Different contributions of thymopoiesis and homeostasis-driven proliferation to the reconstitution of naive and memory T cell compartments (original) (raw)
Abstract
Following transfer into lymphopenic hosts, naive CD8 T cells proliferate and acquire memory phenotype. Although the acquired phenotype is stable in recombination activating gene-1-deficient (RAG−/−) recipients, in sublethally irradiated mice naive CD8 T cells of donor origin gradually accumulate. The naive cells have been attributed to phenotypic reversion of homeostatic memory cells, implying instability of memory phenotype and restoration of the naive T cell compartment by homeostasis-driven proliferation. We show here that (i) the accumulation of naive CD8 T cells of donor origin only occurs in recipients that have been irradiated and have an intact thymus; (ii) the apparent reversion of memory to naive cells actually results from de novo T cell development of hematopoietic stem cells, present in the donor spleen or lymph node cell populations, in the thymus of irradiated recipients; and (iii) the number of homeostatic memory cells generated in both RAG−/− and irradiated hosts reaches a plateau value and their phenotype is stably maintained even after retransfer into nonirradiated normal mice for 30 days. These findings demonstrate that homeostatic memory T cells do not revert to naive cells. After severe T cell depletion homeostasis-driven proliferation restores only the memory T cell compartment, whereas thymopoiesis is required for the reconstitution of the naive T cell compartment.
The number of T cells in a normal individual is maintained at around a constant level (T cell homeostasis; refs. 1 and 2). When T cells are depleted by irradiation, chemotherapy, or infection, the residual T cells can undergo proliferation (3, 4). Similarly, when a small number of naive T cells are adoptively transferred into syngeneic lymphopenic hosts, such as mice deficient in recombination activating gene-1 (RAG−/−) or sublethally irradiated normal C57BL/6 (B6) mice, the transferred T cells can also undergo proliferation (5–9). T cell proliferation in lymphopenic hosts in the absence of exogenous antigen stimulation is referred to as homeostasis-driven or lymphopenia-induced proliferation.
Recently, it was shown that during homeostatic proliferation, naive CD8 T cells acquire the cell surface markers and functional properties of antigen-stimulated memory cells (10–14). Thus, after transfer into RAG−/− recipients persisting CD8 T cells express high levels of CD44, IL-2Rβ, and Ly-6C, and can be induced to express cytolytic activity and IFN-γ rapidly. The acquired memory phenotype is stable in RAG−/− recipients (12). However, in two studies in irradiated B6 recipients disparate findings on the stability of homeostatic memory cells have been reported. In one, cells with the memory phenotype were stable, as in RAG−/− recipients (14). But, in the other study, the acquired memory phenotype was only transient and naive T cells of donor origin accumulated starting around 3 weeks after transfer of spleen and lymph node cells (13). Because the accumulation of naive cells correlated in time with the cessation of homeostatic proliferation in the irradiated B6 recipients, the finding was attributed to phenotype reversion by homeostatic memory T cells when they cease to proliferate. This interpretation has two profound implications. One is that the memory phenotype may not be as stable as once thought. Another is that the phenotype reversion could provide a thymus-independent mechanism for restoring the naive T cell compartment after severe T cell depletion.
What is responsible for the apparent difference in the fates of transferred naive CD8 T cells in irradiated B6 recipients in the two studies? Is there a difference in the stability of the memory T cell phenotype acquired in RAG−/− and irradiated B6 recipients? To answer these questions, we undertook to reexamine the apparent phenotype reversion of homeostatic memory T cells in sublethally irradiated B6 recipients. Our findings demonstrate that the accumulation of donor-derived naive T cells in irradiated recipients results from de novo T cell development in the thymus. Thus, after severe T cell depletion homeostatic proliferation restores only the memory T cell compartment, whereas thymopoiesis is required for the reconstitution of the naive compartment.
Materials and Methods
Mice.
Transgenic mice [2C T cell receptor (TCR)] were on the recombination activating gene-1 deficient (RAG−/−) background (2C/RAG) and had been backcrossed with C57BL/6 (B6, H-2b) mice for ten generations. RAG−/− mice were backcrossed with B6 mice for 13 generations. B6 mice at 5–6 weeks of age were thymectomized under anesthesia by vacuum suction, and were allowed to recover for at least 3 weeks. For adoptive transfer, RAG−/− recipients were used without irradiation or irradiated (400 rads) 2 days before transfer. Irradiated B6 mice and thymectomized B6 mice received 650 rads 2 days before transfer. For lethal irradiation, B6 mice were irradiated with 1,200 rads on the day of transfer. All mice were kept in a specific pathogen-free facility and used between 5 and 10 weeks of age.
Adoptive Transfer.
Lymph node cells (3–4 × 106) from 2C/RAG mice were transferred i.v. into RAG−/−, irradiated RAG−/−, irradiated B6, and irradiated and thymectomized B6 recipients. CD8+CD44−/lo 2C cells were purified (>98%) by cell sorting and TCR−CD11c−CD11b−lymph node cells were purified first by depleting CD11c+ and CD11b+ cells by using magnetic beads (Miltenyi Biotech, Auburn, CA), followed by FACS sorting for TCR− cells (>98%). CD8+CD44−/lo (2 × 106) and TCR−CD11c−CD11b−(1 × 106) cells were transferred.
Antibodies, Intracellular Staining, and Flow Cytometry.
Antibodies to CD8, CD44, IL-2Rβ, Ly-6C, and IFN-γ conjugated to fluorochrome were from PharMingen. The 1B2 antibody (specific for the 2C TCR) was conjugated to biotin. Spleen and lymph node cells were first blocked with unconjugated anti-FcR antibody, stained in PBS containing 0.5% BSA and 0.1% NaN3, and then analyzed on a FACScaliber, collecting 10,000–1,000,000 live cells (PI−) per sample. To detect intracellular IFN-γ, cells were stimulated in vitro with PMA (25 ng/ml) and ionomycin (500 ng/ml) at 37°C for 4 h. Two hours before harvest, brefeldin A was added to the culture. The harvested cells were surface-stained for 2C TCR, CD8, and CD44 before being fixed and stained for IFN-γ. Spleen and lymph node cells from BrdUrd-pulsed mice were surface stained for 2C TCR and CD8 or CD44, and then fixed and stained with anti-BrdUrd antibody (PharMingen).
Proliferation Assays.
To assess proliferation, lymph node cells from 2C/RAG mice were labeled with carboxyfluorescein diacetate-succinimydyl ester (CFSE) and then transferred into different recipients. Proliferation of the transferred T cells in the lymph nodes and spleens was assayed between day 5 and day 14 after transfer. Alternatively, mice were injected once with 5bromo-2′-deoxyuridine (BrdUrd, 1 mg per mouse) i.p. and then fed with BrdUrd-containing water (0.8 mg/ml) for 3 or 7 days before the indicated time point.
Intrathymic FITC Injection.
Mice were anesthetized with avertin (Sigma). An incision was made in the sternum to reveal the thymus, and approximately 10–20 μl of FITC solution (1 mg/ml) was injected into each thymic lobe with a 30-gauge needle. Control mice were injected with PBS. The chest was closed with surgical clips in the overlying skin.
Results
Accumulation of Naive 2C T Cells in Irradiated B6 but Not in RAG−/− Recipients.
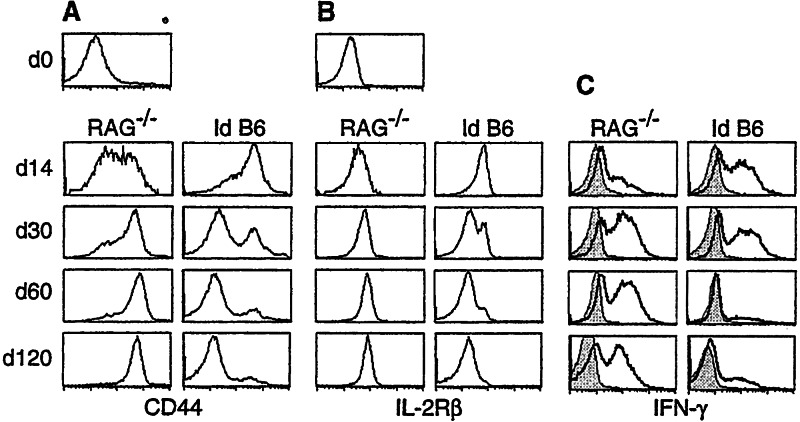
The phenotypic “reversion” of homeostatic memory cells in irradiated B6 recipients was initially observed with CD8 T cells expressing the OT-I TCR (13). To determine whether the same reversion occurs with T cells expressing a different TCR, we used CD8 T cells expressing the 2C TCR for which, like the OT-I TCR, H-2b is syngeneic. Thus, lymph node cells from 2C/RAG mice, consisting of >95% naive 2C cells, were adoptively transferred into nonirradiated RAG−/− hosts and into sublethally irradiated B6 hosts. The clonotypic antibody, 1B2 (15), specific for the 2C TCR, was used to distinguish the transferred 2C cells from the endogenous T cells. In RAG−/− hosts, 2C cells progressively acquired high levels of CD44, IL-2Rβ, and Ly-6C from days 14–30 (Fig.1 A and B, and data not shown). After day 60, persisting 2C cells uniformly expressed the same high levels of these markers as antigen-stimulated memory 2C cells (12, 16). In contrast, in irradiated B6 recipients, most 2C cells expressed high levels of CD44, IL-2Rβ, and Ly-6C only transiently: the levels were high 14 days after transfer, but from days 30–120 increasing proportions of 2C cells recovered from the recipients expressed no or very low levels of these markers, like naive 2C cells from the 2C/RAG donor mice. Similarly, much higher percentages of 2C cells were induced to express IFN-γ 30 or more days after transfer into the RAG−/− recipients (Fig.1D). Large fractions of 2C cells expressed IFN-γ 14 and 30 days after transfer into the irradiated B6 recipients, but only a small fraction was induced to express IFN-γ 60 or more days after transfer. The few cells that expressed IFN-γ all displayed the CD44hi memory phenotype (data not shown). Thus, as with OT-I T cells, the memory phenotype of 2C T cells acquired in RAG−/− recipients is stable, whereas after transfer into sublethally irradiated B6 recipients, increasing percentages of 2C cells over time express the naive phenotype (“reversion”).
Figure 1.
Comparison of 2C cell phenotype in RAG−/− and irradiated B6 recipients at different time points after cell transfer. Total lymph node cells from 2C/RAG mice were adoptively transferred into syngeneic RAG−/− and sublethally Id B6 mice. Lymph node cells from the recipients were assayed 14, 30, 60, and 120 days later for 2C TCR, CD8, plus CD44 or IL-2Rβ. The expression of CD44 (A) and IL-2Rβ (B) is shown for 2C TCR CD8+ 2C cells. CD44 and IL-2Rβ expression by 2C TCR CD8+ 2C cells before transfer is shown by the day 0 (d0). (C) Pooled spleen and lymph node cells from the recipients were incubated in medium alone (shaded area) or stimulated (bold line) with PMA and ionomycin for 4 h, and then stained for 2C TCR, CD8, and intracellular IFN-γ. IFN-γ expression by 2C TCR CD8+ 2C cells is shown. Data shown are from one representative recipient of a total of six per group per time point.
The observed difference between RAG−/− and irradiated B6 recipients was attributed to a difference in proliferation of persisting T cells in the two types of recipients (13). There are, however, many other differences between RAG−/− and irradiated B6 recipients that may have also contributed to the observed difference in the phenotype of the persisting T cells. First, B6 recipients were sublethally irradiated, whereas RAG−/− hosts were not. Whole body irradiation, which eliminates >95% of lymphocytes, not only creates “space” in the secondary lymphoid organs for homeostatic expansion of residual or transferred T cells, but also triggers production of inflammatory cytokines (17) and promotes reconstitution of diverse cell populations of hematopoietic origin by transferred stem cells. Second, endogenous T and B cells are present at low numbers in irradiated B6 mice but entirely absent in RAG−/− mice. The presence of B cells and particularly regulatory T cells, such as those expressing CD25, may affect the appearance of the donor-derived naive T cells. Third, even without the transfer of exogenous T cells, the total T cell number of sublethally irradiated B6, but not RAG−/−, mice is eventually restored by homeostatic proliferation of residual T cells in the periphery and de novo T cell differentiation in the thymus.
2C Cells of Memory Phenotype Proliferate at Similar Rates in RAG1−/− and Irradiated B6 Hosts.
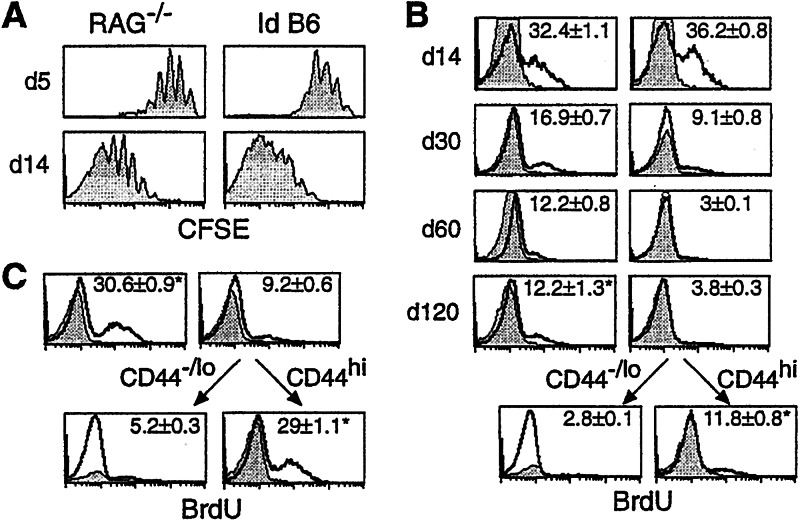
To determine the rate of cell proliferation of persisting T cells, 2C cells were labeled with CFSE and transferred into RAG−/− and irradiated B6 recipients for 5 and 14 days. 2C cells proliferated at similar rates in both types of hosts within 14 days of transfer (Fig.2A). To measure cell proliferation at later times after transfer, recipients were pulsed with BrdUrd for 3 days and the percentages of 2C cells that incorporated BrdUrd were assayed. Consistent with CFSE profiles, ≈35% of 2C cells incorporated BrdUrd in RAG−/− and irradiated B6 hosts by day 14 (Fig.2B). From days 30–120, however, the percentages of BrdUrd positive 2C cells decreased dramatically, indicating a decrease in 2C cell proliferation in both hosts. By days 60 and 120, ≈12% of 2C cells were BrdUrd-positive in RAG−/−recipients, whereas in irradiated B6 hosts only ≈3% were positive.
Figure 2.
Proliferation of 2C cells at different time points after transfer into the RAG−/− and irradiated B6 recipients. (A) CFSE-labeled lymph node cells from 2C/RAG mice were transferred into RAG−/− and irradiated B6 recipients. Lymph node cells from the recipients were harvested 5 and 14 days after transfer and assayed for 2C TCR, CD8, and CFSE. CFSE profiles of CD8+ 2C cells are shown. (B) Lymph node cells from 2C/RAG mice were adoptively transferred into RAG−/− (Left) and irradiated B6 recipients (Right). Mice were given BrdUrd for 3 days. Pooled lymph node and spleen cells from individual recipients were assayed for BrdUrd incorporation by using anti-BrdUrd antibody (bold line) or an isotype control (shaded area). BrdUrd intensities are shown for CD8+1B2+, CD44hi1B2+, or CD44−/lo1B2+ 2C cells at days 14, 30, 60, and 120 after transfer from one representative recipient. The analysis was done twice with three mice per time point per group. The numbers indicate the average of percentages of BrdUrd-positive cells from all six mice ± SD. *, P value of student T test is 0.58. (C) The same as in B, except that mice were given BrdUrd for 7 days before day 60 of transfer. *, P value of student T test is 0.26.
At first glance, difference in percentages of BrdUrd-positive 2C cells 30 or more days after transfer suggests a slower 2C cell proliferation in irradiated B6 than in RAG−/− hosts. However, this comparison is flawed because memory cells are known to proliferate more rapidly than naive cells (5, 18–20), and by 30 or more days after transfer all 2C cells displayed the memory phenotype in RAG−/− hosts, whereas most of the persisting 2C cells were of naive phenotype in irradiated B6 mice. To overcome this problem, BrdUrd incorporation in 2C cells was assayed in the CD44−/lo (naive) and CD44hi (memory) fractions 120 days after the transfer into the irradiated B6 recipients. As shown in Fig.2B, the percentage of BrdUrd-positive 2C cells in the CD44hi memory fraction was ≈12%, within the same range of BrdUrd-positive 2C cells in RAG−/− hosts. When mice were given BrdUrd for 7 days, ≈31% of 2C cells incorporated BrdUrd in RAG−/− mice (Fig. 2C), similar to the level reported (13). Although only ≈9% of total 2C cells incorporated BrdUrd in irradiated B6 hosts, ≈29% of the CD44hi memory 2C cells were BrdUrd-positive, which is not statistically different from 31% (P = 0.26).
Together, these data show that (i) 2C cells of the memory phenotype proliferate at similar rates in both RAG−/− and irradiated B6 hosts and (ii) 2C cells of the naive phenotype are mostly nondividing in irradiated B6 hosts 30 or more days after transfer.
Thymus and Irradiation Are Required for the Accumulation of Donor-Derived Naive T Cells.
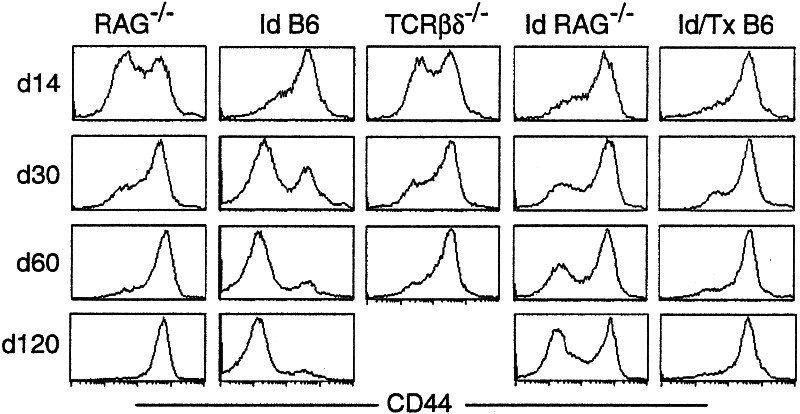
To investigate the role of the thymus in the accumulation of donor-derived naive T cells, we thymectomized B6 mice 3 weeks before irradiation and transfer of 2C cells. From days 14–120 after transfer, increasing proportion of 2C cells in the irradiated and thymectomized (Id/Tx) B6 mice expressed high levels of CD44, IL-2Rβ, and Ly-6C (Fig. 3 and data not shown). By day 120, almost all persisting 2C cells were uniformly high for CD44, in contrast to the persisting 2C cells in irradiated B6 recipients having an intact thymus. Because Id/Tx B6 mice have residual endogenous B and T cells but no new T cell development in the thymus, these results demonstrate that the thymus, not existing B and T cells (including regulatory T cells), is critical for the accumulation of naive 2C cells in the irradiated B6 recipients.
Figure 3.
Phenotype of 2C cells at different time points after transfer into various recipients. Pooled lymph node and spleen cells from 2C/RAG mice were transferred into nonirradiated RAG−/−, irradiated B6, nonirradiated TCRβδ−/−, irradiated RAG−/−, and Id/Tx B6 recipients. Lymph node cells from various recipients were analyzed for 2C TCR, CD8, and CD44 at days 14, 30, 60, and 120 after transfer. Three recipients were analyzed per time point per group. CD44 expression on CD8+ 2C cells is shown for one representative recipient.
To determine the role of irradiation in the accumulation of donor-derived naive T cells, we transferred 2C cells into sublethally irradiated RAG−/− mice. Fourteen days after transfer, as many 2C cells were high for CD44, IL-2Rβ, and Ly-6C (Fig. 3 and data not shown) as in irradiated B6 and Id/Tx B6 recipients. However, as time progressed, increasing proportions of 2C cells became low or negative for CD44, IL-2Rβ, and Ly-6C, reaching ≈60% by day 120. Thus, irradiation also promotes the accumulation of donor-derived naive T cells in RAG−/− recipients.
We also quantified the number of naive and memory 2C cells recovered from spleen and lymph nodes of various recipients at different time points after transfer. Although the number of CD44hi memory 2C cells was quite variable among different types of recipients 14 and 30 days after transfer, the numbers were similar by day 60 and 120 (1–2 × 106; see Table 1_A_, which is published as supporting information on the PNAS web site,www.pnas.org). As expected, the number of CD44−/lo naive 2C cells was low in RAG−/−, TCRβδ−/−, and Id/Tx B6 recipients at all time points analyzed but increased slowly over time in irradiated RAG−/− mice (see Table 1_B_). In irradiated B6 recipients, the number of CD44−/lo naive 2C cells increased steadily from days 14–120, to a total of ≈10 × 106 per recipient. Similarly, there was a significant increase in the number of host T cells from day 14 to day 30 following sublethal irradiation of B6 mice (see Table 1_C_). As with memory 2C cells, the number of CD44hi memory CD8 T cells of host origin was also relatively constant 30 days after irradiation.
Development of Donor-Derived T Cells in the Thymus of Irradiated Recipients.
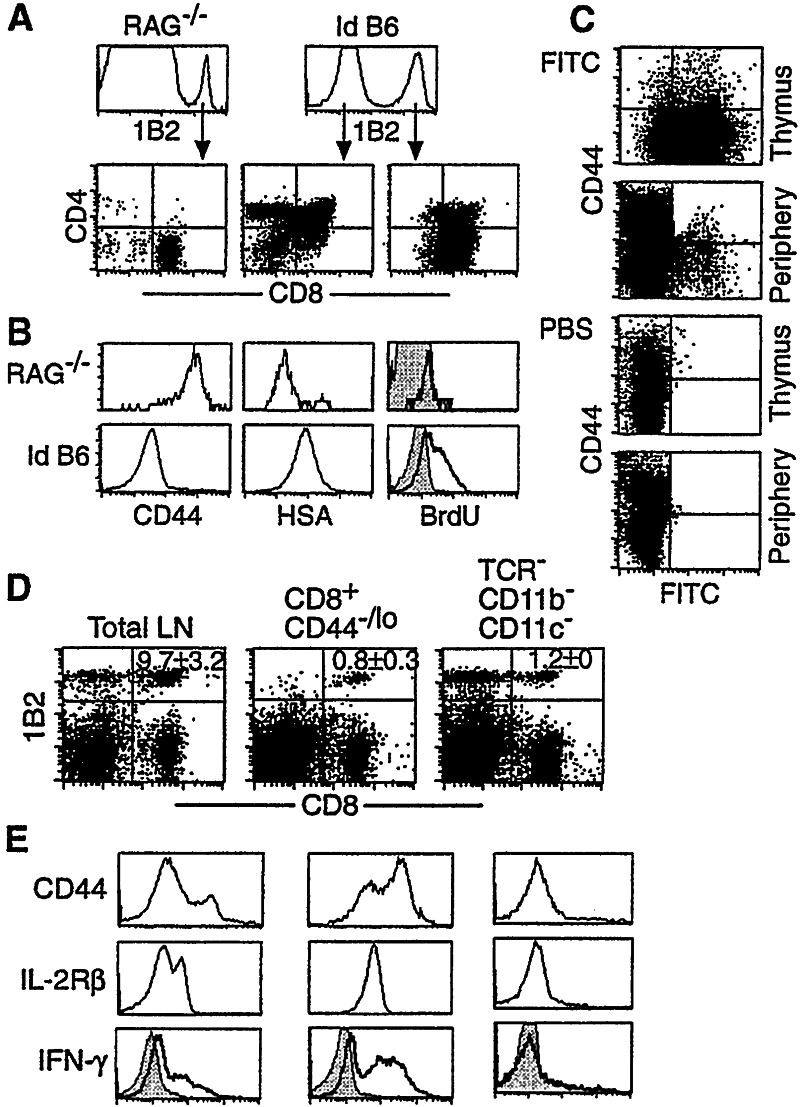
That thymus and irradiation are both required for the accumulation of donor-derived naive T cells in irradiated recipients indicates that these cells are newly generated in the thymus. To examine this possibility, we analyzed T cells in the thymus of various recipients at days 30 and 60 after cell transfer. In the thymus of nonirradiated RAG−/− recipients, only 0.5% of cells were 2C cells (Fig. 4A Left). These 2C cells were CD8 single-positive (SP), CD44hi, and HSAlo, and did not incorporate much BrdUrd during a 3-day labeling (Fig.4B), indicating that they are memory 2C cells circulating through the thymus.
Figure 4.
De novo 2C cell development in the thymus of irradiated RAG−/− and B6 recipients. (A_0 Sixty days after transfer of lymph node cells from 2C/RAG mice, thymocytes from RAG−/− (Left) and irradiated B6 (Right) recipients were assayed for 2C TCR, CD4, and CD8. (Upper) Expression of 2C TCR by total thymocytes; (Lower) CD4 and CD8 expression by 1B2+ or 1B2− thymocytes. The 1B2− thymocytes in RAG−/− mice were CD4−CD8− (not shown). Representative data from one of six recipients per group are shown. (B) Thymocytes from the same recipients as in_A were assayed for 2C TCR, CD8, CD4, plus CD44, HSA, or BrdUrd. Staining intensities for CD44, HSA, and BrdUrd by 1B2+CD8+CD4− thymocytes are shown. Bold line, anti-BrdUrd; shaded area, isotype control. (C) Export of naive 2C cells from thymus to the periphery in irradiated B6 recipients. Sixty days after the adoptive transfer, irradiated B6 recipients were injected intrathymically with FITC or PBS. Two days later, thymocytes and pooled lymph node and spleen cells were stained for 2C TCR, CD8, and CD44. FITC versus CD44 profiles are shown for CD8+ 2C cells. (D and_E_) Presence of hematopoietic stem cells in the transferred lymph node population. Total cells, purified CD8+CD44−/lo 2C cells (>98%), or purified TCR−CD11b−CD11c− cells (>98%) from the lymph nodes of 2C/RAG mice were transferred into irradiated B6 mice. Thirty days later, pooled lymph node and spleen cells from individual recipients were stained for 2C TCR, CD8, plus CD44, and IL-2Rβ. Data shown are from one representative of four recipients per group. The numbers indicate the average percentages of CD8+2C cells ± SD. (D) Dot-plots showing the presence of CD8+ 2C cells in the periphery. (E) CD44, IL-2Rβ, and intracellular IFN-γ expression by 1B2+CD8+ 2C cells. Bold line, anti-IFN-γ; shaded area, isotype control.
In the thymus of irradiated B6 recipients, in contrast, 31% of the T cells were of donor origin (Fig. 4A Right). Among the host-derived (1B2−) thymocytes, 88% were CD4+CD8+, 8% were CD4+, and 3% were CD8+. Among the donor-derived (1B2+) thymocytes, approximately 59% were CD8+ and notably, 35% were CD4+CD8+. It is also notable that unlike CD8+ 2C cells in the thymus of nonirradiated RAG−/− recipients, the CD8+ 2C cells in the thymus of irradiated B6 recipients were CD44lo and HSAhi and a large fraction of the cells incorporated BrdUrd during a 3-day labeling period (Fig.4B). Thus, the CD8+ 2C cells in the thymus of the irradiated B6 recipients have the characteristic feature of immature, developing thymocytes.
To determine whether the newly generated naive 2C cells in the thymus of irradiated B6 hosts are exported to peripheral lymphoid organs, FITC was injected into the thymus of the irradiated B6 recipients 60 days after transfer. Forty-eight hours later, ≈80% of 2C thymocytes in the recipients were labeled by FITC (Fig. 4C), and in the lymph nodes and spleen ≈0.4% of 2C cells were FITC-positive. These FITC-positive 2C cells in the periphery were CD44−/lo, as expected for naive cells.
Together, these results suggest that naive 2C T cells are continuously generated in the thymus of irradiated B6 recipients and that their emigration accounts for the gradual accumulation of donor-derived naive T cells in the periphery. Similar events also occur, although to a lesser degree, in RAG−/− recipients if they have been irradiated.
Hematopoietic Stem Cells Are Present in the Transferred Population.
In both our study and previous studies by others, total lymph node cells and occasionally total spleen cells from TCR transgenic mice on the RAG−/− background were used as the source of donor T cells. De novo differentiation of donor-derived T cells in the thymus of irradiated recipients implies that hematopoietic stem cells are present in the transferred cell population. To address this issue, total cells, purified CD8+CD44−/lo 2C cells, or purified TCR−CD11c−CD11b−(1B2−) cells from lymph nodes of 2C/RAG mice were transferred into irradiated B6 recipients. Thirty days after transfer of purified CD8+CD44−/lo 2C cells, the majority of CD8+ 2C cells in the periphery of irradiated B6 hosts expressed characteristic memory T cell markers and function (Fig. 4 D and E). Strikingly, CD8+ 2C T cells were also detected in the periphery of irradiated B6 recipients that had been given purified 1B2− lymph node cells (TCR−CD11c−CD11b−, Fig. 4D), indicating transfer of hematopoietic stem cells. Furthermore, the 2C cells arising in these recipients expressed the naive phenotype of CD44−/loIL-2RβloLy-6Clo, and did not produce IFN-γ after 4-h stimulation (Fig. 4E), suggesting that they are newly differentiated in the thymus of the recipients.
To further confirm the presence of hematopoietic stem cells in the transferred cell populations, bone marrow cells from nonirradiated RAG−/−, irradiated B6, and Id/Tx B6 recipients (primary recipients), which had received total lymph node cells from 2C/RAG donors 120 days earlier, were depleted of 2C TCR 2CTCR cells and then transferred into lethally irradiated B6 hosts (secondary recipients). Four weeks after the secondary transfer, 2C T cells were detected in the periphery of the secondary recipients only if they had received bone marrow cells from the irradiated B6 and Id/Tx B6 primary recipients, but not if they had received such cells from nonirradiated RAG−/− recipients (data not shown). These results suggest that repopulation of bone marrow by transferred stem cells occurs in irradiated but not in nonirradiated recipients; hence, the requirement for irradiation for the accumulation of donor-derived naive T cells in both B6 and RAG−/− recipients.
Homeostatic Memory T Cells Do Not Revert to Naive Phenotype.
So far, our results clearly show that the accumulation of donor-derived naive T cells in the irradiated hosts results from de novo T cell differentiation in the thymus. However, it is important to demonstrate that homeostatic memory T cells do not revert to naive phenotype. Thus, long-term antigen-stimulated (4 months after antigen stimulation) and homeostatic (3 months after adoptive transfer) memory 2C cells were adoptively transferred into nonirradiated B6 mice. 30 days after transfer, persisting 2C cells in the recipients expressed the same high level of CD44 regardless of whether antigen-stimulated or homeostatic memory 2C cells were initially transferred (data not shown).
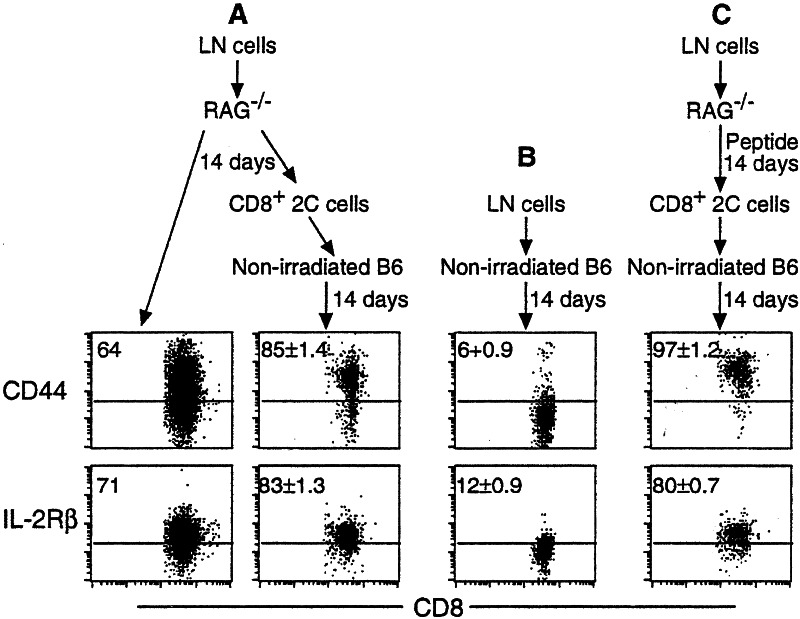
To examine whether T cells that have initiated homeostasis-driven proliferation and differentiation can undergo phenotype reversion, total lymph node cells from 2C/RAG mice were transferred into nonirradiated RAG−/− recipients, some of which were stimulated with antigen (Fig. 5). Fourteen days later, spleen and lymph node cells were harvested from the recipients and some cells were assayed immediately for the phenotype of the recovered 2C cells. As indicated by the proportion of cells that were high for CD44, IL-2Rβ, and Ly-6C (Fig. 5 _A_and C, and data not shown), 2C cells were in the process of acquiring the memory phenotype. From the rest of the harvested cells CD8+ 2C cells were purified and the enriched population was then transferred into nonirradiated B6 mice. Fourteen and 30 days after the secondary transfer, persisting 2C cells in the nonirradiated B6 mice expressed high levels of CD44, IL-2Rβ, and Ly-6C (Fig. 5 A and C, and data not shown). In contrast, when lymph node cells from 2C/RAG mice were directly transferred into nonirradiated B6 mice, most of the persisting 2C cells expressed no or only low levels of CD44, IL-2Rβ, and Ly-6C 14 days later (Fig. 5B). These results suggest that once naive CD8 T cells have initiated the differentiation program in response to either homeostatic or antigenic signals, they will continue to differentiate into memory cells even in an intact recipient.
Figure 5.
Homeostatic memory 2C cells do not revert to naive phenotype. (A) Lymph node cells from 2C/RAG mice were transferred into nonirradiated RAG−/− mice. 14 days later, spleen and lymph node cells were harvested from the recipients. A fraction of cells was assayed immediately. CD8+ 2C cells were enriched by anti-CD8 magnetic beads from the rest of the harvested cells and then transferred into nonirradiated B6 mice. Fourteen days after the secondary transfer, spleen and lymph node cells from the secondary recipients were assayed. CD44 and IL-2Rβ expression by CD8+ 2C cells is shown. Two nonirradiated B6 recipients were analyzed after the secondary transfer. The numbers indicate the average percentages of positive cells ± SD. (B) Lymph node cells from 2C/RAG mice were transferred into nonirradiated B6 mice and 14 days later, the persisting 2C cells in the spleen and lymph nodes were analyzed as in A. Two recipients were used. (C) The same as in A except that RAG−/− recipients were immunized with SIYRYYGL peptide in CFA.
Discussion
The fundamental difference between naive and memory T cells lies in their different pattern of gene expression, which is achieved through modulating chromatin structures by epigenetic mechanisms, such as DNA methylation, histone acetylation, and binding of transcription factors. The expectation that the memory phenotype is stable is based on extensive evidence that immunological memory persists for prolonged periods, and is consistent with the stable propagation of epigenetic changes of chromatin structures over time and over cell divisions (21–23). This expectation is also in accord with findings that during homeostatic proliferation of naive CD8 T cells in RAG−/− hosts, the acquired memory phenotype is stable for both polyclonal T cells and monoclonal T cells expressing the 2C or the OT-I TCR (12, 13). It was therefore of considerable interest when Goldrath et al. reported that after transfer of lymph node cells from OT-1 TCR transgenic mice into sublethally irradiated B6 recipients, the transferred naive T cells only transiently acquired the memory phenotype: starting around 3 weeks after the transfer, naive OT-I T cells accumulated progressively in the recipients, as though memory cells reverted to naive cells. As we show here, the same happens to 2C cells when total lymph node cells from 2C/RAG mice are transferred into sublethally irradiated B6 mice (Fig.1). Thus, the accumulation of naive T cells of donor origin in irradiated B6 recipients is a general phenomenon.
Accumulation of Donor-Derived Naive T Cells Results from de Novo T Cell Development in the Thymus.
What is responsible for the observed difference in the phenotype of donor T cells in RAG−/− and irradiated B6 recipients? Our studies demonstrate that the accumulation of naive donor T cells depends on three conditions: First, the presence of an intact thymus is critical; no naive T cells of donor origin were generated in irradiated B6 mice if they had been thymectomized (Fig.3). Large numbers of CD4+CD8+ and immature CD8+ thymocytes expressing the 2C TCR were found in the thymus of irradiated B6 recipients (Fig. 4). Second, irradiation of recipients is required. Naive 2C cells accumulated gradually in irradiated but not in nonirradiated RAG−/−recipients (Fig. 3). Thus, RAG−/− hosts, if irradiated, behave like B6 hosts. Third, the accumulation of naive 2C cells in the irradiated B6 recipients also required the transfer of total lymph node cells or a purified subset of cells (TCR−CD11c−CD11b−; 1B2−) that contain stem cells (Fig. 4). Transfer of highly purified CD8+CD44lo 2C cells, in which stem cells had probably been removed, did not result in the accumulation of naive 2C cells in the irradiated B6 recipients. Consistently, no accumulation of naive donor T cells was observed 150 days after transfer of purified P14 T cells into sublethally irradiated B6 recipients (14).
It has been estimated that up to 105hematopoietic stem cells circulate in the blood everyday in an adult mouse (24). Our results show that stem cells indeed repopulated the recipient bone marrow, because naive 2C cells were generated in the lethally irradiated secondary B6 recipients after they received 2C (1B2+)-depleted bone marrow cells from the primary irradiated B6 recipients, but not when they received similarly treated bone marrow cells from nonirradiated RAG−/− recipients. DP and immature 2C cells were not detected in the thymus of irradiated B6 mice until 2 weeks after they received lymph node cells. Correspondingly, the appearance of naive 2C cells in the periphery began to become apparent about 3 weeks after initial transfer, in accordance with the time required for T cell reconstitution after transfer of bone marrow stem cells into lethally irradiated hosts (25).
Together, these findings clearly illustrate how donor-derived naive T cells accumulate in irradiated recipients following transfer with total spleen or lymph node cells: Stem cells present in the inoculum repopulate the bone marrow of irradiated recipients and give rise to precursor T cells that undergo further differentiation into single-positive (SP) T cells in the thymus. The export of newly differentiated T cells from the thymus results in the gradual accumulation of donor-derived naive T cells in the periphery.
Homeostatic Memory T Cells Do Not Revert to Naive Phenotype.
Our finding that the de novo T cell differentiation from transferred stem cells results in the accumulation of donor-derived naive T cells in irradiated B6 recipients strongly suggests that homeostatic memory T cells do not revert to naive phenotype. This view is further supported by several other findings. First, the phenotype of homeostatic memory 2C T cells is stably maintained for 120 days in RAG−/− and Id/Tx B6 recipients (Fig. 3). Similarly, the phenotype of homeostatic memory P14 T cells derived from purified naive donor cells (93%) from which stem cells had been likely eliminated was stably maintained for 150 days in irradiated B6 recipients (14). Second, homeostatic memory 2C cells proliferated at similar rates in both RAG−/− and irradiated B6 recipients (Fig. 2), indicating that the accumulation of donor-derived naive T cells in irradiated B6 hosts but not in RAG−/− hosts cannot be due to difference in homeostatic memory cell proliferation. Finally, if phenotypic reversion occurs, 2C cells that had resided in RAG−/−recipients for 2 weeks and were in the process of acquiring the memory phenotype would be expected to undergo phenotype reversion when retransferred into nonirradiated B6 mice. On the contrary, the retransferred 2C cells exhibited the same memory phenotype as antigen-stimulated 2C cells (Fig. 5), indicating that homeostatic proliferation of naive CD8 T cells triggers an irreversible program of memory T cell differentiation, just as does antigen-stimulated memory cell differentiation (26).
Role of Homeostatic Proliferation and Thymopoiesis in Maintaining T Cell Numbers.
It has been suggested that constancy of total T cell numbers is maintained by two broad mechanisms: homeostasis of existing T cells in the periphery and de novo T cell differentiation from stem cells in the thymus. Because the T cell population in the periphery consists of naive and memory compartments, the relative contributions of the two mechanisms have been unclear. Our findings shed some light on this issue: Because homeostasis drives naive T cells to proliferate and differentiate into memory cells without phenotype reversion, the homeostatic expansion can only restore the memory compartment, but not the naive compartment, in T cell-depleted hosts. The restoration of the naive T cell compartment, as we show, can only be accomplished by_de novo_ T cell differentiation in the thymus. Although the latter idea has been proposed (25, 27, 28), the earlier studies were not able to determine whether homeostatic proliferation also contributes to restoration of a depleted naive T cell compartment, because the naive H-Y T cells used happen not to proliferate after transfer into syngeneic lymphopenic hosts (5, 29); and it was also not known then that the homeostatic proliferation of naive CD8 T cells results in their differentiation into memory cells. The functional and kinetic differences between homeostatic proliferation and thymopoiesis in restoring the respective T cell compartments explains why the memory T cell compartment is reconstituted earlier than the naive compartment in adult humans who suffer from T cell depletion as a result of irradiation, chemotherapy, or infection (3).
The low number of homeostatic memory T cells recovered in RAG−/− recipients 120 days after transfer seems surprising considering the relatively high rate of memory cell proliferation, the absence of competing host T cells, and the apparent lymphopenic status of the hosts. Although similar numbers of memory 2C cells were recovered from irradiated B6 recipients, the latter contained substantial numbers of naive T cells of both the donor and host origin (see Table 1). These findings suggest that naive T cells and homeostatic memory T cells may not respond to the same lymphopenic condition and further support the view that naive and memory T cell compartments are regulated independently (25, 30). The relatively high rate of memory 2C cell proliferation in RAG−/−recipients (≈30% BrdUrd-positive cells after a 7-day labeling) is not host-specific or T cell-specific. It also occurs with memory 2C cells in irradiated B6 hosts, with memory OT-1 T cells in RAG−/− hosts (13), and with memory CD8 T cells in normal hosts (5, 18–20). Because the numbers of memory T cells do not continue to increase significantly despite the relative high rate of proliferation, both homeostasis- and antigen-induced memory CD8 T cells probably undergo a high rate of apoptosis during steady state conditions.
In summary, our present findings clearly show that (i) the phenotype of homeostatic memory cells is stable, (ii) homeostatic proliferation contributes only to restoration and maintenance of the memory T cell compartment, and (iii) thymopoiesis appears to be the only way to reconstitute the naive T cell compartment.
Supplementary Material
Supporting Table
Acknowledgments
We thank Dr. Wojciech Swat for performing thymectomy; Tara Schimdt for technical assistance; Glenn Paradis for cell sorting; Dr. David Kranz for 2C/RAG mice; Drs. Ananda Goldrath and Luk Van Parijs for discussion and review of the manuscript; and members of the Chen and Eisen laboratories for helpful discussions. This work was supported in part by National Institutes of Health Grants AI44478 and AI50631 (to J.C.), and AI44477 and CA60686 (to H.N.E.); and a Cancer Center Core Grant (to Richard Hynes). Q.G. is partly supported by an Anna Fuller postdoctoral fellowship.
Abbreviations
TCR
T cell receptor
RAG
recombination activating gene-1
CFSE
carboxyfluorescein diacetate-succinimydyl ester
B6
C57BL/6 mice
Id
irradiated
Tx
thymectomized
References
- 1.Goldrath A W, Bevan M J. Nature (London) 1999;402:255–261. doi: 10.1038/46218. [DOI] [PubMed] [Google Scholar]
- 2.Marrack P, Bender J, Hildeman D, Jordan M, Mitchell T, Murakami M, Sakamoto A, Schaefer B C, Swanson B, Kappler J. Nat Immunol. 2000;1:107–111. doi: 10.1038/77778. [DOI] [PubMed] [Google Scholar]
- 3.Mackall C L, Hakim F T, Gress R E. Semin Immunol. 1997;9:339–346. doi: 10.1006/smim.1997.0091. [DOI] [PubMed] [Google Scholar]
- 4.Tanchot C, Fernandes H V, Rocha B. Philos Trans R Soc London B. 2000;355:323–328. doi: 10.1098/rstb.2000.0569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tanchot C, Lemonnier F A, Pérarnau B, Freitas A A, Rocha B. Science. 1997;276:2057–2062. doi: 10.1126/science.276.5321.2057. [DOI] [PubMed] [Google Scholar]
- 6.Ernst B, Lee D-S, Chang J M, Sprent J, Surh C D. Immunity. 1999;11:173–181. doi: 10.1016/s1074-7613(00)80092-8. [DOI] [PubMed] [Google Scholar]
- 7.Viret C, Wong F S, Janeway J C A. Immunity. 1999;10:559–568. doi: 10.1016/s1074-7613(00)80055-2. [DOI] [PubMed] [Google Scholar]
- 8.Goldrath A W, Bevan M J. Immunity. 1999;11:183–190. doi: 10.1016/s1074-7613(00)80093-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bender J, Mitchell T, Kappler J, Marrack P. J Exp Med. 1999;190:367–373. doi: 10.1084/jem.190.3.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kieper W C, Jameson S C. Proc Natl Acad Sci USA. 1999;96:13306–13311. doi: 10.1073/pnas.96.23.13306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oehen S, Brduscha-Reim K. Eur J Immunol. 1999;29:608–614. doi: 10.1002/(SICI)1521-4141(199902)29:02<608::AID-IMMU608>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 12.Cho B, Varada R, Ge Q, Eisen H N, Chen J. J Exp Med. 2000;192:549–556. doi: 10.1084/jem.192.4.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldrath A W, Bogatzki L Y, Bevan M J. J Exp Med. 2000;192:557–564. doi: 10.1084/jem.192.4.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murali-Krishna K, Ahmed R. J Immunol. 2000;165:1733–1737. doi: 10.4049/jimmunol.165.4.1733. [DOI] [PubMed] [Google Scholar]
- 15.Kranz D M, Sherman D H, Sitkovsky M V, Pasternack M S, Eisen H N. Proc Natl Acad Sci USA. 1984;81:573–577. doi: 10.1073/pnas.81.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cho B K, Wang C, Sugawa S, Eisen H N, Chen J. Proc Natl Acad Sci USA. 1999;96:2976–2981. doi: 10.1073/pnas.96.6.2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hill G R, Crawford J M, Cooke K R, Brinson Y R, Pan L, Ferrara J L M. Blood. 1997;90:3204–3213. [PubMed] [Google Scholar]
- 18.Tough D F, Sprent J. J Exp Med. 1994;179:1127–1135. doi: 10.1084/jem.179.4.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sprent J, Tough D F. Science. 1994;265:1395–1400. doi: 10.1126/science.8073282. [DOI] [PubMed] [Google Scholar]
- 20.Ku C C, Murakami M, Sakamoto A, Kappler J, Marrack P. Science. 2000;288:675–678. doi: 10.1126/science.288.5466.675. [DOI] [PubMed] [Google Scholar]
- 21.Fitzpatrick D R, Shirley K M, Kelso A. J Immunol. 1999;162:5053–5057. [PubMed] [Google Scholar]
- 22.Agarwal S, Rao A. Immunity. 1998;9:765–775. doi: 10.1016/s1074-7613(00)80642-1. [DOI] [PubMed] [Google Scholar]
- 23.Shibahara K-I, Stillman B. Cell. 1999;96:575–585. doi: 10.1016/s0092-8674(00)80661-3. [DOI] [PubMed] [Google Scholar]
- 24.Wright D E, Wagers A J, Gulati A P, Johnson F L, Weissman I L. Science. 2001;294:1933–1936. doi: 10.1126/science.1064081. [DOI] [PubMed] [Google Scholar]
- 25.Tanchot C, Rocha B. Eur J Immunol. 1995;25:2127–2136. doi: 10.1002/eji.1830250802. [DOI] [PubMed] [Google Scholar]
- 26.Kaech S M, Ahmed R. Nat Immunol. 2001;2:415–422. doi: 10.1038/87720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tanchot C, Rocha B. J Exp Med. 1997;1986:1099–1106. doi: 10.1084/jem.186.7.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berzins S P, Boyd R L, Miller J F. J Exp Med. 1998;187:1839–1848. doi: 10.1084/jem.187.11.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bruno L, Kirberg J, von Boehmer H. Immunity. 1995;2:37–43. doi: 10.1016/1074-7613(95)90077-2. [DOI] [PubMed] [Google Scholar]
- 30.Tanchot C, Rosado M M, Agenes F, Freitas A A, Rocha B. Semin Immunol. 1997;9:331–337. doi: 10.1006/smim.1997.0090. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Table