Ability of the hydrophobic FGF and basic TAT peptides to promote cellular uptake of recombinant Cre recombinase: A tool for efficient genetic engineering of mammalian genomes (original) (raw)
Abstract
Conditional mutagenesis is a powerful tool to analyze gene functions in mammalian cells. The site-specific recombinase Cre can be used to recombine loxP-modified alleles under temporal and spatial control. However, the efficient delivery of biologically active Cre recombinase to living cells represents a limiting factor. In this study we compared the potential of a hydrophobic peptide modified from Kaposi fibroblast growth factor with a basic peptide derived from HIV-TAT to promote cellular uptake of recombinant Cre. We present the production and characterization of a Cre protein that enters mammalian cells and subsequently performs recombination with high efficiency in a time- and concentration-dependent manner. Histidine-tagged Cre recombinase transduced inefficiently unless fused to a nuclear localization signal (NLS). Fusion of NLS-Cre to the fibroblast growth factor transduction peptide did not improve the transducibility, whereas fusion with the TAT peptide significantly enhanced cellular uptake and subsequent recombination. More than 95% recombination efficiency in fibroblast cells, as well as murine embryonic stem cells, was achieved after His-TAT-NLS-Cre transduction. Efficient recombination could also be obtained in primary splenocytes ex vivo. We expect that application of His-TAT-NLS-Cre, which can be produced readily in large quantities from a bacterial source, will expand our abilities to manipulate mammalian genomes.
Conditional mutagenesis in mammalian cells has become an important means for the analysis of gene function in vivo (for review, see refs. 1–3). Site-specific recombinases such as the bacteriophage P1 recombinase Cre have been used to gain control over the mutation in a spatial (4–6) and/or temporal manner (7, 8). An increasing number of studies have demonstrated the efficacy of Cre-mediated conditional mutagenesis in mice and cell lines (9–12). Usually a mouse or cell line is generated, in which an essential part of the gene of interest is flanked by two loxP sites. The loxP sites represent Cre recombinase recognition sites and can be used to delete the respective gene segment upon Cre recombination, resulting in a conditioned inactivation or mutation of the gene of interest. To gain temporal control over this mutation event two different approaches have been applied: (i) Cre is delivered into cultured cells either by transfection (13) or adenoviral infection (14, 15); (ii) Cre recombinase activity is induced by application of an exogenous inducer. Induction can be carried out either at the transcriptional level [e.g., Mx-Cre (7) or tetracycline-controlled Cre expression (16)] or posttranslational level employing fusion proteins of Cre with mutated ligand-binding domains of steroid receptors (8). Although a number of studies demonstrated the efficacy of Cre-mediated inducible mutagenesis, still the system could profit substantially from technical advance concerning three major aspects: leakiness of the system before induction, efficiency of induced recombination, and requirement of extensive mouse breeding causing the experiments to be time consuming and costly. The leakiness of the system represents a critical factor because a Cre recombinase that is undesirably active before induction often leads to unwanted side effects such as mosaic recombination and/or selection of recombined or nonrecombined cells both in vivo and in vitro (9, 17, 18). Moreover, the widely used inducers IFN, hydroxy-tamoxifen, and doxycycline are known to display toxic side effects (19, 20) and/or induce also unwanted physiological effects that may interfere with the experimental phenotype of the conditional mutation to be analyzed (21, 22). In cultured cells, Cre-mediated recombination is limited by transfection efficiencies and putative toxicity of the protein (13, 23, 24). Thus, traditional delivery of Cre—either by Cre transgenics, viral vectors, or transfection—represents a limiting step of conditional mutagenesis employing Cre/loxP technology.
Protein transduction is a recently developed method to introduce biologically active proteins directly into mammalian cells with high efficiency (for review, see refs. 25 and 26). Recombinant technology has been used to modify biophysical properties of proteins, particularly with respect to their cell permeability, by employing so-called protein transduction domains (PTDs) (27–29). It has been demonstrated that a basic 11-aa peptide, derived from HIV-TAT, renders β-galactosidase (β-gal) into a cell-permeable form. β-gal activity can be detected in organs such as liver, kidney, lung, brain, and spleen of mice after i.p. injection of TAT-β-gal (28). Besides the basic TAT peptide, other peptides or protein (fragments) also have been reported to enhance cellular uptake of proteins, such as a hydrophobic peptide modified from Kaposi fibroblast growth factor (FGF) (29, 30). Recently it has been reported that a fusion protein of a nuclear localization signal (NLS), Cre recombinase, and a FGF peptide displays cell permeability. After direct application of the recombinant protein to cultured cells of a T lymphocyte line containing a loxP-modified substrate, Cre-mediated recombination was observed in ≈80% of the cells (31). However, it remained unclear from this study whether the FGF peptide is essential for transduction or even contributes significantly to the cellular uptake of Cre.
Our study was aimed at evaluating the actual potency of two prominent PTDs, namely FGF and TAT peptides, to promote the translocation of biologically active Cre across the plasma membrane of mammalian cells. We generated expression vectors encoding Cre recombinase fused to FGF and TAT peptides, respectively. The potentials of the recombinant proteins to transduce and subsequently recombine loxP-flanked targets in mammalian cells were compared side by side with Cre lacking any particular PTD.
Materials and Methods
Plasmid Constructions.
A TAT-encoding fragment was generated by PCR, using primers NPTatU and NeuUS. This fragment was cloned into the pTriEx-1 vector (Novagen) via _Nco_I and _Xho_I restriction sites, resulting in pTriEx-TAT-U-H (F.E. and K.R., unpublished data). This vector encodes, in addition to TAT, a second protein fragment designated as “U,” which was not used in this study. The corresponding DNA fragment was deleted by digestion with _Spe_I and subsequent religation, resulting in pTriEx-TAT-H. The TAT-encoding fragment was removed, resulting in pTriEx-H by _Pst_I digestion. To obtain pTriEx-CH, a Cre-encoding PCR product was generated from the template pPGK-Cre-bpA (32) by using primers 5H3cre and 3X1cre. This fragment was subsequently cloned into the pTriEx-H by using _Hin_dIII and _Xho_I sites. To generate pTriEx-NCH, a NLS-Cre-encoding fragment was amplified using primers 5H3NLScre and 3X1cre and cloned as above. To construct pTriEx-FNCH, annealed oligonucleotides 5PstFGF and 3PstFGF were cloned into the _Pst_I restriction site of pTriEx-NCH. To generate pTriEx-HTNC, a NLS-Cre-STOP encoding PCR product was generated using primers 5H3NLScre and 3creStopXho and subsequently cloned into pTriEx-TAT-H via _Hin_dIII and _Xho_I restriction sites; the His tag was introduced by cloning annealed oligonucleotides NcoHis5 and NcoHis3 into the _Nco_I site. pTriEx-HNCF was constructed by cloning annealed oligonucleotides NcoHis5 and NcoHis3, as well as 5XhoFGF and 3XhoFGF, into the _Nco_I and _Xho_I restriction sites, respectively, of pTriEx-NCH. From pTriEx-HTNC a 700-bp fragment was prepared using _Bam_HI/_Xho_I restriction sites and cloned into pTriEx-HNCF, resulting in pTriEx-HNC. All PCR-generated fragments were confirmed by sequencing. NPTatU (5′-CTTGGCCATGGGCGCTGCAGGTCGCAAGAAACGTCGCCAACGTCGCCGTCCGCCTGCAGGCACTAGTCAGATTTTCGTCAAGACTTTGAC-3′); NeuUS (5′-CCATTACTAGTTGCGCCCATACCACCACGTAGCCTTAGCACAAGATG-3′); 5H3cre (5′-CGTCCAAGCTTGTCCAATTTACTGACCGTACACC-3′); 3X1cre (5′-CTGAACTCGAGACCATCGCCATCTTCCAGCAGGC-3′); 5H3NLScre (5′-CATGGAAGCTTGAAGAAGAAGAGGAAGGTGTC-3′); 5PstFGF(5′-GTATTACTTCCGGTTCTGTTAGCGGCACCGGGTGCA-3′); 3PstFGF (5′-CCCGGTGCCGCTAACAGAACCGGAAGTAATACTGCA-3′); 3creStopXho (5′-CTAATCTCGAGCTAATCGCCATCTTCCAGCAG-3′); NcoHis5 (5′-CATGGGCCATCACCATCACCATCACGG-3′); NcoHis3 (5′-CATGCCGTGATGGTGATGGTGATGGCC-3′); 5XhoFGF (5′-TCGAGGGTGCAGCTGTATTACTTCCGGTTCTGTTAGCGGCACCGTGAT-3′); 3XhoFGF (5′-TCGAATCACGGTGCCGCTAACAGAACCGGAAGTAATACAGCTGCACCC-3′).
Expression and Purification of Recombinant Proteins.
pTriEx plasmids were used to transform Escherichia coli strain TUNER (DE3)pLacI (Novagen), allowing isopropyl β-D-thiogalactoside (IPTG)-inducible expression of His-tagged proteins. An enriched medium of LB + 1% Glucose containing 50 μg/ml carbenicillin and 34 μg/ml chloramphenicol was used to inoculate overnight cultures. Usually, 500-ml cultures of LB containing 100 μg/ml ampicillin and 34 μg/ml chloramphenicol were inoculated 1:50 and grown at 37°C until an OD600 of 0.7. Overexpression was induced by adding IPTG to a final concentration of 0.5 mM and an additional incubation of 3–4 h at 37°C. Cells were harvested by centrifugation and frozen in dry ice/ethanol and stored at −20°C. Cell pellets were thawed by resuspending in lysis buffer [100 mM NaH2PO4/10 mM Tris (pH 8.0)/300 mM NaCl/10 mM imidazole] at 5 ml per gram wet weight. Cleared lysate was obtained after incubation with 2 mg/ml lysozyme (Sigma) and benzonase (Novagen) and centrifugation for 25 min at 30,000 × g at 4°C. One milliliter of 50% Ni-NTA slurry (Qiagen) was added to 5 ml of cleared lysate and mixed gently by shaking at 4°C for 1 h. The slurry was packed into a column and washed with 10 bed volumes [100 mM NaH2PO4/10 mM Tris (pH 8.0)/300 mM NaCl/20 mM imidazole]. Recombinant protein was eluted [100 mM NaH2PO4/10 mM Tris (pH 8.0)/300 mM NaCl/250 mM imidazole] and either dialyzed against appropriate media (see below) for immediate use or frozen at −20°C for storage up to 8 weeks without significant loss of activity. Protein concentrations were measured using Bradford reagent (Sigma) and/or Warburg method. Cre activities were determined by a cell-free assay as described (31, 33), except for our use of 250 ng of substrate DNA (33). For quantification reactions were transformed into E. coli and colonies counted. A commercially available Cre protein (New England Biolabs) was used as a standard.
SDS/PAGE and Immunoblot Analysis.
Samples were separated by SDS/PAGE (10%), transferred to nitrocellulose membranes (Amersham Pharmacia), and probed with anti-Cre (Novagen) or anti-Penta-His (Qiagen) antiserum. Anti-Cre was incubated 1:5,000 in PBS containing 5% dry milk, and anti-Penta-His was used 1:2,000 in PBS containing 3% BSA. Blocking was carried out in corresponding buffers without antibody for 1 h at room temperature. As secondary antibodies anti-rabbit-POD (Vector Laboratories) or anti-mouse-POD conjugates (Amersham Pharmacia) were used both at a 1:10,000 dilution in PBS containing 5% milk powder. Blots were washed using PBST (PBS containing 0.5% Tween 20). Detection was carried out using chemiluminescence (ECL, Amersham Pharmacia).
Cell Culture, and Transfection of and Transduction into CV Fibroblasts.
CV1–5B (8) cells were cultured in DMEM containing glutamax and 10% FCS and 100 units/ml penicillin and 0.1 mg/ml streptomycin. Transfection experiments were carried out in 24-well plates by using 1 μg of plasmid as described (32). For Cre transduction experiments, 2 × 106 cells were plated on a 24-well plate and grown for 24 h, or until 90% confluency. Cre protein was dialyzed against a 1:1 mixture of DMEM and PBS containing 0.1% pluronic F-68 (Sigma) and sterilized by filtration, using a 0.2-μm filter disk (Millipore). Cells were incubated with Cre containing medium at indicated concentrations for indicated times. After transduction cells were washed using PBS and cultured 60 h in normal medium. For determination of β-gal activity, cells were fixed and stained as described (8). For Southern blot analysis genomic DNA was extracted from cells by proteinase K digestion and isopropanol precipitation. DNA was digested overnight by using _Eco_RI restriction enzyme and separated on an agarose gel. DNA was immobilized on a Hybond nitrocellulose membrane (Amersham Pharmacia) and probed with a 32P-labeled lacZ fragment. Quantification of bands was carried out by phosphor imaging technique (FUJIX BAS 1000; TINA 2.09 software, Raytest, Straubenhardt, Germany).
Immunofluorescence.
CV1–5B Fibroblasts were grown on 24-well tissue culture plates until 90% confluency. After a 4-h incubation in Cre fusion protein containing DMEM/PBS (1:1), cells were washed twice with PBS, trypsinized, and plated on coverslips. After 16 h in culture, cells were fixed with cold methanol (−20°C). The samples were washed three times with PBS and blocked for 30 min. Blocking and antibody incubations were carried out in PBS containing 1% dry milk. Antibody detection was performed using polyclonal rabbit anti-Cre antibody (Babco, Richmond, VA, 1:1,000 dilution; 90 min) and FITC-anti-rabbit IgG (Sigma, 1:50 dilution; 60 min). The nuclei were stained by adding 0.01 mg/ml 4,6-di-amino-2-phenylindol (DAPI) in PBS before mounting on glass slides. Cells were observed in an epifluorescence microscope (Zeiss Axiophot) and documented on color slide film. For reproduction images were scanned directly from slides.
Cre Transduction into Mouse Embryonic Stem Cells.
ES cell culture was carried out as described (13). Single-cell suspensions of subconfluent plates were prepared by trypsinization. Cells were washed in PBS and resuspended in Cre containing medium. Cre protein was dialyzed against a 1:1 mixture of DMEM and PBS and sterilized by filtration. After resuspension in Cre-containing medium, ES cells were plated on appropriate plates containing mitomycin C treated embryonic feeder cells. In a typical experiment 2.5 × 105 cells were used in a volume of 0.5 ml plated in a well of a 24-well plate. After transduction, cells were washed and cultured at least 3 days in normal ES medium. Genomic DNA was extracted and used for Southern blot analysis as described above, except the use of _Eco_RV digestion and a ROSA26-specific probe.
Ex Vivo Cre Transduction into Mouse Splenocytes.
For transduction into primary splenocytes we used polβ-flox (6) mice. Freshly prepared splenic cells were washed in PBS and resuspended in Cre-containing medium. Cre protein was dialyzed first against PBS, then against protein-free medium (HyClone, catalog no. SH 30349.01), and sterilized by filtration. Cells (5 × 106) were resuspended in 1 ml of Cre-containing medium and plated in a well of a 24-well plate. After transduction cells were harvested, resuspended in normal B cell medium (DMEM containing 10% FCS, 1 mM sodium pyruvate, 2 mM L-glutamine, 1× nonessential aa, 0.1 mM 2-β-mercaptoethanol; GIBCO) and plated in a well of a 24-well plate. After incubation for 16 h, genomic DNA was extracted and used for Southern blot analysis as described above, except for our use _Bam_HI digestion and a polβ-specific probe (6). Splenocytes were stained with fluorochrome-conjugated monoclonal antibodies [FITC; phycoerythrin (PE)]. Antibodies against CD19 (PharMingen) and Thy1.2(CFO1; in house) were used. For determination of cell viability propidium iodide (PI) staining was performed. FACS analysis was carried out using a FACScan (Becton Dickinson); for sorting a FACStar+ was used (Becton Dickinson).
Results and Discussion
Design of Expression Vectors, and Expression and Purification of Recombinant Cre Fusion Proteins.
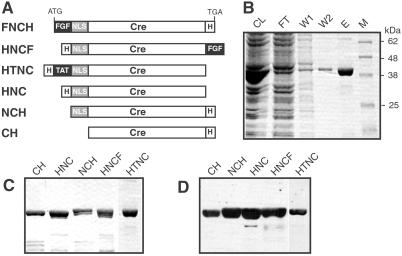
To evaluate the potency of the PTDs FGF and TAT to promote cellular uptake of Cre recombinase into mammalian cells we generated expression vectors encoding FNCH (FGF-NLS-Cre-His), HNCF (His-NLS-Cre-FGF), and HTNC (His-TAT-NLS-Cre) (Fig. 1A). All constructs include a His-tag for single-step purification of recombinant proteins from a bacterial source. As controls we also generated Cre expression constructs lacking any particular transduction domain, but carrying the His-tag either at the N terminus (His-NLS-Cre, abbreviated HNC) or C terminus (NLS-Cre-His, NCH) of Cre. Finally we generated the expression construct Cre-His (CH) which lacks NLS and encodes a C-terminally His-tagged Cre recombinase (Fig. 1A). Analysis of expression revealed that one recombinant protein, namely FNCH, displayed poor solubility under native conditions (data not shown), whereas the other recombinant proteins could be highly enriched under native conditions (Fig. 1 B and C). HTNC displayed reproducibly the highest level of expression (≈30 mg/l culture), whereas the other proteins showed expression levels of about 10 mg/l. All tested proteins were detected by antibodies directed against Cre (Fig. 1D) and the His-tag (data not shown), respectively, and displayed the expected size of ≈41 kDa. All proteins, except HTNC, are soluble in physiological buffers such as PBS up to a concentration of ≈12 μM (≈0.5 mg/ml). HTNC can be concentrated up to 16 μM (≈0.65 mg/ml).
Figure 1.
Design of expression cassettes and purification of Cre fusion proteins. (A) Six expression constructs were generated. All constructs encode Cre recombinase and a His-tag as represented by white boxes. Black boxes represent PTDs (aa sequence): FGF (AAVLLPVLLAAP) and TAT (GRKKRRQRRR). The gray box represents a NLS (KKKRKV) derived from SV40. Abbreviations of the constructs are given on the left. (B) SDS/PAGE analysis of the purification of HTNC employing Ni(II)-affinity chromatography. CL, cleared lysate; FT, flow through; W, wash fraction, E, eluted fraction; M, marker. (C) Comparison of purified fusion proteins on a Coomassie stained gel. (D) Immunoblot analysis of purified Cre fusion proteins employing an anti-Cre antibody.
FGF Peptide Is Dispensable for Transduction of His-NLS-Cre.
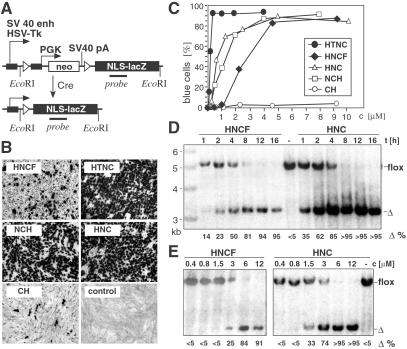
To assess the capacity of recombinant Cre fusion proteins to invade eukaryotic cells and subsequently perform recombination of a loxP-flanked substrate, we used the well established reporter cell line CV1–5B (8). This fibroblast cell line contains a single copy of a stably integrated reporter construct. Cre activity is monitored by deletion of a loxP-flanked stop segment resulting in the activation of a lacZ gene (Fig. 2A). Reporter cells were incubated for 16 h with medium containing 2.5 μM (≈100 μg/ml) of HNCF, HNC, NCH, CH, and HTNC. Indeed, β-gal activity was detected in cells incubated with fusion proteins containing a PTD, namely HNCF and HTNC, indicative of cellular uptake and subsequent recombination of the reporter gene. Unexpectedly, cells incubated with HNCF displayed only a low percentage of blue cells, whereas incubation of the control proteins lacking the PTD, namely HNC and NCH, resulted in a significantly higher percentage of stained cells (Fig. 2B). A dose–response analysis revealed that transduction of HNCF and HNC, as well as NCH and subsequent recombination, is strictly dependent on concentration and reaches a level of saturation of more than 90% efficiency at ≈5 μM (Fig. 2C). Half-maximal recombination is achieved for NCH and HNC between 1 and 2 μM, whereas ≈3–4 μM HNCF are needed for the same level of recombination. To demonstrate Cre-mediated recombination not only by activation of a reporter gene but also at the DNA level, we performed Southern blot analysis of Cre-transduced CV1–5B reporter cells. We compared HNCF side by side with HNC to directly assess the function of the FGF peptide. A time course experiment revealed that incubation of the cells with 12 μM HNCF for 1 h results in about 15% recombination efficiency. Half-maximum recombination is achieved after 4 h of incubation (Fig. 2D). Application of 12 μM HNC protein is more efficient; half-maximal recombination is achieved already after 1–2 h, and 8 h are sufficient to perform recombination in more than 95% of the cells. To assess the concentration dependency of transduction, we incubated reporter cells in serial dilutions of HNCF and HNC down to 0.4 μM. Southern blot analysis demonstrated that applications of up to 0.8 μM of both either HNCF or HNC did not result in any detectable recombination (Fig. 2E). However, application of 1.5 μM HNC resulted in substantial recombination (≈30%), whereas at the same concentration HNCF is almost inactive (≈2%). Recombination in almost every cell is achieved by using 6 μM HNC and 12 μM HNCF, respectively (Fig. 2E). Recently it has been reported that a cell permeable Cre, namely His-NLS-Cre-MTS, which is almost identical to the HNCF protein used in our study, can enter eukaryotic cells and perform recombination (31). However, the actual ability of FGF to promote this process was not investigated. Our comparative analysis of HNC and HNCF demonstrated that transduction of His-NLS-Cre and subsequent recombination is independent of the hydrophobic FGF peptide. Therefore, the FGF peptide is dispensable for efficient transduction of His-NLS-Cre. Moreover, the analysis of the time and concentration dependency of HNCF as compared with HNC demonstrated that application of a NLS-Cre fusion protein lacking the transduction peptide FGF is more effective.
Figure 2.
Transduction of Cre fusion proteins into a fibroblast Cre reporter cell line. (A) Schematic representation of the reporter gene construct of the fibroblast reporter cell line CV1–5B (8). Cre deletes a loxP-flanked segment, thereby activating a lacZ gene. (B) CV1–5B cells were incubated for 16 h in medium containing 2.5 μM (≈100 ng/μl) purified Cre fusion proteins as indicated; as a control, cells were incubated with normal medium. After incubation cells were washed, incubated with normal medium, fixed, and stained for β-gal activity. (C) Quantification of β-gal activities of reporter cells incubated for 16 h with various concentrations of Cre fusion proteins as indicated. Percentages of blue cells are given. (D and E) Southern blot analysis of transduction and subsequent recombination. (D) Time kinetics of HNCF and HNC transduction. Reporter cells were incubated with either 12 μM HNCF or HNC for indicated periods of time. (E) Concentration dependency of HNCF and HNC transduction. Reporter cells were incubated for 16 h with increasing concentrations of either HNCF or HNC, as indicated. Calculated deletion efficiencies in % are given at bottom (Δ%). flox, loxP-flanked gene; Δ, deleted gene.
NLS Promotes Cellular Uptake of Recombinant Cre.
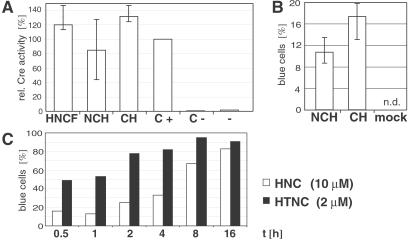
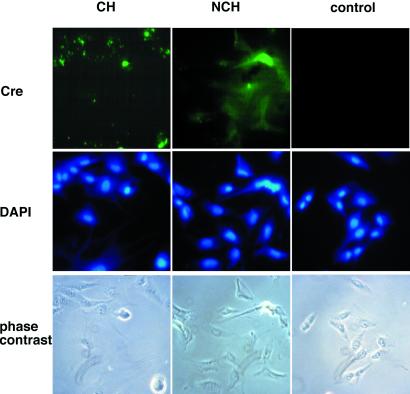
All recombinant Cre fusion proteins analyzed in this study resulted in highly efficient transduction and subsequent recombination, with one exception, namely CH (Fig. 2B). Recombination efficiency after transduction using CH did not increase over 5%, whereas NCH and HNC, the corresponding constructs carrying a NLS, resulted in efficiencies of more than 90% (Fig. 2C). There are three possible explanations for this observation: (i) the enzymatic activity of HNC and NCH, respectively, is higher than that of CH; this is unlikely because CH and NCH displayed comparable specific activities in a cell-free assay (Fig. 3A). (ii) Nuclear translocation of CH is inefficient because of the lack of the NLS resulting in a low recombination efficiency; this explanation is also unlikely because recombination efficiencies of NCH and CH after transfection were comparable (Fig. 3B), indicating that nuclear translocation is not a limiting factor. This observation is consistent with the previously published finding that addition of a NLS to Cre confers no increase of recombination (34). (iii) The NLS itself might function as a PTD or at least contributes to the cellular uptake of Cre fusion proteins. To investigate the function of NLS during cellular uptake independently of the recombination event we performed an immunohistochemical analysis. Localization of CH and NCH after transduction was monitored by fluorescence microscopy (Fig. 4). NCH was detectable in most of the cells both in the cytoplasm and nucleus, whereas in the case of the CH-treated cells only a low percentage was found to be Cre-positive. Moreover, in the latter case the Cre-specific signals were mainly concentrated in extracellular spot-like structures, whereas NCH staining was more diffuse, dispersing over the whole cell body.
Figure 3.
(A) Comparison of Cre recombinase activities of HNCF, NCH, and CH by using a cell-free activity assay. Eighty nanograms of either HNCF, NCH, or CH were used to recircularize a linearized substrate vector as described (31, 33). The specific activity of a commercially available control was set to 100% (C+). Further controls were: 80 ng heat-inactivated Cre protein (C−) and no protein (−). Vertical lines represent ranges of values. (B) Quantitative analysis of recombinase activities of NCH and CH after transfection. CV1–5B cells were transfected with corresponding pTriEx expression vectors or a mock control and stained for β-gal activity. Given are mean values of three transfections. n.d., not detectable. (C) Comparison of the time dependency of HNC and HTNC transduction. CV1–5B cells were incubated with either 10 μM HNC or 2 μM HTNC between 30 min and 16 h, as indicated. After incubation, cells were washed, incubated with normal medium, stained for β-gal activity, and counted. Percentages of blue cells are given.
Figure 4.
Detection of Cre in cells after transduction employing an anti-Cre antibody. CV1–5B cells were incubated for 4 h with 5 μM of either CH or NCH or medium only as a control. After incubation, cells were examined for immunofluorescence by using anti-Cre antiserum or by phase contrast microscopy. Cells were counterstained using 4,6-di-amino-2-phenylindol (DAPI). See Materials and Methods for details.
TAT Peptide Enhances Transduction of His-NLS-Cre.
The comparative analysis of all proteins analyzed in this study demonstrated that the TAT peptide containing HTNC is the most effective protein in transduction and subsequent recombination. One can achieve recombination in more than 90% of fibroblast reporter cells with only 0.5 μM HTNC, whereas ≈5 μM of HNC is necessary to achieve the same level of recombination within the same time (Fig. 2C). Moreover, using HTNC shorter incubation times as compared with HNC are needed to obtain the same recombination efficiency (Fig. 3C). Incubation with 2 μM HTNC for 30 min resulted in about 50% recombination; incubation with 10 μM HNC induced recombination only in ≈15% of the cells within the same time. Highly efficient recombination of about 80% was achieved after 2 h incubation with HTNC, whereas 16 h were needed to obtain the same level of recombination when using HNC (Fig. 3C).
Transduction into Embryonic Stem Cells and Splenocytes ex Vivo.
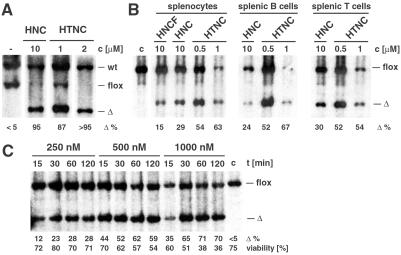
To evaluate further the use of HTNC for genetic engineering we tested transduction into murine ES cells. We used the RDR-IB1N cell line containing a loxP-modified substrate in the ROSA26 locus (F. T. Wunderlich, F.E., and K.R., unpublished data). We incubated ES cells in either HNC- or HTNC-containing medium for 20 h. Southern blot analysis of genomic DNA demonstrated that Cre-mediated recombination was achieved in almost every cell when using 2 μM HTNC (Fig. 5A). Eighty-seven-percent deletion was achieved by using only 1 μM HTNC. A 10-fold higher concentration of HNC was necessary to obtain a similar level of deletion. No difference in colony numbers were observed 2 days after transduction between the cell suspensions incubated with 10 μM HNC and 1 μM HTNC, respectively, as compared with control cells incubated in normal medium. A significant decrease of colony numbers was observed in the case of cells treated with more than 2 μM HTNC, indicative of a cytotoxic effect of HTNC at high concentrations. Indeed, growth of cells was almost completely inhibited in the presence of 5 μM HTNC. However, at concentrations that are sufficient to induce deletion in about 90% of the cells (1–2 μM HTNC) no growth inhibition was observed; recombination efficiencies of more than 95% after either HTNC (2 μM) or HNC (10 μM) transduction were reproducibly confirmed also by employing a different ES-cell line containing a loxP-substrate (data not shown). Additionally, we also wanted to assess the potential of HTNC transduction into resting cells such as primary splenocytes. We therefore incubated splenocytes of mice carrying a loxP-flanked segment in the 5′ region of the polβ gene (6) either in HNCF-, HNC-, or HTNC-containing medium. After 60 min of incubation cells were incubated for additional 16 h in normal medium providing time for Cre-mediated recombination. Cells were then sorted into B and T cells. Southern blot analysis of extracted genomic DNAs demonstrated that treatment with 10 μM HNCF results in 15% deletion in total splenocytes (Fig. 5B Left). As already observed in fibroblasts, the same concentration of HNC is more efficient (29%). However, the most efficient recombination was obtained with 1 μM HTNC (63%). Similar values were obtained from sorted B and T cells (Fig. 5B Middle and Right). 10 μM HNC resulted in 20–30% recombination, whereas a 10-fold lower concentration of HTNC induced Cre-mediated recombination in 52–67% of the B and T cells. Even treatment with only 500 nM of HTNC resulted in more than 50% recombination in either total splenocytes, or sorted B or T cells (Fig. 5B). To assess the time dependency of HTNC transduction and the viability of the treated cells we incubated splenocytes for 15, 30, 60, and 120 min in 250 nM, 500 nM, and 1 μM HTNC (Fig. 5C). Southern blot analysis revealed that substantial recombination occurred already after 15 min incubation (12–44% recombination, depending on the concentration). Recombination efficiency increased with longer incubation times and reached saturation between 30 and 60 min. No further increase was observed after 120 min. The highest recombination efficiency was obtained after 60 min of incubation with 1 μM HTNC (71%); cell viability of this population was 38%. Higher viabilities were obtained with lower concentrations: after 60 min in 500 nM HTNC 57% of the splenocytes were still viable and 70% viability was determined after incubation with 250 nM HTNC; this value is comparable to cells cultured in normal medium displaying a viability of 75% (Fig. 5C).
Figure 5.
HTNC transduction into (A) murine embryonic stem cells and (B and C) splenocytes ex vivo analyzed by Southern blot. (A) RDR-IB1N ES cells were incubated for 20 h in medium containing indicated concentrations of either HNC or HTNC. After 3 additional days of incubation in normal ES medium, genomic DNAs were examined by Southern blot analysis. (B) Splenocytes of a polβ-flox/flox mouse (6) were incubated for 60 min in medium containing indicated concentrations of either HNCF, HNC, or HTNC. After transduction, cells were incubated for 16 h in normal medium. Shown is Southern blot analysis of sorted B cells (CD19+/PI−), T cells (Thy1.2+/PI−), and total splenocytes. (C) Splenocytes of a polβ-flox/flox mouse were incubated with medium containing 250 nM, 500 nM, and 1000 nM of HTNC for indicated periods of time. Calculated deletion efficiencies (Δ%) and cell viabilities in % are given at the bottom. wt, wild type allele; flox, loxP-flanked allele; Δ, deleted allele.
Conclusions
The aim of this study was to evaluate the potency of two prominent PTDs, namely the hydrophobic FGF and the basic TAT peptide, to promote cellular uptake of recombinant Cre. Transduction of His-NLS-Cre and subsequent recombination of a loxP-modified substrate in reporter cells turned out to be independent of the FGF peptide. This may also be true for the His-NLS-Cre-MTS fusion protein, which has recently been reported to allow Cre transduction (31) and is very similar to the HNCF protein used in the present experiments. If not the FGF peptide, what else drives the translocation of the His-NLS-Cre across the cellular membrane? The only recombinant protein analyzed in this study displaying no or weak transduction potential was Cre-His—i.e., the only construct without NLS. From this observation we conclude that the basic NLS (see legend of Fig. 1A) itself can function as a PTD at least in the context of Cre recombinase. This conclusion is consistent with the observation that basic peptides in general are able to enhance cellular uptake of heterologous proteins or peptides (35–37). We further extended this observation by generating a fusion of NLS-Cre with the longer basic TAT-derived peptide, known to act efficiently as a PTD in some experimental settings (26–28). Indeed, our studies demonstrated that the TAT-modified variant His-TAT-NLS-Cre transduced much more efficiently than His-NLS-Cre. More than 90% of fibroblast cells undergo Cre-mediated recombination after treatment with 500 nM HTNC; a 10-fold higher concentration of HNC is needed to obtain the same level of recombination. HTNC transduction is more efficient also in murine embryonic stem cells (5–10-fold) and primary splenocytes (10–20-fold) as compared with HNC. Thus, application of HTNC turns out to be a powerful tool to rapidly and efficiently perform Cre-mediated recombination in cultured mammalian cells.
To examine the potential of HTNC transduction in vivo as well, we performed a series of experiments in mice employing HTNC, HNC, and HNCF. To rule out the possibility that an indirect read-out system (e.g., Cre-mediated activation of a reporter gene) could lead to an overestimation of the actual recombination efficiency, we decided to directly assess the recombination product at the DNA level by Southern blot analysis. Although three different mouse lines each containing different loxP-modified loci were used, we did not detect any significant Cre-mediated recombination by Southern blot after i.p. injection of the same amount as described (31) of HTNC, HNC, and HNCF, respectively. We observed only a weak signal representing ≈10% deletion in a single mouse in the peritoneum close to the site of injection (data not shown). We thus failed to induce substantial Cre-mediated recombination in mice by Cre transduction—not even when using the very efficient HTNC protein. It has recently been reported that i.p. administration of His-NLS-Cre-MTS, almost identical to HNCF, results in efficient recombination in mice as well (31). In this study, recombination was determined indirectly by activation of a LacZ reporter gene. We infer that there might be some recombination activity in vivo, especially close to the site of injection. This activity could be strong enough to activate reporter genes in some experimental settings, but seems insufficient to result in recombination of the loxP targets in the majority of the cells.
In conclusion, in our collection of Cre fusions HTNC turned out to be most efficiently transducible into mammalian cells. Every cell line examined in this study, either primary cells ex vivo or stable cell lines, could be modified genetically by HTNC transduction at low, seemingly nontoxic concentrations, with at least a 50% (and up to 95%) efficiency, depending on the cell type. We expect that HTNC transduction will be applicable for a much larger variety of cells, thereby overcoming limitations of conventional techniques such as transfection and adenoviral infection. Because HTNC can be readily produced in and purified from E. coli in large quantities, we expect HTNC to serve as a powerful tool for genetic manipulation in mammalian cells.
Acknowledgments
We are indebted to G. Schütz for providing the reporter cell line CV1–5B. We thank F. T. Wunderlich, R. Kühn, F. Schwenk, M. Alimzhanov, N. Uyttersprot, and A. Waisman for helpful discussions, and C. Goettlinger for technical help. This work was supported by funds from the Volkswagen Foundation (I/76353), the European Union (QLG1-1999-00202), and Artemis Pharmaceuticals, Cologne, Germany.
Abbreviations
aa
amino acid(s)
β-gal
β-galactosidase
ES
embryonic stem cell(s)
FGF
fibroblast growth factor
NLS
nuclear localization signal
PTD
protein transduction domain
References
- 1.Rajewsky K, Gu H, Kühn R, Betz U A K, Müller W, Roes J, Schwenk F. J Clin Invest. 1996;98:S51–S53. doi: 10.1172/JCI118828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nagy A. Genesis. 2000;26:99–109. [PubMed] [Google Scholar]
- 3.Lewandoski M. Nat Rev Genet. 2001;2:743–755. doi: 10.1038/35093537. [DOI] [PubMed] [Google Scholar]
- 4.Orban P C, Chui D, Marth J D. Proc Natl Acad Sci USA. 1992;89:6861–6865. doi: 10.1073/pnas.89.15.6861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lakso M, Sauer B, Mosinger B, Jr, Lee E J, Manning R W, Yu S H, Mulder K L, Westphal H. Proc Natl Acad Sci USA. 1992;89:6232–6236. doi: 10.1073/pnas.89.14.6232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gu H, Marth J D, Orban P C, Mossmann H, Rajewsky K. Science. 1994;265:103–106. doi: 10.1126/science.8016642. [DOI] [PubMed] [Google Scholar]
- 7.Kühn R, Schwenk F, Aguet M, Rajewsky K. Science. 1995;269:1427–1429. doi: 10.1126/science.7660125. [DOI] [PubMed] [Google Scholar]
- 8.Kellendonk C, Tronche F, Monaghan A P, Angrand P O, Stewart A F, Schütz G. Nucleic Acids Res. 1996;24:1404–1411. doi: 10.1093/nar/24.8.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kellendonk C, Tronche F, Casanova E, Anlag K, Opherk C, Schütz G. J Mol Biol. 1999;285:175–182. doi: 10.1006/jmbi.1998.2307. [DOI] [PubMed] [Google Scholar]
- 10.Kulkarni R N, Bruning J C, Winnay J N, Postic C, Magnuson M A, Kahn C R. Cell. 1999;96:329–339. doi: 10.1016/s0092-8674(00)80546-2. [DOI] [PubMed] [Google Scholar]
- 11.Li M, Indra A K, Warot X, Brocard J, Messaddeq N, Kato S, Metzger D, Chambon P. Nature (London) 2000;407:633–636. doi: 10.1038/35036595. [DOI] [PubMed] [Google Scholar]
- 12.Maruyama M, Lam K P, Rajewsky K. Nature (London) 2000;407:636–642. doi: 10.1038/35036600. [DOI] [PubMed] [Google Scholar]
- 13.Torres R M, Kühn R. Laboratory Protocols for Conditional Gene Targeting. Oxford: Oxford Univ. Press; 1997. [Google Scholar]
- 14.Rohlmann A, Gotthardt M, Willnow T E, Hammer R E, Herz J. Nat Biotechnol. 1996;14:1562–1565. doi: 10.1038/nbt1196-1562. [DOI] [PubMed] [Google Scholar]
- 15.Shibata H, Toyama K, Shioya H, Ito M, Hirota M, Hasegawa S, Matsumoto H, Takano H, Akiyama T, Toyoshima K, et al. Science. 1997;278:120–123. doi: 10.1126/science.278.5335.120. [DOI] [PubMed] [Google Scholar]
- 16.Utomo A R, Nikitin A Y, Lee W H. Nat Biotechnol. 1999;17:1091–1096. doi: 10.1038/15073. [DOI] [PubMed] [Google Scholar]
- 17.Minamino T, Gaussin V, DeMayo F J, Schneider M D. Circ Res. 2001;88:587–592. doi: 10.1161/01.res.88.6.587. [DOI] [PubMed] [Google Scholar]
- Fuhrmann-Benzakein, E., Garcia-Gabay, I., Pepper, M. S., Vassalli, J. D. & Herrera, P. L. (2000) Nucleic Acids Res.28, e99. [DOI] [PMC free article] [PubMed]
- 19.Vasioukhin V, Degenstein L, Wise B, Fuchs E. Proc Natl Acad Sci USA. 1999;96:8551–8556. doi: 10.1073/pnas.96.15.8551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Danielian P S, Muccino D, Rowitch D H, Michael S K, McMahon A P. Curr Biol. 1998;8:1323–1326. doi: 10.1016/s0960-9822(07)00562-3. [DOI] [PubMed] [Google Scholar]
- 21.Kuzin I I, Snyder J E, Ugine G D, Wu D, Lee S, Bushnell T, Jr, Insel R A, Young F M, Bottaro A. Int Immunol. 2001;13:921–931. doi: 10.1093/intimm/13.7.921. [DOI] [PubMed] [Google Scholar]
- 22.Lin Q, Dong C, Cooper M D. J Exp Med. 1998;187:79–87. doi: 10.1084/jem.187.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loonstra A, Vooijs M, Beverloo H B, Allak B A, van Drunen E, Kanaar R, Berns A, Jonkers J. Proc Natl Acad Sci USA. 2001;98:209–214. doi: 10.1073/pnas.161269798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Silver D P, Livingston D M. Mol Cell. 2001;8:233–243. doi: 10.1016/s1097-2765(01)00295-7. [DOI] [PubMed] [Google Scholar]
- 25.Hawiger J. Curr Opin Chem Biol. 1999;3:89–94. doi: 10.1016/s1367-5931(99)80016-7. [DOI] [PubMed] [Google Scholar]
- 26.Schwarze S R, Ho A, Hruska K A, Dowdy S F. Trends Cell Biol. 2000;10:290–295. doi: 10.1016/s0962-8924(00)01771-2. [DOI] [PubMed] [Google Scholar]
- 27.Nagahara H, Vocero-Akbani A M, Snyder E L, Ho A, Latham D G, Lissy N A, Becker-Hapak M, Ezhevsky S A, Dowdy S F. Nat Med. 1998;4:1449–1452. doi: 10.1038/4042. [DOI] [PubMed] [Google Scholar]
- 28.Schwarze S R, Ho A, Vocero-Akbani A, Dowdy S F. Science. 1999;285:1569–1572. doi: 10.1126/science.285.5433.1569. [DOI] [PubMed] [Google Scholar]
- 29.Rojas M, Donahue J P, Tan Z, Lin Y Z. Nat Biotechnol. 1998;16:370–375. doi: 10.1038/nbt0498-370. [DOI] [PubMed] [Google Scholar]
- 30.Lin Y Z, Yao S Y, Veach R A, Torgerson T R, Hawiger J. J Biol Chem. 1995;270:14255–14258. doi: 10.1074/jbc.270.24.14255. [DOI] [PubMed] [Google Scholar]
- 31.Jo D, Nashabi A, Doxsee C, Lin Q, Unutmaz D, Chen J, Ruley H E. Nat Biotechnol. 2001;19:929–933. doi: 10.1038/nbt1001-929. [DOI] [PubMed] [Google Scholar]
- Wunderlich, F. T., Wildner, H., Rajewsky, K. & Edenhofer, F. (2001) Nucleic Acids Res.29, e47. [DOI] [PMC free article] [PubMed]
- 33.Cantor E J, Chong S. Protein Expression Purif. 2001;22:135–140. doi: 10.1006/prep.2001.1428. [DOI] [PubMed] [Google Scholar]
- 34.Le Y, Gagneten S, Tombaccini D, Bethke B, Sauer B. Nucleic Acids Res. 1999;27:4703–4709. doi: 10.1093/nar/27.24.4703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wender P A, Mitchell D J, Pattabiraman K, Pelkey E T, Steinman L, Rothbard J B. Proc Natl Acad Sci USA. 2000;97:13003–13008. doi: 10.1073/pnas.97.24.13003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matsushita M, Tomizawa K, Moriwaki A, Li S T, Terada H, Matsui H. J Neurosci. 2001;21:6000–6007. doi: 10.1523/JNEUROSCI.21-16-06000.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Han K, Jeon M J, Kim S H, Ki D, Bahn J H, Lee K S, Park J, Choi S Y. Mol Cells. 2001;12:267–271. [PubMed] [Google Scholar]