Identification of a protein essential for a major pathway used by human cells to avoid UV- induced DNA damage (original) (raw)
Abstract
When DNA replication stalls at a fork-blocking lesion, cells use damage tolerance pathways to continue replication. One pathway, “translesion synthesis,” involves specialized DNA polymerases that can use damaged DNA as a template. Translesion synthesis can result in mutations (i.e., can be error-prone), but it can also be error-free. An alternative pathway has been hypothesized (sometimes called “damage avoidance”), by which cells make temporary use of an undamaged copy of the blocked sequence as a template, i.e., the newly synthesized daughter strand of the sister duplex or the allelic copy. This pathway is error-free. Evidence of the use of the daughter strand of the sister duplex as a template in intact mammalian cells has not been available heretofore. To determine whether hMms2, a ubiquitin-conjugating enzyme-like protein, plays a critical role in such damage avoidance, a human fibroblast cell strain in which both error-prone translesion synthesis and error-free damage avoidance can be detected and quantified simultaneously, and several derivative strains in which expression of hMms2 protein had been eliminated or greatly decreased, were compared for their ability to avoid translesion synthesis past UV254nm-induced DNA photoproducts. Loss of hMms2 protein eliminated the ability of the latter strains to use an allelic copy of a target gene for damage avoidance, i.e., to produce a wild-type gene from two nonfunctional allelic copies of that gene. Molecular analysis of the wild-type gene showed that this process involves gene conversion unassociated with crossing-over. That the loss of hMms2 also eliminated use of the daughter strand of the sister duplex as a template for damage avoidance could be inferred from the fact that the frequency of mutations induced by UV in the single copy HPRT gene of the derivative strains was significantly higher than that observed in the parental strain. These data indicate that hMMS2 is essential for human cells to carry out damage avoidance by using either type of homolog, and that damage avoidance and translesion synthesis are alternative pathways for tolerating fork-blocking photoproducts.
DNA is constantly exposed to damaging agents. If the damage is not removed, e.g., by excision repair, before the onset of S-phase, certain kinds of lesions can block replication by the major DNA polymerase complex (1). The damage tolerance mechanisms developed by prokaryotic and eukaryotic cells to overcome such replication blocks fall into two categories: translesion synthesis and damage avoidance. Evidence suggests that translesion synthesis is a process in which specialized, distributive DNA polymerases take over for the major DNA polymerase complex to carry out DNA replication by using the damaged DNA as a template (2–5). After distributive incorporation of nucleotides past the damage, the major DNA replication complex resumes its replication activity. Translesion synthesis can be either “error-prone” or “error-free,” depending on such factors as the type of damage, its sequence context in the DNA, and the availability of specialized distributive translesion synthesis polymerases, e.g., hPol ζ, hPol η, Pol ι (6–9) or their auxiliary proteins (10). The alternative pathway to tolerate DNA damage, sometimes referred to as damage avoidance, temporarily uses a homologous undamaged copy of DNA instead of the damaged DNA as a template to continue replication (11–13). By its very nature, damage avoidance is error-free. The mechanisms involved in mammalian cells' avoidance of fork-blocking lesions are not well characterized, but unlike what occurs in bacteria (12), mammalian cells do not transfer parental DNA strands containing UV photoproducts into daughter strands (14). It has been proposed that in mammalian cells, the 3′ end of a blocked leading strand separates from its original template and makes use of an intact homologous copy of the DNA as a template for continuing replication past the block. If the newly synthesized daughter strand of the sister duplex is to provide the temporary template for the blocked leading strand, synthesis of the lagging strand must have continued for a short distance beyond where the leading strand was blocked, and this lagging strand must become dissociated from its partner. After replication has proceeded past the block, the paired structure must uncouple so that normal DNA replication using the original template can resume. Although the newly synthesized daughter strand of the sister duplex may provide the most convenient homologous copy for the blocked fork, the homologous allelic gene can also serve as an alternative template for circumventing such a replication block. Evidence that intact mammalian cells make use of the newly synthesized daughter strand of the sister duplex to circumvent fork-blocking lesions has not been available.
Xiao and coworkers (15) cloned a gene from S. cerevisiae that complements the sensitivity of yeast mutant mms2–1, originally identified (16) for its sensitivity to methyl methanesulfonate. This gene, MMS2, codes for a ubiquitin-conjugating enzyme-like protein (15) that forms a stable complex with Ubc13 protein (17–19), enabling in vitro di-ubiquitin formation via Lys-63 instead of the conventional Lys-48 chain assembly (20). Loss of Mms2 significantly increases the rate of spontaneous mutations in S. cerevisiae and moderately increases the cytotoxic and mutagenic effects of UV254nm radiation (15), indicating that it plays a role in an error-free pathway.
Xiao et al. (21) isolated the human homolog of the S. cerevisiae MMS2 gene, designated hMMS2. The present study was designed to determine whether the hMMS2 gene plays a critical role in the ability of human cells to tolerate DNA damage, and if so, to identify the pathway in which it functions and determine the nature of the molecular events involved. To do so, an infinite life span, chromosomally stable human fibroblast cell strain, designated MSU-1.2, derived in the Carcinogenesis Laboratory (22) was engineered to contain a chromosomally integrated substrate that allows detection of the production of a selectable wild-type gene from two nonfunctional homologous genes (23). Three strains in which expression of hMms2 protein was eliminated or greatly reduced by using antisense hMMS were identified and compared with the parental strain for their ability to carry out damage avoidance after the exposure to UV radiation. The results indicate that human cells can use both kinds of homologous templates to avoid fork-blocking lesions, and that hMms2 protein is essential for the damage avoidance process.
Materials and Methods
Media Used.
Cells to be used for experiments involving cell survival assays, or selection for resistance to 6-thioguanine (TG, Sigma), or hygromycin (Calbiochem), were cultured in Eagle's essential minimal medium supplemented with l-aspartic acid (0.2 mM), l-serine (0.2 mM), sodium pyruvate (1 mM), hydrocortisone (1 μg/ml), and supplemented calf serum (10% vol/vol) (culture medium). This medium containing the appropriate selection drug, i.e., TG (40 μM) or hygromycin (100 units/ml) was used for selection of TG resistant or hygromycin-resistant cells, respectively. The parental cell strain, which contains a transfected tTAk gene that was transfected on a plasmid carrying the hisD gene as its selectable marker (see text), was cultured in McM medium (24) lacking histidine but containing histidinol (1 mM), supplemented calf serum (10% vol/vol) (HyClone), penicillin (100 units/ml), streptomycin (100 μg/ml), and hydrocortisone (1 μg/ml). Cell strains that contain a transfected antisense hMMS2 gene that was transfected on a plasmid carrying the gene coding for puromycin resistance (see text) as its selectable marker were cultured in this same McM medium with the addition of puromycin (1 μg/ml).
DNA Transfection.
Cells were transfected with Lipofectamine (GIBCO/BRL). After 48 h, transfectants were selected in medium containing the appropriate drug. The selection medium was renewed every 5 days. When drug-resistant clones had formed, they were isolated, expanded in the appropriate selection medium, and stored frozen until needed.
Preparation of Purified hMms2 and Mouse Polyclonal hMms2 Antibodies.
The human hMMS2 ORF was PCR amplified and cloned into pGEX6 (Amersham Pharmacia). The GST-hMms2 fusion protein was over-produced in Stratagene's Epicurian coli BL21-CodonPlus-RIL cells, and standard protocols were followed to purify the fusion protein with a 5-ml GSTrap column (Amersham Pharmacia). After PreScission protease (Amersham Pharmacia) cleavage, hMms2 was eluted from a GSTrap column and further purified by using a Superdex-75 size column (Amersham Pharmacia). Polyclonal mouse serum was raised against the purified hMms2 protein in five BALB/c mice. Three weeks after the second boost, the mice were bled, and the anti-serum was pooled and characterized by ELISA and Western analysis by using the purified protein.
Northern and Western Analysis.
For Northern analysis, RNA from each cell strain was extracted with RNAzol B (TEL-TEST, Friendswood, TX) according to the manufacturer's recommendations. For each sample, 15 μg of total RNA was fractionated in 1.2% formaldehyde agarose gel and transferred to Hybond-N+ (Amersham Pharmacia) nylon membranes. The RNA was crosslinked to the membrane with a UV Stratalinker 2400 (Stratagene). To determine the level of antisense RNA expression, hybridization was performed according to the standard procedures with a probe specific to hMMS2. The membrane was stripped and reprobed with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) to compare the relative amounts of total RNA loaded for each sample. For Western analysis, nuclear protein was extracted from the cell strains as described (25). For each cell strain, 50 μg of nuclear protein was subjected to Western analysis with hMms2 mouse polyclonal antibody. Purified hMms2 protein was included as the positive control, and Ku-80 protein was detected with rabbit polyclonal Ku-80 antibody to compare the relative amounts of total nuclear extracts loaded for each sample.
Assay for Cytotoxicity.
Cells in exponential growth were detached from the dishes with trypsin, plated at cloning density, and allowed 12 h to attach before being irradiated by UV254nm, as described (26). The culture medium was renewed 1 day after irradiation and again after 7 days. After 14 days, when the surviving cells had formed clones, they were stained. The survival was determined by comparing the cloning efficiency of the irradiated cells with that of the sham-irradiated control cells, and the value was expressed as a percent of the efficiency of the control cells.
Assay for the Frequency of UV-Induced Wild-Type Hygromycin-Resistant Cells.
This assay was carried out as described (27). Briefly, sufficient sets of cells plated at 0.5 × 106 cells per dish were used to have at least 2 × 106 surviving target cells per dose and were allowed 12 h to attach. The number of target cells attached to the dishes at the time of irradiation was determined electronically. Forty-eight hours after irradiation, the culture medium was changed to medium containing hygromycin (100 units/ml). This selection medium was replaced every 5 days, and the clones were stained 3 weeks after irradiation. The frequency was calculated as follows: total hygromycin-resistant clones divided by the total number of surviving cells, determined as described in the above paragraph, taking into account the number of attached target cells. The induced frequencies were calculated by subtracting the background frequencies
Characterization of hyg Genes.
Hygromycin-resistant clones were analyzed by using PCR and _Hin_dIII digestion, as described (23). Briefly, cells from each clone were used for PCR analysis with a set of primers that amplify a 1,167-bp fragment from each copy of the hyg gene. The PCR products were digested with _Hin_dIII and subjected to gel electrophoresis. PCR products from wild-type copies of the hyg gene are resistant to _Hin_dIII digestion. When gene conversion events occur, one or the other of the mutant hyg genes is restored to wild type, whereas the second mutant hyg gene remains unchanged. _Hin_dIII digestion of PCR products generates 1,167-bp, 278-bp, and 889-bp fragments, or 1,167-bp, 695-bp, and 472-bp fragments, depending on which hyg gene has been restored to the wild type. However, if a single reciprocal exchange were to occur between two mutant hyg genes, it would result in a single wild-type copy of the hyg gene.
Assay for the Frequency of UV-Induced Mutations in the HPRT Gene.
The methods used to determine the frequency of UV-induced TG resistant (TGR) cells have been described (28). Briefly, sufficient sets of cells, plated at 0.5 × 106 to 2 × 106 per 150-mm diameter dish, were used in order to have at least 1 × 106 surviving target cells per dose. The culture medium was changed the day after irradiation and again after 7 days. Cells used to detect induced mutations were allowed an 8-day expression period before at least 1 × 106 cells were plated at 25,000 cells per 100-mm diameter dish to assay for TG resistance. Selection medium containing 40 μM of TG was replaced after 7 days. At the time of selection, for each dose, a separate set of cells, plated at cloning density and fed with medium lacking TG, was used to determine the cloning efficiency of the cells. The frequency of mutants was calculated as follows: total TG-resistant clones divided by the total number of clonable cells selected. The induced frequencies were calculated by subtracting the background frequencies in the sham-irradiated control population.
Results and Discussion
Assay System for Detecting Damage Tolerance in the Same Target Cell Strains.
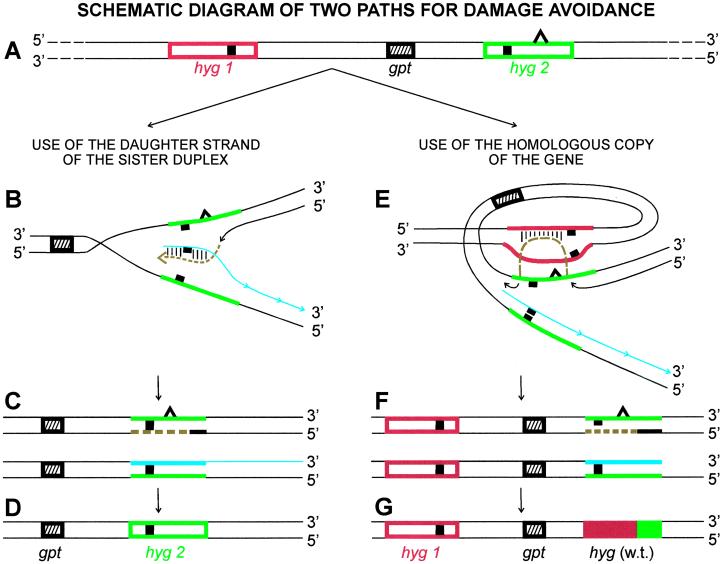
Damage tolerance pathways allow human cells with fork-blocking lesions to continue DNA replication and avoid cell death. To detect use of translesion synthesis, we measured the frequency of error-prone translesion synthesis by using the HPRT gene as the target for mutations and quantifying the frequency of induced TGR-resistant cells (28). (Error-free translesion synthesis cannot be detected directly but can be inferred by comparing the frequency of mutations induced in cells that lack such a process with that of cells that have it.) To detect the use of damage avoidance, we employ a substrate (stably integrated into the genome of the target cells) that allows quantification of the frequency with which cells produce a selectable wild-type copy of a gene by combining information from two defective homologous genes. The substrate (diagrammed in Fig. 1A) consists of two copies of the hygromycin phosphotransferase (hyg) gene conferring resistance to hygromycin, each inactivated by a 10-bp _Hin_dIII linker inserted at a different site (27). The genes, separated by ≈6,000 bp (23), are arranged in cis with one another, mimicking the situation for allelic genes in human cells. If a fork-blocking DNA lesion (represented by the triangle in Fig. 1 A_–_C, E, and F) interrupts replication of one of the genes, e.g., the hyg2 gene shown in Fig. 1A, use of the corresponding section of the newly synthesized daughter strand (shown in blue in Fig. 1B) of the sister duplex as a template will allow the cell to avoid the damage and continue DNA replication. However, the newly synthesized DNA strand (shown in brown) of the hyg2 gene resulting from such damage avoidance will still contain the 10-bp _Hin_dIII linker (Fig. 1C Upper), even after the lesion has been removed or after another round of replication has occurred (Fig. 1D). Therefore, this process will not yield a cell that is resistant to hygromycin. However, as diagrammed in Fig. 1E, if cells use the homologous copy of the gene, hyg1, as a template to avoid the blocking lesion, the newly synthesized strand (shown in brown) can obtain the information from the corresponding section of the hyg1 gene. The strand will no longer contain the 10-bp _Hin_dIII linker (Fig. 1F Upper). The next round of replication of that strand will yield a selectable wild-type copy of the hyg gene (Fig. 1G). An added value of this substrate is that it allows one to infer the nature of the mechanisms involved by analyzing the structure of the hyg gene(s) in the hygromycin-resistant (hygR) cells.
Figure 1.
Schematic diagram of the two possible paths for avoiding DNA damage by making use of an undamaged homolog to continue replication, i.e., use of a daughter strand of the sister duplex or use of the homologous copy of the blocked gene. (A) The intrachromosomal substrate contains two copies of the hyg gene (hyg1, red; hyg2, green), orientated in the same direction. Each copy is mutated by a _Hin_dIII linker insertion (■) at a different position. The gpt gene in the intervening segment is a selectable marker used to transfect the substrate into target cell strains. The open triangle on one strand of the hyg2 gene represents a UV-induced fork-blocking lesion. (B) The blocked strand of the hyg2 gene is shown copying information from the newly synthesized daughter strand (blue) of the sister duplex rather than using the damaged template. The section of DNA synthesized in this process is shown in brown. The base pairs formed between this DNA and the newly synthesized daughter strand are shown as parallel short lines. (C) The outcome of this process. The newly replicated leading strand (brown) of the hyg2 gene from this damage-avoidance process still contains the _Hin_dIII linker sequence (Upper). The replicated product not containing the lesion also retains the original _Hin_dIII linker DNA sequence (Lower). For the sake of clarity, the section containing the hyg1 gene (red) is not shown for these products. (D) The outcome after one round of replication. The hyg2 gene has been replicated but still contains the _Hin_dIII linker. (E) The hyg2 gene is shown copying information from the homologous copy, hyg1, rather than using the damaged strand. The section of DNA synthesized in this process is shown in brown. The base pairs formed between this DNA and one strand of the hyg1 gene are shown as parallel short lines. (F) The outcome of this process. The DNA sequence of the hyg1 gene remains intact. The newly replicated leading strand of the hyg2 gene (brown) has obtained information from the corresponding section of the hyg1 gene so it no longer contains the _Hin_dIII linker sequence (Upper). The replicated product not containing the lesion retains its original DNA sequence (Lower). (G) The outcome after one round of replication. A wild-type copy of the hyg gene has been generated. (The red portion represents information copied from the hyg1 gene; the green portion represents information from the hyg2 gene).
Preparation and Identification of Human Cells Expressing Antisense hMMS2 and Devoid of hMms2 Protein.
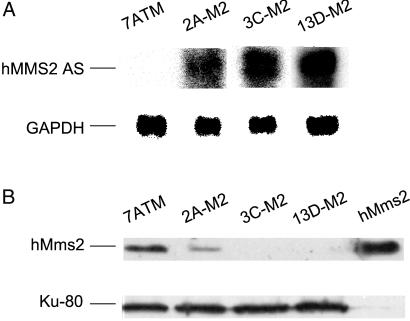
We successfully used an antisense hMMS2 RNA strategy to deplete human cells of hMms2 protein. To do so, we transfected an MSU-1.2 human fibroblast-derived cell strain, designated E7.2 (23), containing the substrate as diagrammed in Fig. 1A, with a plasmid designated pTet-tTak containing the tetracycline promoter (tetP; refs. 29 and 30), the tetracycline transactivator (tTA) gene under the control of tetP, and the hisD gene coding for histidinol resistance. A clone of histidinol-resistant cells that expressed tTA at a high level and exhibited the same UV-induced frequency of TGR cells and hygR cells as cell strain E7.2 was chosen as the parental strain for the present study. This parental cell strain expressing tTA was transfected with a plasmid carrying antisense hMMS2 under control of the tetP promoter. The hMMS2 antisense expression construct contains a fragment consisting of 20 bp of the 5′ untranslated region and the first 400 bp of the ORF of hMMS2 cDNA, cloned into the _Spe_I-_Hin_dIII sites of pTet-Puro plasmid (6, 10) in antisense orientation. The plasmid also contains a gene coding for puromycin resistance. Sixty puromycin-resistant transfectants were isolated and screened for expression of antisense hMMS2 RNA by RT-PCR by using a set of antisense-specific primers; i.e., one primer was specific for the plasmid. Sixteen clones were found to express hMMS2 antisense RNA. These 16 clones were expanded into large populations, and the cell strains were further analyzed by Northern blotting to quantify the level of expression of antisense RNA and identify those expressing the highest amount (data not shown). Nuclear extracts from the parental cell strain and the 16 antisense RNA-expressing cell strains were prepared and analyzed by Western blotting for the level of hMms2 protein. Purified hMms2 protein was included as the positive control. Three cell strains, designated 2A-M2, 3C-M2, and 13D-M2, which express high levels of antisense hMMS2 RNA (Fig. 2A), showed an undetectable level or a very low level of expression of hMms2 protein compared with the parental cell strain (Fig. 2B). We verified that these three cell strains lacking hMms2 protein still retained an intact copy of the substrate shown in Fig. 1A, and then used them to investigate the role of hMms2 in damage tolerance.
Figure 2.
Northern and Western blots showing the correlation between expression of antisense hMMS2 RNA and loss of hMms2 protein. (A) Northern analysis showing that clones 2A-M2, 3C-M2, and 13D-M2 express high levels of hMMS2 antisense RNA. GAPDH transcript was used as the loading control for all samples. hMMS2 AS indicates the transcript of hMMS2 antisense. (B) Western analysis showing the level of expression of hMms2 protein in nuclear extracts from these hMMS2 antisense-expressing transfectants and their parental strain. Ku-80 was used as the loading control. Purified hMms2 was included as the positive control.
Effect of Depletion of hMms2 Protein on Cell Survival.
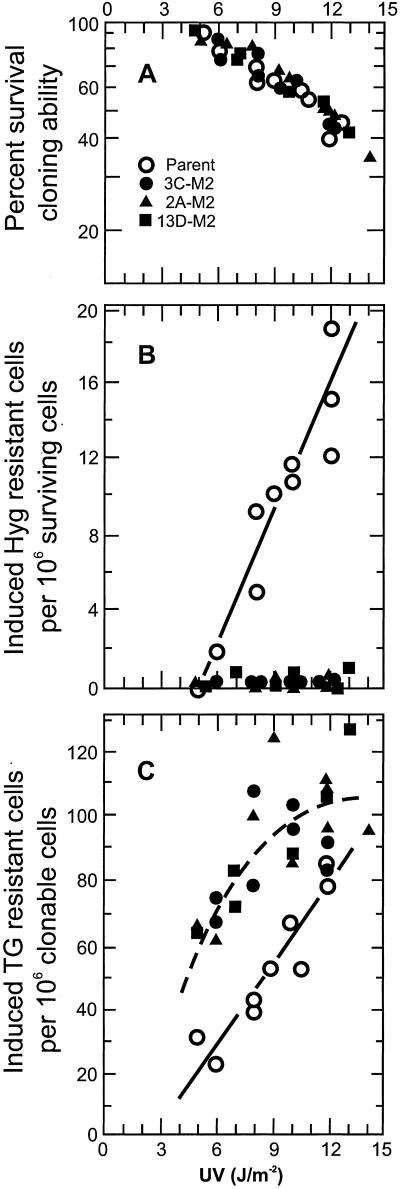
These three cell strains were compared with their parental strain for the effect of loss of hMms2 on UV-induced cytotoxicity. As shown in Fig. 3A, there was no significant difference between cells expressing or not expressing hMms2 protein in their sensitivity to the cytotoxic effect of UV. This finding indicated that cells expressing or not expressing hMms2 protein were equally capable of tolerating the UV photoproducts. The implications of this finding are discussed below in connection with the data indicating decreased use of damage avoidance and increased use of translesion synthesis. The majority of the data points shown in Fig. 3A were derived from experiments in which cell strains also were assayed for the frequency of UV-induced hygR cells and TGR cells (see below).
Figure 3.
The biological effects of inhibition of hMMS2 expression. White symbol, the parental cell strain; black symbols, transfectant cell strains showing an undetectable or very low level of hMms2 protein: ○, strain 3C-M2; ▴, strain 2A-M2; and ■, strain 13D-M2. (A) The UV-induced cytotoxicity in these cell strains. Some points have been offset slightly to make them visible. The cloning efficiency of all four unirradiated strains was comparable. (B) The frequency of hygR cells induced by UV in the parental cell strain and the three cell strains lacking hMms2 protein. Some points have been offset slightly to make them visible. The background frequencies for the unirradiated cell strains were: ≈10 × 10−6 for the parent and ≤1 × 10−6 for the strains lacking hMms2. (C) The frequency of TGR cells induced by UV in the parental cell strain and the three cell strains lacking hMms2 protein. For the majority of the data points generated, at least 2 × 106 cells were assayed for TG resistance. The observed frequency of TGR clones has been corrected for the cloning efficiency of the cells at the time of selection (28). For the majority of the data, these cloning efficiency values ranged from 45–65%, so the correction factors used on the observed frequencies were <2.3. The induced frequencies were calculated by subtracting the background frequencies, observed with the sham-irradiated populations. The latter were ≤10 × 106 clonable cells. The solid line represents the least-squares line for the data from the parental cell strain; the dashed line represents the least-squares line for the data points generated from the three cell strains deficient in hMms2 protein, taken together. (The solid line does not extrapolate through the origin, reflecting the effect of excision repair. The dashed line may extrapolate through the origin. The cells were not tested at such low doses.)
Effect of Depletion of hMms2 Protein on the Frequency of UV-Induced HygR Cells.
As expected from earlier studies in this laboratory (27), there was a dose-dependent increase in the frequency of UV-induced hygR cells in the parental cell strain, indicating use of a homologous copy of the gene as a template (Fig. 3B). (Production of a wild type hyg gene does not occur in the absence of the allelic copy; ref. 31.) However, in the three cell strains lacking hMms2 protein, that response to UV was virtually eliminated. The frequency of UV-induced hygR cells was not significantly greater than the background frequency, i.e., <1 × 10−6. This decrease in frequency is not the result of mere expression of antisense rather than the loss of hMms2 protein, because a fourth cell strain, exhibiting a very high level of antisense hMMS2 RNA, but yet showing a normal level of hMms2 protein, gave a UV-induced frequency of hygR cells equal to that found in the parental strain (data not shown). The data in Fig. 3B indicate that hMms2 protein plays an essential role in the damage-avoidance pathway dealing with photoproducts; i.e., during DNA replication, human cells can make temporary use of the allelic copy of a gene as a template to avoid fork-blocking lesions, but cells lacking hMms2 protein are virtually incapable of using this damage avoidance pathway. PCR and restriction enzyme digestion were used to analyze, for the structure of their hyg genes, 30 of the hygR clones induced by UV or arising spontaneously. The results showed that each wild-type hyg gene had been generated by a gene conversion event unaccompanied by crossing-over, as illustrated in Fig. 1G.
Effect of Depletion of hMms2 Protein on the Frequency of UV-Induced TGR Cells.
As expected, UV radiation caused a dose-dependent increase in the frequency of TGR cells in the parental cell strain (Fig. 3C), indicating that these cells had carried out error-prone translesion synthesis. The three cell strains lacking hMms2 protein also gave a dose-dependent induction of TGR cells for doses up to 8 J/m2, but the frequency of TGR cells was twice as high as that induced in the parental cells, indicating that the strains lacking hMms2 carried out translesion synthesis more frequently than did the parent cells. This increased use of translesion synthesis as a means of tolerating photoproducts by cells that cannot make use of damage avoidance can explain why such cells lacking hMms2 were not more sensitive than the parental cells to the cytotoxic effect of UV. Increased cell killing by UV photoproducts in cells lacking hMms2 would be expected if the cells were less able than the parent cells to continue to replicate DNA containing fork-blocking lesions. However, if the cells lacking hMms2 were to make increased use of their various specialized translesion synthesis polymerases, they could continue to replicate their DNA, although use of an undamaged homologous copy of the DNA as a template had been eliminated. S. cerevisiae cells lacking Mms2 were found to be somewhat more sensitive to the cytotoxic effect of UV than are wild-type yeast cells, i.e., ≤2-fold (15). However, because human cells have at least two more forms of translesion synthesis polymerases than do yeast cells, i.e., Pol ι and Pol κ (3), it is not surprising that they are more capable than yeast of replicating past fork-blocking lesions.
At higher doses of UV, the frequency of mutants in the three cell strains lacking hMms2 leveled off. The dashed line is the least-squares line for all of the data points obtained from these three cell strains. One possible explanation for why cells lacking hMms2 protein did not exhibit a dose-dependent increase in mutant frequency at higher UV doses is that at those doses, the number of mutations continued to increase, but some of these occurred within the same cell. We tested that possibility by sequencing the coding region of the HPRT gene of eight unequivocally independent mutants from these cell stains irradiated with the highest doses of UV. There was only one mutation in each gene. Another possible explanation is that, at those higher doses, the level of a DNA polymerase(s) involved in error-free translesion synthesis past photoproducts is increased in cells lacking hMms2 protein. Western analysis of the level of expression of polymerase η after UV-irradiation with these doses did not demonstrate such induction (data not shown).
Indirect Evidence That Human Cells Use the Newly Synthesized Daughter Strand of the Sister Duplex for Damage Avoidance.
The data in Fig. 3 A and C show that the parental cells expressing hMms2 and the three strains lacking hMms2 are equally able to tolerate UV-induced damage, but the latter are twice as sensitive to the induction of mutations in the HPRT gene. Because HPRT is located on the X chromosome, and the target cells are from a male donor, there is only one copy of this gene in the cell. Therefore, cells have only two options for tolerating fork-blocking lesions in the HPRT gene: (i) use the newly synthesized daughter strand of the sister duplex of the HPRT gene as the template to avoid the damage (error-free), or (ii) carry out potentially mutagenic translesion synthesis. The data in Fig. 3C strongly suggest that the parental cells used both options, but the first option was not available in cells lacking hMms2 protein.
We recognize that UV induces several kinds of fork-blocking photoproducts, and that some types of lesions are more likely than others to result in mutations. However, for the sake of simplicity, let us assume there is only one type of fork-blocking lesion, and that cells make equal use of the two major pathways for tolerating such lesions, i.e., translesion synthesis and damage avoidance. If the latter pathway is completely eliminated, e.g., by knocking out of expression of hMms2 protein, the number of fork-blocking lesions that have to be dealt with by translesion synthesis polymerases should double. Although translesion synthesis might not result in mutations every time, the frequency of mutations introduced during translesion synthesis should double because the total number of lesions to be passed has doubled. This is the extent of increase we observed when we eliminated hMms2 protein.
Possible Role of hMms2 in Damage Avoidance.
The role that hMms2 plays in the observed loss of ability of the human cells to carry out gene conversion events to avoid DNA fork-blocking lesions remains elusive. Hofmann and Pickart (17) showed that in yeast, Mms2 forms a complex with Ubc13, and the complex is required for the assembly of polyubiquitin chains linked through Lys-63 in a Ubc13-dependent manner. These investigators proposed that this complex acts as a signal transducer to recruit target proteins to the site of DNA damage. Later, Ulrich and Jentsch (18) showed that in yeast, Rad5 recruits the Mms2/Ubc13 complex to DNA through its RING finger domain, and that Rad5 interaction with Rad18 brings the Mms2/Ubc13 complex into contact with the Rad6/Rad18 complex. Likewise, Xiao et al. (32) proposed that in yeast, the Mms2/Ubc13 complex is required to promote both _RAD5_- and _POL30_-dependent error-free replication pathways. Recently, McKenna et al. (33) showed that in human cells, hMms2 forms a complex with hUbc13, and this interaction is required for hUbc13-mediated polyubiquitination through Lys-63. The crystal structure of this complex also was determined recently (34). Our data support the hypothesis that the hMms2/hUbc13 complex is required to recruit or activate proteins needed by human cells to be able to make use of an undamaged homologous copy of DNA as an alternative template to avoid fork-blocking lesions and continue DNA replication.
In summary, our data provide direct evidence that human cells can carry out damage avoidance by using an allelic copy of a gene as a temporary template for DNA replication, and that this process involves gene conversion, unaccompanied by crossing-over. Our mutagenesis data provide indirect evidence to support the model, originally proposed by Strauss and coworkers (11), that the newly synthesized daughter strand of the sister duplex also can be used as a temporary template for DNA replication to avoid DNA damage. Our data indicate that the product of the hMMS2 gene is essential in both cases. Our assay system provides a basis for identifying other proteins involved in the damage avoidance pathway dealing with fork-blocking lesions.
Acknowledgments
We thank L. Pastushok for hMms2 protein purification and generation of hMms2 mouse polyclonal antibody, Dr. H. Zhang for preparing and characterizing the parental cell strain, L. Qin for assisting with RNA extraction and Northern analysis, and J. Dao, M. Battle, and Y. Wang for helpful suggestions on Western blotting. We thank Dr. K. Meek for providing rabbit polyclonal Ku-80 antibody and Dr. S. Kleff and M. Hanna for critical reading of the manuscript. This work was supported by Department of Health and Human Services/National Institutes of Health Grants ES-09822 and CA 91490 (to V.M.M.) and Grant MOP-53240 from the Canadian Institutes of Health Research (to W.X.)
Abbreviations
HPRT
hypoxanthine phosphoribosyltransferase gene
hyg, hygromycin phosphotransferase gene
hygR, hygromycin resistant
TG
6-thioguanine
TGR
6-thioguanine resistant
References
- 1.Friedberg E C, Walker G C, Siede W. DNA Repair and Mutagenesis. Washington, DC: Am. Soc. Microbiol.; 1995. [Google Scholar]
- 2.Nelson J R, Lawrence C W, Hinkle D C. Science. 1996;272:1646–1649. doi: 10.1126/science.272.5268.1646. [DOI] [PubMed] [Google Scholar]
- 3.Woodgate R. Genes Dev. 1999;13:2191–2195. doi: 10.1101/gad.13.17.2191. [DOI] [PubMed] [Google Scholar]
- 4.Friedberg E C, Feaver W J, Gerlach V L. Proc Natl Acad Sci USA. 2000;97:5681–5683. doi: 10.1073/pnas.120152397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pham P, Bertram J G, O'Donnell M, Woodgate R, Goodman M F. Nature (London) 2001;409:366–370. doi: 10.1038/35053116. [DOI] [PubMed] [Google Scholar]
- 6.Gibbs P E, McGregor W G, Maher V M, Nisson P, Lawrence C W. Proc Natl Acad Sci USA. 1998;95:6876–6880. doi: 10.1073/pnas.95.12.6876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Masutani C, Araki M, Yamada A, Kusumoto R, Nogimori T, Maekawa T, Iwai S, Hanaoka F. EMBO J. 1999;18:3491–3501. doi: 10.1093/emboj/18.12.3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Masutani C, Kusumoto R, Iwai S, Hanaoka F. EMBO J. 2000;19:3100–3109. doi: 10.1093/emboj/19.12.3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tissier A, McDonald J P, Frank E G, Woodgate R. Genes Dev. 2000;14:1642–1650. [PMC free article] [PubMed] [Google Scholar]
- 10.Gibbs P E, Wang X-D, Li Z, McManus T P, McGregor W G, Lawrence C W, Maher V M. Proc Natl Acad Sci USA. 2000;97:4186–4191. doi: 10.1073/pnas.97.8.4186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Higgins N P, Kato K, Strauss B. J Mol Biol. 1976;101:417–425. doi: 10.1016/0022-2836(76)90156-x. [DOI] [PubMed] [Google Scholar]
- 12.West S C, Cassuto E, Howard-Flanders P. Nature (London) 1981;294:659–662. doi: 10.1038/294659a0. [DOI] [PubMed] [Google Scholar]
- 13.Echols H, Goodman M F. Annu Rev Biochem. 1991;60:477–511. doi: 10.1146/annurev.bi.60.070191.002401. [DOI] [PubMed] [Google Scholar]
- 14.Spivak G, Hanawalt P C. Biochemistry. 1992;31:6794–6800. doi: 10.1021/bi00144a021. [DOI] [PubMed] [Google Scholar]
- 15.Broomfield S, Chow B L, Xiao W. Proc Natl Acad Sci USA. 1998;95:5678–5683. doi: 10.1073/pnas.95.10.5678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prakash L, Prakash S. Genetics. 1977;86:33–55. doi: 10.1093/genetics/86.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hofmann R M, Pickart C M. Cell. 1999;96:645–653. doi: 10.1016/s0092-8674(00)80575-9. [DOI] [PubMed] [Google Scholar]
- 18.Ulrich H D, Jentsch S. EMBO J. 2000;19:3388–3397. doi: 10.1093/emboj/19.13.3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.VanDemark A P, Hofmann R M, Tsui C, Pickart C M, Wolberger C. Cell. 2001;105:711–720. doi: 10.1016/s0092-8674(01)00387-7. [DOI] [PubMed] [Google Scholar]
- 20.Dubiel W, Gordon C. Curr Biol. 1999;9:R554–R557. doi: 10.1016/s0960-9822(99)80353-4. [DOI] [PubMed] [Google Scholar]
- 21.Xiao W, Lin S L, Broomfield S, Chow B L, Wei Y F. Nucleic Acids Res. 1998;26:3908–3914. doi: 10.1093/nar/26.17.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin C C, Wang Q, Maher V M, McCormick J J. Cell Growth Differ. 1994;5:1381–1387. [PubMed] [Google Scholar]
- 23.Zhang H, Tsujimura T, Bhattacharyya N P, Maher V M, McCormick J J. Carcinogenesis. 1996;17:2229–2235. doi: 10.1093/carcin/17.10.2229. [DOI] [PubMed] [Google Scholar]
- 24.Ryan P A, Maher V M, McCormick J J. Exp Cell Res. 1987;172:318–328. doi: 10.1016/0014-4827(87)90390-9. [DOI] [PubMed] [Google Scholar]
- 25.Schreiber E, Matthias P, Muller M M, Schaffner W. Nucleic Acids Res. 1989;17:6419. doi: 10.1093/nar/17.15.6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patton J D, Maher V M, McCormick J J. Carcinogenesis. 1986;7:89–93. doi: 10.1093/carcin/7.1.89. [DOI] [PubMed] [Google Scholar]
- 27.Tsujimura T, Maher V M, Godwin A R, Liskay R M, McCormick J J. Proc Natl Acad Sci USA. 1990;87:1566–1570. doi: 10.1073/pnas.87.4.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maher V M, McCormick J J. In: Technologies for Detection of DNA Damage and Mutagenesis. Pfeifer G, editor. New York: Plenum; 1996. pp. 381–390. [Google Scholar]
- 29.Gossen M, Bujard H. Proc Natl Acad Sci USA. 1992;89:5547–5551. doi: 10.1073/pnas.89.12.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shockett P, Difilippantonio M, Hellman N, Schatz D G. Proc Natl Acad Sci USA. 1995;92:6522–6526. doi: 10.1073/pnas.92.14.6522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liskay R M, Stachelek J L, Letson A. Cold Spring Harbor Symp Quant Biol. 1984;49:183–189. doi: 10.1101/sqb.1984.049.01.021. [DOI] [PubMed] [Google Scholar]
- 32.Xiao W, Chow B L, Broomfield S, Hanna M. Genetics. 2000;155:1633–1641. doi: 10.1093/genetics/155.4.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McKenna S, Spyracopoulos L, Moraes T, Pastushok L, Ptak C, Xiao W, Ellison M J. J Biol Chem. 2001;276:40120–40126. doi: 10.1074/jbc.M102858200. [DOI] [PubMed] [Google Scholar]
- 34.Moraes T F, Edwards R A, McKenna S, Pastushok L, Xiao W, Glover J N, Ellison M J. Nat Struct Biol. 2001;8:669–673. doi: 10.1038/90373. [DOI] [PubMed] [Google Scholar]