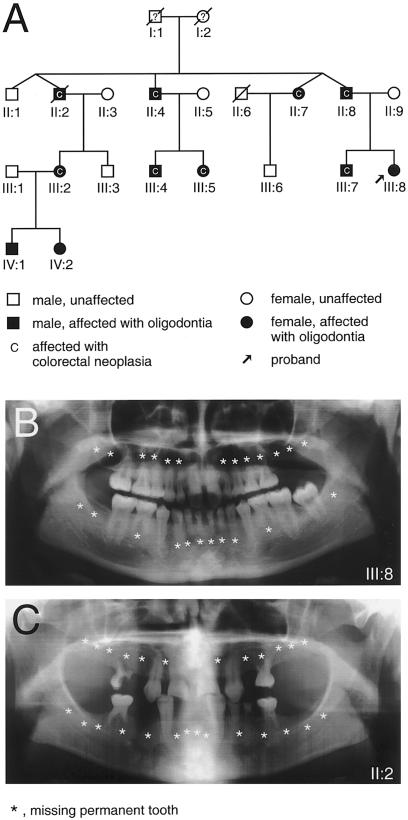
Here, we describe a Finnish four-generation family in which oligodontia segregated as an autosomal dominant trait (fig. 1_A_). The proband and her father were orthodontic patients at the Institute of Dentistry, University of Helsinki. The diagnosis of oligodontia was based on clinical examination, panoramic radiographs, and interviews. The study was approved by the Ethics Committee of the Institute of Dentistry, and informed consent was obtained from all participating individuals.
Figure 1.
Pedigree of the four-generation family and oligodontia phenotypes. A, Pedigree showing autosomal dominant inheritance of oligodontia and colorectal neoplasia. B and C, Panoramic radiographs of the proband (III:8) at age 15 years (B) and her uncle (II:2) at age 34 years (C). Stars indicate congenitally missing permanent teeth. Note that both family members have many persisting deciduous teeth. A persistent deciduous tooth is an often-encountered finding when the corresponding permanent tooth has not developed.
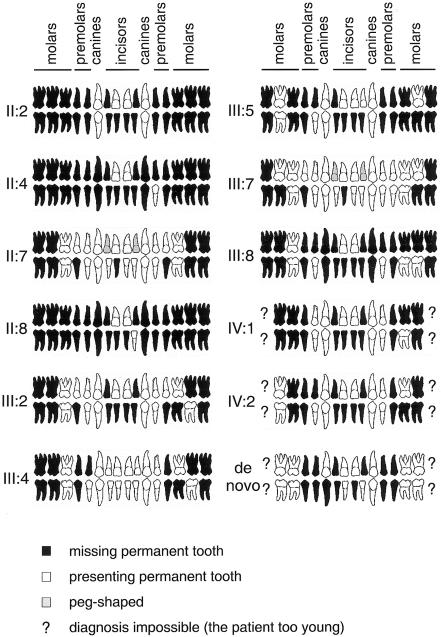
Altogether, 11 family members lacked at least eight permanent teeth, and, in 2 of them, only three permanent teeth had developed (figs. 1_B_, 1_C_, and 2). The oligodontia phenotype appeared completely penetrant. The majority of the affected family members lacked most permanent molars, premolars, lower incisors, and upper lateral incisors. Three also lacked all canines. Most often present were upper central incisors (always present), canines, first premolars, and first molars. One affected individual (IV:1) also lacked four deciduous incisors. Other individuals reported no defects in primary dentition, but dental records were not always available. The considerable variation in the number and type of missing teeth is typical for familial oligodontia (Vastardis et al. 1996; Stockton et al. 2000; Lammi et al. 2003; Nieminen et al. 2003). However, the tooth phenotypes in this family were more severe than and different from oligodontia in families with mutations reported elsewhere. MSX1 mutations are associated especially with agenesis of the second premolars and third molars and PAX9 mutations with molar tooth agenesis (Vastardis et al. 1996; Stockton et al. 2000; Nieminen et al. 2001, 2003; Lammi et al. 2003). The phenotype also differed from hypohidrotic ectodermal dysplasia (HED [MIM 305100]), in which both deciduous and permanent teeth are severely affected and the teeth that develop are often conical in shape. Furthermore, the other ectodermal symptoms in nails, hair, or skin typical for HED were not found in our patients.
Figure 2.
Agenesis of permanent teeth caused by AXIN2 mutations. The number of missing permanent teeth ranged from 8 to 29. Agenesis of deciduous teeth was found in only one individual (IV:1) (not shown).
The patient records initially revealed two patients with oligodontia from the oldest generation (individuals II:2 and II:7) who had a history of colorectal neoplasia (fig. 1_A_; table 1). During this study, 10 other family members (7 subjects with oligodontia and 3 healthy individuals) were studied through use of colonoscopy or sigmoidoscopy. Colorectal neoplasia was found in six family members with oligodontia (individuals II:4, II:8, III:2, III:4, III:5, and III:7). No signs of neoplasia were found in family members with normal dentition. In the family, risk for colorectal neoplasia thus appeared to be associated with oligodontia. The colorectal findings were variable, since polyposis was found in a few patients, whereas it was not otherwise evident, even in the patients with severe neoplasia (table 1).
Table 1.
Colorectal Findings in Family Members
| Patient | Agea(years) | Colorectal Findings |
|---|---|---|
| II:1b | 60 | No abnormal findings in colonoscopy |
| II:2 | 54 | A widely metastasized adenocarcinoma originating from the hepatic flexure of the colon |
| II:4 | 62 | Two hyperplastic polyps and two tubular adenomas with mild dysplasia (cecum and ascending colon) |
| II:7 | 57 | 68 adenomatous polyps of different sizes in colon, with severe dysplastic changes |
| II:8 | 58 | A tubular adenoma in the rectum and the hepatic flexure of colon showing mild dysplasia |
| III:2 | 35 | A 5–6-cm-wide sessile polypous tumor of the ascending colon, histopathology showing a tubulovillous adenoma with severe dysplasia but no invasive carcinoma |
| III:3b | 29 | No abnormal findings in colonoscopy |
| III:4 | 31 | 2 6-mm polyps removed at colonoscopy (cecum and upper rectum); histology showed hyperplastic polyp mixed with tubular adenoma, mild dysplasia |
| III:5 | 27 | A sessile hyperplastic polyp of 10 mm in diameter removed at colonoscopy from the ascending colon |
| III:6b | 36 | No abnormal findings in sigmoidoscopy |
| III:7 | 35 | 10–20 hyperplastic polyps in the ascending colon; a large sessile hyperplastic polyp in cecum; 2 small adenomas in the sigmoid colon |
| III:8 | 26 | No abnormal findings in colonoscopy |
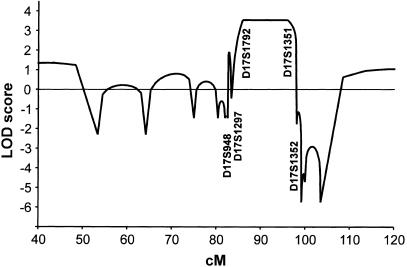
Seventeen members of the family were available for molecular analysis. DNA was isolated from venous blood samples with the Puregene DNA purification system (Gentra). Sequencing of the coding regions of the oligodontia candidate genes PAX9 (Nieminen et al. 2001) and MSX1 of the proband and his father did not reveal mutations. For a genomewide search with linkage analysis, tooth agenesis was modeled as an autosomal dominant trait with 95% penetrance and a gene frequency of 0.0001. Linkage analysis excluded loci for PAX9, MSX1, LEF1, and PITX2. Analysis of chromosomes of the patients with colorectal neoplasia excluded also the APC locus. In pairwise analysis with Mlink (Lathrop et al. 1984), highest pairwise LOD scores were obtained in chromosome 17 for two neighboring markers, 3.07 for D17S944 and 3.23 for D17S949. No recombinants were found for those markers and the disease. Genotyping more markers defined a maximal recombination-free region of ∼15 cM between markers D17S948 and D17S1352, according to Généthon and Marshfield genetic maps (University of California–Santa Cruz Web site). Multipoint analysis with Simwalk (Sobel and Lange 1996) gave a maximum LOD score of 3.55 between markers D17S1792 and D17S1351 (fig. 3), providing evidence for linkage of oligodontia to a locus in that region.
Figure 3.
Multipoint linkage analysis of oligodontia and genetic markers on chromosome 17. LOD scores along chromosome 17 are presented as a function of the genetic distance. The positions of the most important markers used in the analysis are indicated. A genomewide search was performed with Linkage Mapping Set MD10 (ABI), with minor modifications (374 STRP markers), at the Finnish Genome Center. Alleles of markers were amplified using standard PCR methods, Ampli_Taq_ Gold (ABI), and GeneAmp 9700 PCR system (ABI) and were analyzed in a MegaBace 1000 sequencer (Amersham). Allele-calling was made in Genetic Profiler 1.5 (Amersham). Alleles of additional STRP markers were amplified using Dynazyme II DNA Polymerase (Finnzymes) and DNA Engine PTC 200 thermal cycler (MJR) and were analyzed in an ABI 377 fluorescent sequencer. Allele-calling was made with ABI Genotyper software.
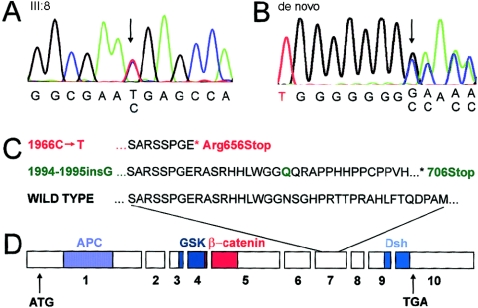
The region includes ⩾80 known or predicted genes. None of these had been previously implicated in tooth agenesis in humans or mice. AXIN2 (MIM 604425) was selected as a candidate gene because it is involved in Wnt signaling and because somatic mutations of AXIN2 have been found in colorectal tumors with defective mismatch repair (Behrens et al. 1998; Liu et al. 2000). Direct sequencing of _AXIN2_-coding regions and flanking intronic sequences (fig. 4; table A [online only]; Dental Genetics Group Web site) revealed a 1966C→T transition in exon 7, leading to a change of arginine 656 to a stop codon and premature termination of translation (fig. 4_A_ and 4_C_). Further sequencing revealed the presence of the same nonsense mutation in all family members who had oligodontia but not in the healthy family members or in >100 unrelated control individuals.
Figure 4.
Sequence analysis of AXIN2. Genomic DNA was amplified with Dynazyme Ext (Finnzymes) and primers for exons 1–10 of AXIN2 (table A [online only]; GenBank). PCR products were purified with ExoSAP-IT (USB) and were sequenced with ABI BigDye terminator reagents, version 3.1 (Applied Biosystems). The reactions were analyzed in an ABI 3730 DNA sequencer in Molecular Medicine Sequencing Laboratory, Biomedicum Helsinki. A, Chromatogram from sequencing of exon 7 of the proband (III:8) of the three-generation family. All affected family members had the same heterozygous 1966C→T mutation, indicated by the arrow. The mutation was not present in the unaffected family members. B, Chromatogram from sequencing of exon 7 of the sporadic case of oligodontia (de novo mutation). Arrow indicates the position of a heterozygous insertion of a G after nucleotide 1994. C, Partial amino acid sequence from exon 7 of human AXIN2 and consequences of two mutations. Red indicates the mutation in the family affected with oligodontia. Green indicates the de novo frameshift mutation. The Green “Q” indicates the position of the insertion. The asterisk (*) denotes the stop codon. D, Genomic and protein structure of AXIN2. Boxes represent exons; the predicted interaction domains of AXIN2 protein are depicted with colors. APC = adenomatous polyposis coli; GSK = glycogen synthase kinase 3β; Dsh = disheveled homology domain. ATG denotes the beginning of the coding region; TGA denotes the stop codon.
Table A.
PCR Primers and Conditions for Sequencing of AXIN2
| Target | Size of Product(bp) | Forward Primer | Reverse Primer | PCR Conditionsa |
|---|---|---|---|---|
| Exon 1 5′ | 727 | TGGGTTTTTGGAAGGTTGTG | GAACAGGTAAGCACCGTCTTG | Hot +59 ×32 |
| Exon 1 3′ | 684 | CATCTCCGGATTCCCCTCT | TCCACCCATCCACCATACTT | Hot +59 ×32 |
| Exon 2 | 465 | GCTGCCTCTGGAATACTCTCTG | TAAGTGCTCAGGTGGCATCC | Hot +59 ×32 |
| Exon 3 | 327 | AGCACCGATGGTATCTGGAG | CCACCACCCATTTCTTTTCTT | Hot +59 ×32 |
| Exon 4 | 549 | GATGGTTGACAACAGTCTTTGAAG | CTAACGCACCCCATGCAC | Hot +62 ×32 |
| Exon 5 | 673 | CTTCTGCTTCCTGGGTCACT | CTGCCGCCCTCTTAGAAACT | Hot +59 ×32 |
| Exon 6 | 330 | AGGAGTCCCGGAGATTTAACC | AACAGCCATTCCCACAATACC | Hot +59 ×32 |
| Exon 7 | 398 | TTCCAGTTCTTCTAACCCAGTTTC | TTGAGACCCAGGCAGAAAGAG | Hot +59 ×32 |
| Exon 8 | 320 | AATTGCTCTGGGGACAACAG | GGACATGGATGGCAACATCT | Hot +59 ×32 |
| Exon 9 | 300 | GCACGTGTGTGTTTGCTTTAG | TCTGGCTCTTGGTTCTGAGC | Hot +59 ×32 |
| Exon 10 | 684 | TCAACAATGTGGAAAATGCAG | AGAAACCATGAACGCACTCC | Hot +59 ×32 |
While screening the AXIN2 gene for mutations in other patients with oligodontia, we identified a heterozygous 1-bp insertion (fig. 4_B_), after nucleotide 1994 in exon 7, in a 13-year-old boy with a very similar tooth phenotype as that in the family described above (fig. 2). The mutation was not found in his healthy parents, indicating that it is a de novo germline mutation in one of the parents. The mutation expands one of the several mononucleotide repeats in exon 7 of AXIN2 and recodes the amino acids starting at Asn666, incorporating a stop codon 40 codons later. This mutation was identical to one of the somatic frameshift mutations described for colorectal cancer tissue (Liu et al. 2000). The finding of this de novo mutation suggests the vulnerability of the mononucleotide repeats in exon 7 of AXIN2 for frameshift mutations also in absence of defective mismatch repair (Liu et al. 2000). The young age of the patient prevents the confirmation of cancer predisposition.
Both mutations introduce a premature stop codon to exon 7 of the AXIN2 gene. According to current knowledge, in-frame stop codons introduced by mutations may lead to degradation of mRNA via the nonsense-mediated mRNA decay mechanism (Wilusz et al. 2001; Cartegni et al. 2002). The mRNAs transcribed from the mutated copies of AXIN2 fulfill the requirement that the premature stop codon be located ⩾50 bp upstream of the last exon-exon junction, suggesting that the mutated AXIN2 mRNAs are guided to the degradation pathway. A protein product translated from the mutated mRNA would be truncated and lacking the C-terminal domain (fig. 4_C_ and 4_D_), which, in Axin1, mediates oligomerization and interactions with protein phosphatase 2A, disheveled, and LRP5/6 (Seidensticker and Behrens 2000; Mao et al. 2001). Since the truncations would cause the predicted oligomerization domain to be lost and, in the case of the frameshift mutation, the length of recoded amino acids would be short, a dominant negative effect would be improbable. Stop-codon–creating exon 7 frameshift mutants of AXIN2 are associated with accumulation of β-catenin in the nucleus of cancer cells and, in transfection experiments, activate a TCF-promoted reporter gene (Liu et al. 2000). C-terminal deletions in mouse Axin1 have been shown to abolish its ability to inhibit Wnt reporter-gene activity (Hsu et al. 1999). These data indicate that Arg656Stop and 1994-1995insG lead to decreased AXIN2 function and most probably represent loss-of-function mutations that cause activation of Wnt signaling.
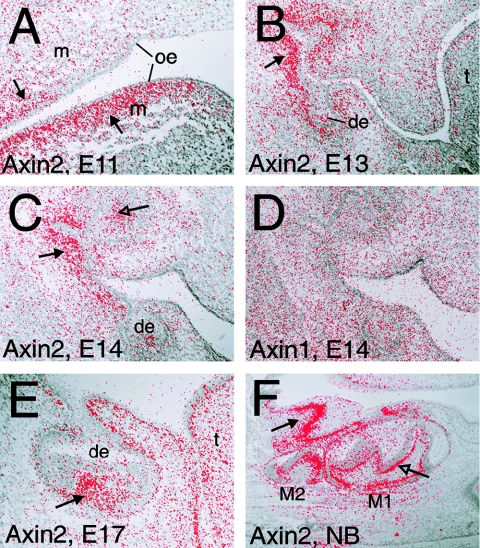
It has been shown that, in mice, Axin2 exhibits tissue- and stage-specific expression patterns during development (Jho et al. 2002; Aulehla et al. 2003). It is expressed in the primitive streak and later in the dorsal neural tube and developing somites, as well as in the branchial arches and the limb buds of mouse embryos (Jho et al. 2002). In the presomitic mesoderm, it is expressed in an oscillating pattern, and an orchestrating role for Axin2 and Wnt3a was suggested in the establishment of the segmental pattern during somitogenesis (Aulehla et al. 2003). In the developing gut, Axin2 expression was found in the intervillus epithelium (Jho et al. 2002). We studied the expression of Axin1 and Axin2 during mouse tooth development by in situ hybridization. Axin1 was expressed uniformly in the developing jaws (fig. 5_D_), whereas Axin2 expression was most intense in the mesenchyme underlying the oral and dental epithelium (fig. 5_A_–5_C_, 5_E_, and 5_F_). At more advanced stages of tooth morphogenesis, the expression was intense in the odontoblasts producing dentine (fig. 5_F_). Axin2 expression was also seen in the enamel knot (fig. 5_C_), an epithelial signaling center that regulates tooth morphogenesis (Jernvall and Thesleff 2000).
Figure 5.
In situ hybridization analysis of Axin2 and Axin1 expression during mouse tooth development. Serial paraffin sections were cut through paraformaldehyde-fixed heads of embryonic and newborn mice. The in situ hybridization with 35S-UTP (Amersham)–labeled riboprobes spanning 1,234 bp of Axin1 and 1,178 bp of Axin2 sequence from exons 1–4 (GenBank) was performed as described elsewhere (Wilkinson and Green 1990). In situ grains in dark-field images of the slides were painted red and superimposed on corresponding bright field images. A_–_E, Frontal sections. F, Sagittal section. A_–_C, E, and F, Axin2. D, Axin1. A, In an E11 embryo, tooth development is initiated. In upper and lower jaws, Axin2 is expressed in the mesenchyme underlying the oral epithelium (arrow). B, At E13, teeth have reached the bud stage. Expression of Axin2 continues in the mesenchyme and is particularly intense adjacent to the tooth buds (arrow). C, In an E14 embryo, teeth are at the cap stage. In addition to the mesenchyme (arrow), expression is seen in the enamel knot (open arrow). D, At E14, Axin1 is expressed weakly and uniformly throughout dental and oral tissues. Similar Axin1 expression was seen at all observed developmental stages (data not shown). E, At E17, tooth development is at the bell stage. Axin2 is expressed in the dental papilla mesenchyme (arrow), as well as in the mesenchymal cells directly underlying all epithelia. F, In a newborn mouse, cell differentiation has started in the molars. Axin2 expression is intense in the layer of mesenchymal odontoblasts and preodontoblasts (open arrow). Intense expression is also seen under the oral epithelium (arrow). oe = oral epithelium; de = dental epithelium; m = mesenchyme; t = tongue; M1 = first molar; M2 = second molar; E = embryonic day.
The involvement of Wnt-signaling genes in carcinogenesis is well established, but relatively little is known about the connection between them and congenital malformations in humans. In addition to the neoplastic manifestations caused by loss-of-function mutations of APC, mutations in two Wnt receptors, LRP5 and frizzled-4, are known to affect regulation of bone density or to disturb eye development (Gong et al. 2001; Robitaille et al. 2002; Van Wesenbeeck et al. 2003). Initially, most genes that participate in Wnt signaling and especially their developmental roles have been identified on the basis of Drosophila and mouse mutants. The understanding of functions of Axins is based on mouse and zebrafish mutants, which show that Axin1 function is necessary for embryonic axis formation and for normal development of neural tube and heart (Zeng et al. 1997; Heisenberg et al. 2001; van de Water et al. 2001). However, the functions of Axin2 remain to be demonstrated. It is interesting that Axin2 expression is induced by Wnt signaling, suggesting a suppressing role for Axin2 through a negative-feedback loop (Jho et al. 2002; Leung et al. 2002). As a consequence of the AXIN2 mutations described in this study, the feedback regulation would be attenuated.
In this study, we present the first evidence for the functions of AXIN2. The Arg656Stop mutation was present in all 11 patients with oligodontia from a multigeneration family, whereas it was not present in the healthy individuals, thus showing complete segregation with the oligodontia phenotype. The mutation was not found in >200 control chromosomes. Another mutation in AXIN2 was found in an unrelated patient with oligodontia. Furthermore, in situ hybridization showed that, in mice, Axin2 is expressed in developing dental tissues. In conclusion, the mutations of AXIN2 are responsible for the severe oligodontia of the patients, showing that AXIN2 function is essential for the development of dentition in humans. Since the deciduous dentition was unaffected in most patients, it appears that AXIN2 function is especially critical for the development of permanent teeth. Considering the importance of Wnt signaling for the development of most organs, it is surprising that congenital malformations caused by mutations in AXIN2 were found only in the dentition.
In mutant mice, tooth development is inhibited when the Wnt pathway is blocked by inactivating Lef1 function or by overexpressing the Wnt inhibitor Dkk (van Genderen et al. 1994; Andl et al. 2002). On the other hand, occurrence of odontomas and supernumerary teeth has been reported in association with FAP (in this case, called “Gardner syndrome”) (Fitzgerald 1943; Gardner 1962; Ida et al. 1981; Wolf et al. 1986). Even though there is no data available as to whether supernumerary teeth occur in those families in which loss-of-function mutations in APC have been identified, it can be speculated that formation of supernumerary teeth in patients with FAP resulted from overactivation of Wnt signaling. Thus, it appears that disruption of Wnt signaling leads to failure of tooth development, whereas increased signaling leads to development of supernumerary teeth. However, our data indicate that overstimulation of the Wnt pathway may also lead to failure of tooth morphogenesis. Recent data based on a mathematical model for Wnt pathway and its experimental confirmation indicate that Axin and APC promote the formation of β-catenin degradation complexes in very different ways (Lee et al. 2003). The different outcomes of loss-of-function mutations in APC and AXIN2 may also be explained if AXIN2 is especially important as a negative-feedback regulator. It is interesting that Axin2 expression during mouse tooth development coincided with that of the TCF-family member Lef1, although Lef1 is much more intensely expressed in the epithelial signaling center enamel knot than Axin2 (Kratochwil et al. 1996). The function of Lef1 is to activate Wnt target genes, and, in the enamel knot, it is necessary for the bud-to-cap transition during tooth morphogenesis (Kratochwil et al. 1996). Our findings suggest that suppression of Wnt function is necessary during specific stages of tooth development in dental mesenchyme and/or in the enamel knot. This underlines the importance of modulating the intensity of signaling for morphogenetic responses. The central role of specific inhibitors of signal pathways in the regulation of embryonic development has become apparent during recent years. For instance, in embryonic axis formation, inhibition of Wnt and BMP signaling is essential, and differential Wnt activities determine the formation of head, trunk, and tail (Zeng et al. 1997; Kiecker and Niehrs 2001).
Our results also provide strong evidence that familial colorectal cancer can be caused by mutations in AXIN2. Colorectal neoplasia in the family described in this study was found only in association with oligodontia and the AXIN2 mutation and affected all those of the oldest generation who had the mutation. The causative role of the AXIN2 mutation is also corroborated by earlier findings of somatic mutations in AXIN2 in tumor tissues (Liu et al. 2000; Wu et al. 2001; Taniguchi et al. 2002). Identification of AXIN2 as a new gene that is responsible for hereditary cancer reduces the remarkable 40% proportion of hereditary colorectal cancers without known molecular causes. It appears that colorectal neoplasia caused by loss of function of AXIN2 has very high or full penetrance. It is interesting that there is a large intrafamilial variation in type and number of colorectal neoplasias and polyps caused by the AXIN2 mutation. The phenotype appears to be distinct from FAP, resembling the attenuated form of FAP (AFAP) described elsewhere (Knudsen et al. 2003).
The AXIN2 Arg656Stop mutation is one of the rare cases in which a mutation simultaneously predisposes to cancer and causes a hypoplastic malformation. Our finding reflects the development of teeth as epithelial appendages regulated by molecular mechanisms similar to those that are involved in colorectal carcinogenesis, thus highlighting commonalities between developmental pathways and tissue homeostasis. It is an intriguing possibility that tooth agenesis may be used as an indicator of susceptibility for colorectal cancer.
Acknowledgments
We are grateful for all the members of the families for their participation in the study. We are indebted to Professor Kaarina Haavikko for the initial diagnosis of oligodontia in several family members. We warmly thank Jonna Jalanka, Elina Järvinen, Elli Kempas, Marjatta Kivekäs, Merja Mäkinen, Carin Sahlberg, and Anneli Sinkkonen for their assistance and advice. We are grateful to Professor Leena Peltonen for education, encouragement, and the opportunity to use the facilities of the Department of Molecular Medicine, National Public Health Institute. The expertise of Pekka Ellonen, Heli Keränen, and Susanna Anjala in the Molecular Medicine Sequencing Laboratory, Biomedicum Helsinki, is gratefully acknowledged. We thank Professor Hannu Sariola for critical reading of the manuscript. This study was financially supported by the Finnish Dental Society, the Academy of Finland, and research grants TYH1335 and TYH2257 from Helsinki University Central Hospital (Erityisvaltionosuus).
Electronic-Database Information
Accession numbers and URLs for data presented herein are as follows:
- Dental Genetics Group, http://www.helsinki.fi/science/dentgen
- GenBank, http://www.ncbi.nih.gov/GenBank/ (for human genomic contig NT_010783, mouse Axin1 mRNA [accession number AF009011], and mouse Axin2 mRNA [accession number NM_015732])
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for FAP/Gardner syndrome, hypodontia, oligodontia, HED, and AXIN2)
- University of California–Santa Cruz Genome Bioinformatics, http://genome.ucsc.edu/ (for positions in Généthon and Marshfield genetic maps)
References
- Alonso L, Fuchs E (2003) Stem cells in the skin: waste not, Wnt not. Genes Dev 17:1189–1200 10.1101/gad.1086903 [DOI] [PubMed] [Google Scholar]
- Andl T, Reddy ST, Gaddapara T, Millar SE (2002) WNT signals are required for the initiation of hair follicle development. Dev Cell 2:643–653 10.1016/S1534-5807(02)00167-3 [DOI] [PubMed] [Google Scholar]
- Arte S (2001) Phenotypic and genotypic features of familial hypodontia. PhD thesis, Institute of Dentistry, University of Helsinki, Helsinki; http://ethesis.helsinki.fi/julkaisut/laa/hamma/vk/arte/ (accessed March 16, 2004)
- Aulehla A, Wehrle C, Brand-Saberi B, Kemler R, Gossler A, Kanzler B, Herrmann BG (2003) Wnt3a plays a major role in the segmentation clock controlling somitogenesis. Dev Cell 4:395–406 10.1016/S1534-5807(03)00055-8 [DOI] [PubMed] [Google Scholar]
- Behrens J, Jerchow BA, Wurtele M, Grimm J, Asbrand C, Wirtz R, Kuhl M, Wedlich D, Birchmeier W (1998) Functional interaction of an Axin homolog, conductin, with β-catenin, APC, and GSK3β. Science 280:596–599 10.1126/science.280.5363.596 [DOI] [PubMed] [Google Scholar]
- Cartegni L, Chew SL, Krainer AR (2002) Listening to silence and understanding nonsense: exonic mutations that affect splicing. Nat Rev Genet 3:285–298 10.1038/nrg775 [DOI] [PubMed] [Google Scholar]
- Fitzgerald GM (1943) Multiple composite odontomes coincidental with other tumorous conditions: report of a case. J Am Dent Assoc 30:1408–1417 [Google Scholar]
- Gardner EJ (1962) Follow-up study of a family group exhibiting dominant inheritance for a syndrome including intestinal polyps, osteomas, fibromas and epidermal cysts. Am J Hum Genet 14:376–390 [PMC free article] [PubMed] [Google Scholar]
- Giles RH, van Es JH, Clevers H (2003) Caught up in a Wnt storm: Wnt signaling in cancer. Biochim Biophys Acta 1653:1–24 [DOI] [PubMed] [Google Scholar]
- Gong Y, Slee RB, Fukai N, Rawadi G, Roman-Roman S, Reginato AM, Wang H, et al (2001) LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell 107:513–523 [DOI] [PubMed] [Google Scholar]
- Groden J, Thliveris A, Samowitz W, Carlson M, Gelbert L, Albertsen H, Joslyn G, Stevens J, Spirio L, Robertson M (1991) Identification and characterization of the familial adenomatous polyposis coli gene. Cell 66:589–600 [DOI] [PubMed] [Google Scholar]
- Heisenberg CP, Houart C, Take-Uchi M, Rauch GJ, Young N, Coutinho P, Masai I, Caneparo L, Concha ML, Geisler R, Dale TC, Wilson SW, Stemple DL (2001) A mutation in the Gsk3-binding domain of zebrafish Masterblind/Axin1 leads to a fate transformation of telencephalon and eyes to diencephalon. Genes Dev 15:1427–1434 10.1101/gad.194301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu W, Zeng L, Costantini F (1999) Identification of a domain of Axin that binds to the serine/threonine protein phosphatase 2A and a self-binding domain. J Biol Chem 274:3439–3445 10.1074/jbc.274.6.3439 [DOI] [PubMed] [Google Scholar]
- Huelsken J, Birchmeier W (2001) New aspects of Wnt signaling pathways in higher vertebrates. Curr Opin Genet Dev 11:547–553 10.1016/S0959-437X(00)00231-8 [DOI] [PubMed] [Google Scholar]
- Ida M, Nakamura T, Utsunomiya J (1981) Osteomatous changes and tooth abnormalities found in the jaw of patients with adenomatosis coli. Oral Surg Oral Med Oral Pathol 52:2–11 [DOI] [PubMed] [Google Scholar]
- Jernvall J, Thesleff I (2000) Reiterative signaling and patterning during mammalian tooth morphogenesis. Mech Dev 92:19–29 10.1016/S0925-4773(99)00322-6 [DOI] [PubMed] [Google Scholar]
- Jho EH, Zhang T, Domon C, Joo CK, Freund JN, Costantini F (2002) Wnt/β-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol Cell Biol 22:1172–1183 10.1128/MCB.22.4.1172-1183.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecker C, Niehrs C (2001) A morphogen gradient of Wnt/β-catenin signalling regulates anteroposterior neural patterning in Xenopus. Development 128:4189–4201 [DOI] [PubMed] [Google Scholar]
- Kinzler KW, Nilbert MC, Su LK, Vogelstein B, Bryan TM, Levy DB, Smith KJ, Preisinger AC, Hedge P, McKechnie D (1991) Identification of FAP locus genes from chromosome 5q21. Science 253:661–665 [DOI] [PubMed] [Google Scholar]
- Knudsen AL, Bisgaard ML, Bulow S (2003) Attenuated familial adenomatous polyposis (AFAP): a review of the literature. Fam Cancer 2:43–55 10.1023/A:1023286520725 [DOI] [PubMed] [Google Scholar]
- Kratochwil K, Dull M, Fariñas I, Galceran J, Grosschedl R (1996) Lef1 expression is activated by BMP-4 and regulates inductive tissue interactions in tooth and hair development. Genes Dev 10:1382–1394 [DOI] [PubMed] [Google Scholar]
- Lammi L, Halonen K, Pirinen S, Thesleff I, Arte S, Nieminen P (2003) A missense mutation in PAX9 in a family with distinct phenotype of oligodontia. Eur J Hum Genet 11:866–871 10.1038/sj.ejhg.5201060 [DOI] [PubMed] [Google Scholar]
- Lathrop GM, Lalouel JM, Julier C, Ott J (1984) Strategies for multilocus linkage analysis in humans. Proc Natl Acad Sci USA 81:3443–3446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E, Salic A, Kruger R, Heinrich R, Kirschner MW (2003) The roles of APC and Axin derived from experimental and theoretical analysis of the Wnt pathway. PLoS Biology 1:E10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung JY, Kolligs FT, Wu R, Zhai Y, Kuick R, Hanash S, Cho KR, Fearon ER (2002) Activation of AXIN2 expression by β-catenin-T cell factor: a feedback repressor pathway regulating Wnt signaling. J Biol Chem 277:21657–21665 10.1074/jbc.M200139200 [DOI] [PubMed] [Google Scholar]
- Liu W, Dong X, Mai M, Seelan RS, Taniguchi K, Krishnadath KK, Halling KC, Cunningham JM, Boardman LA, Qian C, Christensen E, Schmidt SS, Roche PC, Smith DI, Thibodeau SN (2000) Mutations in AXIN2 cause colorectal cancer with defective mismatch repair by activating β-catenin/TCF signalling. Nat Genet 26:146–147 10.1038/79859 [DOI] [PubMed] [Google Scholar]
- Lustig B, Behrens J (2003) The Wnt signaling pathway and its role in tumor development. J Cancer Res Clin Oncol 129:199–221 [DOI] [PubMed] [Google Scholar]
- Mai M, Qian C, Yokomizo A, Smith DI, Liu W (1999) Cloning of the human homolog of conductin (AXIN2), a gene mapping to chromosome 17q23–q24. Genomics 55:341–344 10.1006/geno.1998.5650 [DOI] [PubMed] [Google Scholar]
- Mao J, Wang J, Liu B, Pan W, Farr GH, Flynn C, Yuan H, Takada S, Kimelman D, Li L, Wu D (2001) Low-density lipoprotein receptor-related protein-5 binds to Axin and regulates the canonical Wnt signaling pathway. Mol Cell 7:801–809 10.1016/S1097-2765(01)00224-6 [DOI] [PubMed] [Google Scholar]
- Nieminen P, Arte S, Tanner D, Paulin L, Alaluusua S, Thesleff I, Pirinen S (2001) Identification of a nonsense mutation in the PAX9 gene in molar oligodontia. Eur J Hum Genet 9:743–746 10.1038/sj.ejhg.5200715 [DOI] [PubMed] [Google Scholar]
- Nieminen P, Kotilainen J, Aalto Y, Knuutila S, Pirinen S, Thesleff I (2003) MSX1 gene is deleted in Wolf-Hirschhorn syndrome patients with oligodontia. J Dent Res 82:1013–1017 [DOI] [PubMed] [Google Scholar]
- Peters H, Neubuser A, Kratochwil K, Balling R (1998) Pax9-deficient mice lack pharyngeal pouch derivatives and teeth and exhibit craniofacial and limb abnormalities. Genes Dev 12:2735–2747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robitaille J, MacDonald ML, Kaykas A, Sheldahl LC, Zeisler J, Dube MP, Zhang LH, Singaraja RR, Guernsey DL, Zheng B, Siebert LF, Hoskin-Mott A, Trese MT, Pimstone SN, Shastry BS, Moon RT, Hayden MR, Goldberg YP, Samuels ME (2002) Mutant frizzled-4 disrupts retinal angiogenesis in familial exudative vitreoretinopathy. Nat Genet 32:326–330 10.1038/ng957 [DOI] [PubMed] [Google Scholar]
- Satokata I, Maas R (1994) Msx1 deficient mice exhibit cleft palate and abnormalities of craniofacial and tooth development. Nat Genet 6:348–356 [DOI] [PubMed] [Google Scholar]
- Seidensticker MJ, Behrens J (2000) Biochemical interactions in the Wnt pathway. Biochim Biophys Acta 1495:168–182 10.1016/S0167-4889(99)00158-5 [DOI] [PubMed] [Google Scholar]
- Sobel E, Lange K (1996) Descent graphs in pedigree analysis: applications to haplotyping, location scores, and marker-sharing statistics. Am J Hum Genet 58:1323–1337 [PMC free article] [PubMed] [Google Scholar]
- Stockton DW, Das P, Goldenberg M, D’Souza RN, Patel PI (2000) Mutation of PAX9 is associated with oligodontia. Nat Genet 24:18–19 10.1038/71634 [DOI] [PubMed] [Google Scholar]
- Taniguchi K, Roberts LR, Aderca IN, Dong X, Qian C, Murphy LM, Nagorney DM, Burgart LJ, Roche PC, Smith DI, Ross JA, Liu W (2002) Mutational spectrum of β-catenin, AXIN1, and AXIN2 in hepatocellular carcinomas and hepatoblastomas. Oncogene 21:4863–4871 10.1038/sj.onc.1205591 [DOI] [PubMed] [Google Scholar]
- Thesleff I (2003) Epithelial-mesenchymal signalling regulating tooth morphogenesis. J Cell Sci 116:1647–1648 10.1242/jcs.00410 [DOI] [PubMed] [Google Scholar]
- van de Water S, van de Wetering M, Joore J, Esseling J, Bink R, Clevers H, Zivkovic D (2001) Ectopic Wnt signal determines the eyeless phenotype of zebrafish Masterblind mutant. Development 128:3877–3888 [DOI] [PubMed] [Google Scholar]
- van Genderen C, Okamura RM, Farinas I, Quo RG, Parslow TG, Bruhn L, Grosschedl R (1994) Development of several organs that require inductive epithelial-mesenchymal interactions is impaired in LEF-1- deficient mice. Genes Dev 8:2691–2703 [DOI] [PubMed] [Google Scholar]
- Van Wesenbeeck L, Cleiren E, Gram J, Beals RK, Bénichou O, Scopelliti D, Key L, Renton T, Bartels C, Gong Y, Warman ML, De Vernejoul M-C, Bollerslev J, Van Hul W (2003) Six novel missense mutations in the LDL receptor-related protein 5 (LRP5) gene in different conditions with an increased bone density. Am J Hum Genet 72:763–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vastardis H (2000) The genetics of human tooth agenesis: new discoveries for understanding dental anomalies. Am J Orthod Dentofacial Orthop 117:650–656 10.1067/mod.2000.103257 [DOI] [PubMed] [Google Scholar]
- Vastardis H, Karimbux N, Guthua SW, Seidman JG, Seidman CE (1996) A human MSX1 homeodomain missense mutation causes selective tooth agenesis. Nat Genet 13:417–421 [DOI] [PubMed] [Google Scholar]
- Wilkinson D, Green J (1990) In situ hybridization and the three-dimensional reconstruction of serial sections. In: Copp AJ, Cole DL (eds) Postimplantation mammalian embryos. Oxford University Press, London, pp 155–171 [Google Scholar]
- Wilusz CJ, Wang W, Peltz SW (2001) Curbing the nonsense: the activation and regulation of mRNA surveillance. Genes Dev 15:2781–2785 [DOI] [PubMed] [Google Scholar]
- Wolf J, Jarvinen HJ, Hietanen J (1986) Gardner’s dento-maxillary stigmas in patients with familial adenomatosis coli. Br J Oral Maxillofac Surg 24:410–416 [DOI] [PubMed] [Google Scholar]
- Wu R, Zhai Y, Fearon ER, Cho KR (2001) Diverse mechanisms of β-catenin deregulation in ovarian endometrioid adenocarcinomas. Cancer Res 61:8247–8255 [PubMed] [Google Scholar]
- Yamamoto H, Kishida S, Uochi T, Ikeda S, Koyama S, Asashima M, Kikuchi A (1998) Axil, a member of the Axin family, interacts with both glycogen synthase kinase 3β and β-catenin and inhibits axis formation of Xenopus embryos. Mol Cell Biol 18:2867–2875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng L, Fagotto F, Zhang T, Hsu W, Vasicek TJ, Perry WL, Lee JJ, Tilghman SM, Gumbiner BM, Costantini F (1997) The mouse fused locus encodes Axin, an inhibitor of the Wnt signaling pathway that regulates embryonic axis formation. Cell 90:181–192 [DOI] [PubMed] [Google Scholar]