Jagged1 signals in the postnatal subventricular zone are required for neural stem cell self-renewal (original) (raw)
Abstract
Neural stem cells (NSCs) in the postnatal mammalian brain self-renew and are a source of neurons and glia. To date, little is known about the molecular and cellular mechanisms regulating the maintenance and differentiation of these multipotent progenitors. We show that Jagged1 is required by mitotic cells in the subventricular zone (SVZ) and stimulates self-renewal of multipotent epidermal growth factor-dependent NSCs. Jagged1-expressing cells line the adult SVZ and are juxtaposed to Notch1-expressing cells, some of which are putative NSCs. In vitro, endogenous Jagged1 acts through Notch1 to promote NSC maintenance and multipotency. In vivo, reducing Jagged1/Notch1 signaling decreases the number of proliferating cells in the SVZ. In addition, soluble Jagged1 promotes self-renewal and neurogenic potential of multipotent neural progenitors in vitro. Our findings suggest a central role for Jagged1 in the NSC niche in the SVZ for maintaining a population of NSCs in the postnatal brain.
Keywords: adult neural stem cells, jagged signaling, neurogenesis
Introduction
Many adult mammalian tissues contain stem cells in niches with complex microenvironments that provide the appropriate determination and differentiation signals for the formation of new cells (van der Kooy and Weiss, 2000; Watt and Hogan, 2000; Blau et al, 2001; Chu and Gage, 2001; Price and Williams, 2001; Toma et al, 2001; Weissman et al, 2001; Wagers et al, 2002; Alvarez-Buylla and Lim, 2004). In the adult mammalian central nervous system (CNS), a population of neural stem cells (NSCs) resides in the periventricular area of the forebrain lateral ventricles (subventricular zone (SVZ)) (Reynolds and Weiss, 1992; Morshead et al, 1994). These NSCs display the principal characteristics of multipotency and self-renewal. In the rodent brain, SVZ is the source of neuroblasts that converge and migrate in chains from the lateral walls of the forebrain along defined ‘glial tubes' to the olfactory bulb throughout life (Alvarez-Buylla and Temple, 1998). Upon reaching the olfactory bulb, the neuroblasts differentiate into granule and periglomerular interneurons.
In the SVZ, four cell types have been defined based on their morphology. Under the ependymal cells lining the lateral ventricle resides a population of periventricular astrocytes (B cells) (reviewed by Doetsch, 2003), and elegant use of transgenic and retroviral approaches suggests that some of these B cells have NSC properties (Doetsch et al, 1999). The B cells are proposed to undergo asymmetric cell divisions to generate transient amplifying cells (C cells). C cells proliferate rapidly, and undergo a limited number of cell cycles before generating neuroblasts (A cells) (reviewed by Doetsch, 2003). Although recent data indicate that glial cells both in rodents as well as in other species can have stem cell-like properties (Malatesta et al, 2000; Noctor et al, 2001), the identity of the mammalian SVZ stem cell remains to be determined unequivocally.
The identification and characterization of mammalian NSCs in vivo has proven to be problematic due to the lack of specific markers. The most commonly used method for identifying NSCs is their self-renewal capacity and multipotency in neurosphere cultures. Within the neurospheres, NSCs self-replicate, and can be induced to differentiate into neurons, astrocytes and oligodendrocytes. In vitro, postnatal SVZ-derived NSCs are dependent upon epidermal growth factor (EGF) (Morshead et al, 1994; Doetsch et al, 1999; Tropepe et al, 1999). Recent data imply that neurospheres can be derived from transient amplifying cells under the influence of EGF (Doetsch et al, 2002).
Notch signaling has been shown to be involved in the maintenance and differentiation of mammalian embryonic neural progenitors in the neural tube (de la Pompa et al, 1997; Hitoshi et al, 2002; Lutolf et al, 2002). Ectopic activation of Notch signaling in the neural tube results in expansion of neural progenitors at the expense of neurogenesis (Lardelli et al, 1996; Gaiano et al, 2000). However, little is known about the mechanisms that control NSC maintenance and differentiation in the postnatal brain. Notch1 is prominently expressed by putative NSCs in the neurogenic regions of the adult mouse brain (Stump et al, 2002; Irvin et al, 2004) and antisense experiments suggest a role for Notch1 in the maintenance of embryonic derived NSC (Chojnacki et al, 2003).
Jagged1 is a member of the Serrate/Jagged family of canonical Notch ligands (Lindsell et al, 1995), and is widely expressed during development and in the adult CNS (Lindsell et al, 1996; Stump et al, 2002; Irvin et al, 2004). Although data suggest that Jagged1/Notch signaling may regulate oligodendrocyte maturation and myelination in the murine brain (Wang et al, 1998; Genoud et al, 2002), its role in the CNS is not clear due to the early embryonic lethality of Jagged1-deficient mice (Xue et al, 1999).
We have shown previously that Jagged1 is expressed in the neurogenic regions of the postnatal brain (Stump et al, 2002). Now we have addressed the role of Jagged1 signaling in the postnatal SVZ and NSC. We found that Jagged1 is expressed by cells in the SVZ and rostral migratory stream (RMS), and is required for maintained proliferation in both regions of the postnatal mouse brain. Additionally, we show that loss of Jagged1 function causes a loss of self-replicating NSCs in vitro. Finally, Jagged1 treatment of NSCs in vitro can substitute for mitogens such as EGF and increase the neurogenic potential. Our experiments identify Jagged1 as a key regulator of NSC self-renewal and differentiation potential within the postnatal brain.
Results
Notch1 and Jagged1 are expressed in the SVZ
To address whether adult-type mammalian NSCs are regulated by Notch signaling, we analyzed the location of Notch1-expressing cells in the mouse SVZ. In situ mRNA hybridization (Supplementary Figure 1A), and immunohistochemistry (Supplementary Figure 1B) using an affinity-purified antibody raised against Notch1 (Stidworthy et al, 2004) revealed Notch1-expressing cells clustered in the SVZ under and within the ventricle lining. In addition, using affinity-purified antibodies, we identified Jagged1-expressing cells lining the lateral ventricle walls adjacent to the SVZ (Figure 1A). These Jagged1-expressing cells are located in and just under the ependymal cell layer (Figure 1B, arrows) and project processes into clusters of tightly packed cells within the SVZ (Figure 1B and H, arrowheads).
Figure 1.
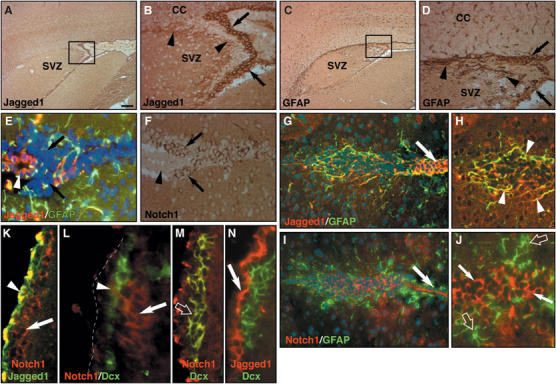
Notch1 and Jagged1 are expressed in the SVZ. (A) Jagged1 is expressed by cells lining the SVZ. (B) Jagged1-expressing cells are associated with the subependymal cells (arrows) and project processes into the SVZ (arrowheads and H). (C) GFAP immune-reactivity is localized to the subependymal cells lining the lateral ventricles (and arrows in G). (D) GFAP-expressing cells extend processes into the SVZ similar to Jagged1-expressing cells (arrowheads and in H). (E) Jagged1 (red) colocalizes with GFAP (green) in cells lining the SVZ (arrowhead) and adjacent to cells clustered close to the ventricular lining (nuclei stained with DAPI, arrows; blue). (F) Consecutive sagittal section to that in E stained with anti-Notch1 antibodies (arrows; brown). Tightly packed, Notch1-positive clusters of cells lie adjacent to the Jagged1/GFAP-positive cells (arrowhead). (G) Double immunostaining shows Jagged1 (red)/GFAP (green)-expressing cells lining the lateral ventricle (arrow) and projecting processes into the SVZ. (H) Higher magnification of the Jagged1 (red)/GFAP (green) double-positive processes (arrowheads) intermingled between clusters of tightly packed cells. (I) Notch1 and GFAP immunostaining on a section consecutive to that in G and H (arrow; ventricle lining). (J) Notch1-expressing cells (arrows) are clustered in the SVZ and adjacent to GFAP-positive cells (open arrows). (K) Notch1-positive cells (arrow) in the subependymal layer of the SVZ do not express Jagged1, whereas the ventricle lining (arrowhead) stains for both Notch1 and Jagged1. (L) Most Notch1-positive cells (arrow) in the caudal SVZ do not express Doublecortin (Dcx; green). However, some Notch1 is occasionally detected at low levels in a few Dcx-positive neuroblasts (arrowhead) under the ependymal lining (dotted line). (M) Dcx expressing neuroblasts in the medial SVZ and RMS express Notch1 (open arrow). (N) Jagged1 (arrow) does not overlap with Dcx expression in the SVZ or RMS. Scale bar in A=500 μm for panels A and C, 50 μm for panels B, D, G, and I, 25 μm for E, F, H, and J, 15 μm for K, and 10 μm for M and N and 5 μm for L.
Immunostaining with anti-glial fibrillary acid protein (GFAP) antibodies identified astrocytes throughout the brain and lining the SVZ (Figure 1C and D, arrows) that project processes into the underlying neurogenic region (Figure 1D and H, arrowheads), similar to Jagged1-expressing cells. In addition, we observed Jagged1-, GFAP- and Notch1-expressing cells along the RMS (data not shown). Double immunofluorescence with anti-Jagged1 and anti-GFAP antibodies revealed coexpression in cells lining the SVZ (Figure 1E, arrowhead) and in processes projecting through clusters of tightly packed cells within the SVZ (Figure 1E, G and H). Immunostaining on consecutive 5-μm paraffin sections showed that some of these clustered cells express Notch1 (Figure 1F, arrows). We then examined the relationship between Jagged1- and Notch1-expressing cells. We performed immunostaining on consecutive sections of adult mouse SVZ with antibodies to Jagged1, GFAP, and Notch1. We found Jagged1 to be prominently expressed by GFAP-positive cells in the SVZ (Figure 1H, arrowheads) and within the ventricular lining (Figure 1G, arrow). Notch1 is expressed by clustered cells (Figure 1I and J, arrows) that are adjacent to GFAP-positive and presumably Jagged1-expressing cells (Figure 1I and J, open arrows). Consecutive immunostaining for Jagged1 and Notch1 revealed that Notch1-expressing cells in the SVZ do not express detectable levels of Jagged1 (Figure 1K, arrow), whereas some coexpression is seen in the ventricle lining (Figure 1K, arrowhead).
We then addressed whether the clustered Notch1-expressing cells also expressed the neuroblast marker Doublecortin (Dcx). Immunostaining showed Dcx-expressing cells in the rostral SVZ and along the RMS (Supplementary Figure 1C–E). Therefore, we analyzed Dcx expression in the caudal SVZ, the region where the majority of the Notch1-positive cells are found. Most of the Notch1-positive cells in the subependymal region of the caudal SVZ were clustered near to, but did not express, Dcx (Figure 1L, arrow). Conversely, the majority of the Dcx-positive cells migrating under the ependymal lining did not express Notch1 (Figure 1L). Occasionally however, we did find a small number of Notch1-positive cells in the caudal SVZ that expressed low levels of Dcx (Figure 1L, arrowhead). Therefore, we addressed whether Notch1 expression in the proximal RMS overlaps with Dcx expression in migratory neuroblast. Indeed, in the proximal and medial RMS, many Notch1-positive cells coexpressed Dcx (Figure 1M, open arrow) although most of the Dcx-expressing cells in the distal RMS did not express Notch1 (Supplementary Figure 1D–F). Jagged1 expression never overlapped with Dcx, whether in the SVZ or RMS (Figure 1N, arrow).
Compound reductions in Jagged1 and Notch1 results in reduced mitosis in the SVZ
To address the function of Jagged1 in the postnatal brain, we generated a conditional allele for Jagged1 (Figure 2A). The first and second coding exons of the Jagged1 gene were flanked with LoxP sequences (Supplementary data). Floxed Jagged1 mice are indistinguishable from control littermates and show no anatomical defects (data not shown). A null allele for Jagged1 was generated by breeding floxed Jagged1 mice with CMV-Cre deleter mice. Mice hemizygous for the _Jagged1_-null allele were identified by PCR (Figure 2B) and showed no obvious anatomical defects in the CNS, whereas mice homozygous for the _Jagged1_-null alleles died in utero as described previously (Xue et al, 1999).
Figure 2.
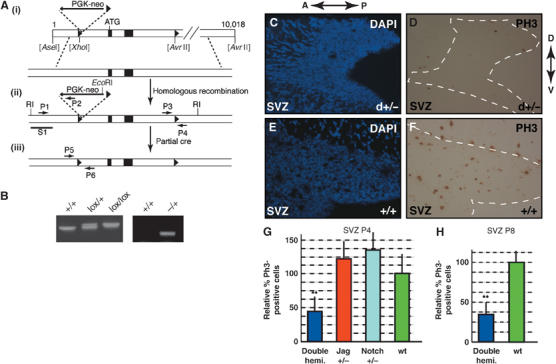
Jagged1 and Notch1 regulate proliferation in the SVZ. (A) Schematic representation of the Jagged1 locus and targeting construct (see Supplementary data). (i–iii) A PGK-neo selection marker flanked by two loxP sites, as well as a third loxP site, were inserted as shown. Black boxes indicate exons 1 and 2, ATG the start codon, and the black triangles the loxP sites. (ii) Homologous recombinants were detected by PCR with primers P1–6, and by Southern blotting of genomic DNA using probe S1. (iii) The PGK-neo cassette was removed by transient Cre-recombinase expression in ES cells. (B) Floxed Jagged1 animals were genotyped with primers P3 and P4 to detect floxed (300 bp) and wt loci (250 bp), and the _Jagged1_-null allele carrying mice with primers P1 and P4. (C, D) At P4, mice double hemizygous for Jagged1 and _Notch1_-null alleles (d+/−) have a reduction in PH3-positive prophase cells (C—DAPI, D—PH3) in the SVZ (outlined with a dotted line) compared to wt littermates (E—DAPI, F—PH3). (G) Quantification revealed a highly significant (**Student's _t_-test P<0.001), approximately 60% reduction in PH3-positive cells in the SVZ of double-hemizygous mutants (_n_=3) compared to wt littermates (_n_=3) at P4. (**H**) P8 double-hemizygous mutants showed >60% reduction in PH3-positive cells compared to wt littermates (**Student's _t_-test P<0.001). Anterior–posterior (A–P) and dorsal–ventral (D–V) axis is shown for panels C–F.
Mammalian Notch signaling has been shown to be sensitive to gene dosage (McCright et al, 2002). Therefore, we reduced Jagged1 and Notch1 signaling in vivo by intercrossing Jagged1 and Notch1 hemizygous null mice to generate animals lacking one Jagged1 and one Notch1 allele (double hemizygotes). Hemizygous _Notch1_-null mice are viable and have no obvious defects (Radtke et al, 1999). Double-hemizygous mice were born but, in contrast to single-hemizygous mutants, the majority died within a few hours of birth and only 10% survived up to 8 days. We performed histological analyses and immunostaining on the brains of postnatal day four (P4) and P8 hemizygous mutant mice. At P4 the brains of double-hemizygous mutants and control wild-type (wt) mice were anatomically similar and histological stains did not reveal obvious abnormalities in the SVZ and RMS (data not shown). To address changes in mitotic cell activity, we performed antiphosphohistone III (PH3) staining and analyzed the proportion of cells in the M phase of the cell cycle within the SVZ (Figure 2C–F) and RMS (Figure 3A–D) of mutant and control mice.
Figure 3.
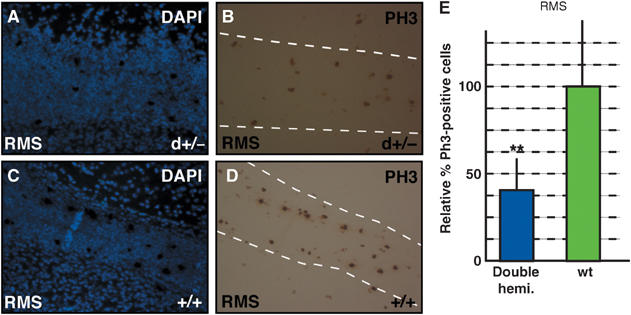
Jagged1 and Notch1 regulate proliferation in the RMS. (A–D) P4 mice double hemizygous for Jagged1 and _Notch1_-null alleles (d+/−) show a reduction of PH3-positive cells in the RMS (outlined with a dotted line) compared to wt (+/+) littermates. (E) Quantification revealed a highly significant (**Student's _t_-test P<0.001) reduction in proliferating cells along the RMS of double-hemizygous mutants compared to wt control littermates at P4.
At P4, double-hemizygous mice displayed a highly significant (Student's _t_-test P<0.001) reduction in the proportion of M-phase cells in the SVZ compared to wt littermates and single-hemizygous mutants (Figure 2G). At P8, we observed a 60% reduction in PH3-positive cells in the SVZ of double-hemizygous mice compared to control mice (Figure 2H). In addition, the SVZ of P8 mutants was thinner, with a reduced cellularity (data not shown). A similar reduction in mitotic cells was also observed in the RMS of P4 double-hemizygous mice (Figure 3E). Hence, loss of function of one Jagged1 and one Notch1 allele results in a significant reduction in the proportion of SVZ cells in the M phase of cell cycle. These data indicate that reduced Jagged1/Notch1 signaling results in a perturbation of mitotic cells in the neurogenic SVZ of the postnatal brain.
Jagged1 and Notch1 are expressed by defined cells in neurospheres
Due to the absence of Cre-recombinase-expressing mouse lines for the specific and conditional inactivation of Jagged1 from the adult SVZ in vivo, we turned to the well-established and versatile neurosphere cell culture system to address Jagged1 signaling in NSCs. We isolated NSCs from the forebrain of perinatal wt mice and maintained them in clonal spherogenic cultures supplemented with EGF to expand adult-type NSCs (Temple and Alvarez-Buylla, 1999). In order to focus on self-replicating spherogenic stem cells, we passaged these primary neurospheres and analyzed the expression of Jagged1 in the spheres formed 5 days later. Jagged1-expressing cells were found on the surface of neurospheres and many also expressed the intermediate filament protein Nestin (Figure 4A–C, arrows). When neurospheres were plated onto culture dishes, Jagged1 expression also colocalized with GFAP (Figure 4D and D, arrows), confirming the expression observed in the SVZ in vivo (Figure 1E, G and H). Whereas many Nestin-expressing cells also stained with the Jagged1 antibodies, only a subpopulation of the GFAP-positive cells expressed Jagged1, as in vivo (Figure 4E). In contrast, Notch1-expressing cells on the surface of neurospheres expressed the progenitor cell marker Nestin, but not detectable levels of GFAP (Figure 3F–H, arrows).
Figure 4.
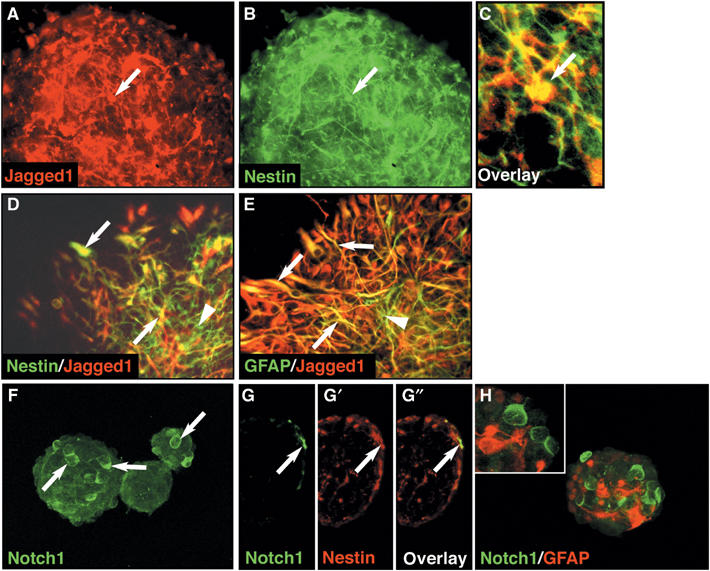
Jagged1 and Notch1 are expressed by neural progenitors. (A) Immunostaining showing Jagged1-expressing cells (arrow) on the surface of intact neurospheres. (B) Coimmunostaining with antibodies against the progenitor cell marker Nestin. (C) Enlarged overlay of Jagged1 and Nestin immunostaining indicating coexpression by a subpopulation of cells (arrow). (D) Acutely plated neurospheres immunostained with anti-Jagged1 and anti-Nestin antibodies showing coexpression by a subpopulation of cells (yellow; arrows), other Nestin-positive cells do not express Jagged1 (arrowhead). (E) Jagged1 and GFAP are coexpressed by many astroglia (yellow; arrows), but not all GFAP-positive cells express Jagged1 (arrowhead). (F) Confocal microscopic analysis of Notch1 expression by cells on the surface of most neurospheres (arrows). (G–G″) Cryosections (8 μm) double immunostained for Notch1 and Nestin. (G) Notch1-expressing cells (arrow) are restricted to the surface of the neurosphere, as are the majority of the Nestin-expressing progenitors (arrow; G′). (G″) All Notch1-expressing cells express Nestin (arrow). (H) Confocal 3D reconstruction of Notch1 and GFAP double-immunostained spheres. The inset shows a single optical section and the absence of Notch1 and GFAP costaining.
Ablation of Jagged1 blocks NSC self-renewal in vitro
To analyze the function of Jagged1 in EGF-dependent NSCs, we inactivated the Jagged1 gene using an Adenoviral-Cre vector (Adeno-Cre; Kalamarides et al, 2002) in dissociated NSCs isolated from postnatal floxed Jagged1 animals carrying the Cre-reporter Rosa26R (Figure 5A and B). The constitutive expression of β-galactosidase from the Rosa26R allele after Cre activity allowed us to address recombination efficiency (Figure 5C). Recombined floxed Jagged1 NSCs, from here on referred to as Jagged1-deficient, formed secondary neurospheres at a rate similar to that seen for control-infected NSCs isolated from Rosa26R mice (Figure 5D). The Jagged1-deficient neurospheres were indistinguishable from infected control spheres in terms of size and morphology (data not shown). RT–PCR analysis of the infected Jagged1-deficient neurospheres showed a lack of Jagged1 mRNA 5 days post-infection, confirming gene ablation (Figure 5F). Other components of the canonical Notch signaling cascade were still present (Figure 5F), and quantitative analysis using real-time PCR showed a downregulation of Hes1 and Hes5 to 40.3±2.8 and 2.0±1.2% of levels in Adeno-Cre-infected Rosa26R cells, respectively (Figure 5H and Supplementary Figure 3).
Figure 5.
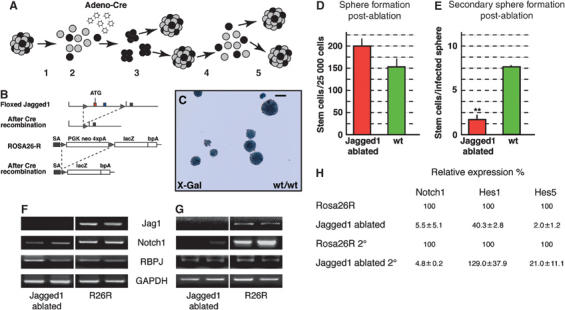
Jagged1 is required for NSC self-renewal. (A) Schematic representation of the experimental approach. Primary EGF-dependent neurospheres were dissociated (1) and infected at a multiplicity of infection of 50 with Adeno-Cre virus (2). Infected NSCs were cultured for 5 days post-infection (3) and used in differentiation assays for RNA isolation, or were dissociated (4) and cultured under spherogenic conditions for a further 5 days (5). (B) Conditional Jagged1 gene rearrangement induced by infection of dissociated, floxed Jagged1 NSCs in vitro with an Adeno-Cre virus. The Rosa26R Cre-reporter allele was included to follow recombination efficiency by monitoring the constitutive expression of β-galactosidase following Cre recombinase activity. (C) X-gal staining for the Rosa26R Cre-reporter allele (R26R) β-galactosidase activity showed 95–99% recombination efficiency in floxed Jagged1 as well as in control cells carrying only the Rosa26R reporter. (D) Quantification of neurospheres generated from 25 000 dissociated primary neurosphere cells. _Jagged1_-ablated NSCs retain their sphere-forming potential after infection comparable to wt cells. (E) Dissociation and secondary neurosphere formation revealed that post-ablation Jagged1-deficient NSCs lose their self-renewal capacity. Graph depicts the number of sphere-forming cells retained in the Adeno-Cre-infected neurospheres post-ablation (**Student's _t_-test P<0.001). (F) RT–PCR analysis of canonical Notch signaling components after Jagged1 ablation. (G) RT–PCR analysis of secondary spheres post-ablation confirmed the loss of Jagged1 and Notch1 mRNA. (H) Quantitative real-time PCR analysis showing downregulation of the Notch1 and its targets Hes1 and Hes5. GAPDH mRNA was used to standardize the cDNA in the reactions. The results shown in (F), (G), and (H) are averages of two representative cDNA preparations from independent samples and experiments. Scale bar in (B)=100 μm.
To test whether Jagged1-deficient cells retain their self-renewing capacity and can be maintained in the spherogenic assay, we passaged the neurospheres 5 days post-infection (Figure 5A). The number of secondary spheres formed from each post-infection neurosphere is a direct reflection of the number of self-replicating stem cells. As expected, Adeno-Cre-infected Rosa26R control cells retained their self-renewal capacity with an average of 7.6±0.16 spherogenic stem cells per infected neurosphere. By contrast, the Jagged1-deficient NSCs showed a striking reduction in their self-replicating ability, with an average of only 1.7±0.53 stem cells present in the neurospheres 5 days post-ablation (Figure 5E). Hence, Jagged1-deficient neurospheres contained only 1/5 of the normal number of stem cells.
RT–PCR analysis of the secondary post-infection neurospheres demonstrated complete ablation of Jagged1 from the infected floxed Jagged1 cells, excluding the possibility that the remaining spherogenic cells had failed to recombine the Jagged1 loci (Figure 5G). Residual Jagged1 protein or molecular compensation may explain the persistence of some self-renewing cells in the Jagged1-deficient neurospheres, although we could not detect Jagged1 protein on recombined cells (Figure 6A–D, red). Conversely, it is possible that Jagged1 ablation results in NSCs forming transient intermediates with a limited spherogenic capacity.
Figure 6.
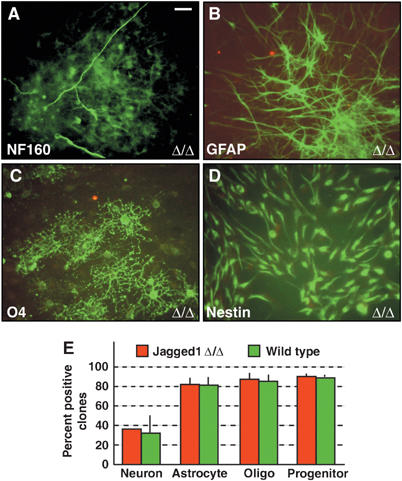
Jagged1 regulates self-renewal, but not cell lineage commitment. Jagged1-deficient neurospheres (Δ/Δ) plated at clonal density give rise to clones containing (A) neurofilament (NF160)-positive neurons, (B) GFAP-positive astroglia, (C) O4-positive oligodendrocytes and (D) Nestin-positive progenitors (green A–D). Jagged1 protein was not detected on ablated cells (red; A–D). (E) Quantification of the percentage of clones containing lineage-specific marker-positive cells in Jagged1-deficient and control Adeno-Cre-infected cells. Scale bar in (A)=10 μm for panels A–D.
The remaining Jagged1-deficient secondary neurospheres also showed a major reduction in the expression of Hes5 compared to infected control spheres (Jagged1-ablated 2°; Figure 5H and Supplementary Figure 3A). Surprisingly, a dramatic reduction in Notch1 mRNA levels was observed in the spheres formed after Jagged1 ablation (Figure 5F, G and H), whereas mRNA levels of other members of the Notch family were not affected (Supplementary Figure 2A). In addition, semiquantitative PCR analysis showed a slight increase in the expression of the proneural genes Mash1 and Math1 following Jagged1 ablation, but not of the later neuronal marker NeuroD, the glial marker GFAP, and the progenitor cell marker Nestin (Supplementary Figure 2A). The remaining secondary Jagged1-deficient neurospheres could not be maintained by further dissociation, indicating that the loss of Jagged1 leads, over time, to a complete loss of self-replicating NSCs.
Jagged1 deficiency does not affect differentiation potential in vitro
To address whether loss of Jagged1 affects not only the self-renewing potential but also the lineage determination of NSCs, we induce differentiation under clonal conditions. Jagged1-deficient neurospheres plated as effectively as control neurospheres and spread onto the coated dishes over the 6-day period. After differentiation, the cells were fixed and immunostained. Jagged1 protein was not detectable on the _Jagged1_-ablated spheres (Figure 6A–D). Surprisingly, Jagged1-deficient neurospheres formed neurons, astroglia, and oligodendroglia as efficiently as Adeno-Cre-infected Rosa26R neurospheres (Figure 6E). Hence, although Jagged1 regulates stem cell maintenance, loss of function does not directly prevent NSCs from adopting specific neural cell fates in vitro.
Jagged1 functions through Notch1 to maintain NSC self-renewal
Our in vivo and in vitro data indicate that Jagged1 is required for NSC maintenance through the activation of Notch1 (Figures 2 and 3). To address this, we performed an Adeno-Cre-mediated conditional gene inactivation of Notch1 from NSCs in vitro, using cells isolated from floxed Notch1 mice also carrying the Rosa26R allele (Figure 7A) (Radtke et al, 1999; Lutolf et al, 2002). Comparable to the observations made following Jagged1 ablation, specific loss of Notch1 function did not prevent sphere formation 5 days post-infection with Adeno-Cre (Figure 7C). However, Notch1 could no longer be detected at the protein or mRNA levels (Figure 7B and E). We observed a 93% reduction in Notch1 mRNA levels by Affymetrix microarray analysis of RNA levels in the Notch1-deficient spheres compared to infected Rosa26R spheres. However, dissociation of the Notch1-deficient neurospheres revealed a dramatic 250-fold reduction in self-replicating NSCs (8.1±1.1 stem cells/sphere for control compared to 0.04±0.01 in Notch1-deficient spheres: Figure 7D).
Figure 7.
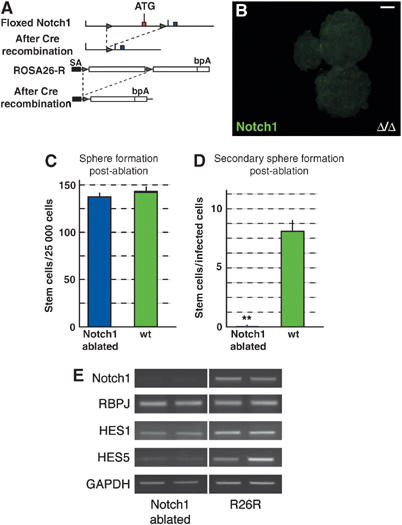
Notch1 mediates the Jagged1 signal for NSC self-renewal. (A) Floxed Notch1 alleles were generated by insertion of loxP sites (triangle) upstream of the first coding exon of the gene. Following Cre-mediated recombination, the first exon and part of the putative promoter of the Notch1 are ablated to generate a null allele (Radtke et al, 1999). The Rosa26R Cre-reporter allele was included in the analysis for lineage tracing. (B) Adeno-Cre infection of dissociated NSCs isolated from floxed Notch1 mice results in a complete loss of Notch1 protein expression after 5 days. (C) _Notch1_-deficient NSCs form neurospheres at the same rate as infected control cells. (D) Dissociation of the neurospheres 5 days post-infection showed an almost complete loss of spherogenic cells in the _Notch1_-ablated population (**Student's _t_-test P<0.001). (E) RT–PCR confirmed a dramatic reduction in the levels of Notch1 mRNA in the _Notch1_-ablated, compared to infected, control neurospheres (R26R) after 5 days, and moderate reductions in Hes1 and Hes5 expression. (E) shows a representative series of results using two RNA samples from independent experiments. GAPDH mRNA was used to standardize the cDNA in the reactions. Scale bar in (B)=50 μm.
Analysis of the canonical Notch pathway components after Notch1 ablation showed a reduction in Hes gene expression, particularly Hes5 (Figure 7E), which, by Affimetrix microarray analysis, was downregulated to 20% of the level seen in Adeno-Cre-infected Rosa26R cells (data not shown). The remaining Hes mRNA could be due to RNA stability or residual Notch1 protein. However, we were unable to analyze changes in gene expression in secondary infected neurospheres, since Notch1-deficient neurospheres could not be dissociated and maintained. Although ablation of either Notch1 or Jagged1 resulted in a drastic loss of NSCs in the neurosphere assay, the expression levels of Notch2 and Notch3 were not significantly affected by ablation of either gene, nor were the levels of the ligands Dll1, Dll2, Dll3, and Jagged2, indicating a lack of compensatory signaling (Supplementary Figure 2A and data not shown). In addition, expression levels of the proneural genes Mash1 and Math1 and the early neuronal marker NeuroD were not affected (Supplementary Figure 2A and Affymetrix microarray; to be presented elsewhere). Hence, Jagged1 and Notch1 are required by NSCs for self-maintenance.
Notch1 deficiency does not affect differentiation potential in vitro
We found that loss of Jagged1 did not affect the differentiation potential of NSCs in vitro (Figure 6). Therefore, we analyzed the potential of Notch1-deficient neurospheres. Clonal analysis showed that Notch1-deficient neurospheres formed neurons, astroglia, and oligodendroglia similar to Adeno-Cre-infected control Rosa26R neurospheres, although Notch1 protein was no longer detectable (Figure 8A–E). Both Jagged1- and Notch1-deficient cultures contained Nestin-positive clones, although their number was slightly reduced in the Notch1-deficient cultures (Figure 8D and E).
Figure 8.
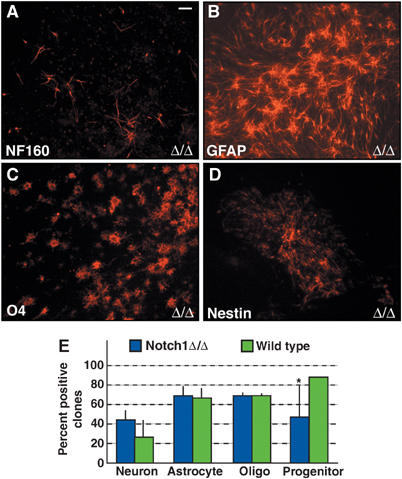
Notch1 ablation does not alter cell lineage commitment. Notch1-deficient NSCs retain the ability to generate (A) neurofilament160-positive neurons, (B) GFAP-positive astrocytes, (C) O4-positive oligodendroglia, and (D) Nestin-positive progenitor cells. Notch1 protein was not detected on the ablated cells (green; A–D). (E) Quantification of clones containing lineage-specific marker-positive cells showed a significant reduction (*Student's _t_-test P<0.05) only in the number of Nestin-containing clones in the _Notch1_-ablated population compared to infected control cells. Scale bar in (A)=20 μm for panels A–D.
NSCs of the adult SVZ are dependent on Jagged1 and Notch1
Our in vitro and in vivo analyses were based on NSCs isolated from the perinatal mouse brain and the SVZ of mutant mice shortly after birth. Due to the postnatal lethality observed in double-hemizygous Jagged1/Notch1 mice, we addressed whether NSCs isolated from the SVZ of adult mice are also dependent upon Jagged1 and Notch1. We generated neurospheres from microdisected SVZs of adult floxed Jagged1 and floxed Notch1 mice and Rosa26R mice. Although the number of neurospheres generated from adults is considerably lower than from perinatal mice, we were able to analyze the NSCs for Jagged1 and Notch1 dependency.
We repeated the spherogenic assays and performed quantitative PCR analysis of gene regulation with NSCs isolated from three individual adult mice of each genotype. In complete agreement with the findings for EGF-dependent cells from perinatal mice, neither ablation of Jagged1 (105.9±7.0% of control) nor Notch1 (96.5±2.9% of control) affected sphere formation 5 days post-infection (Figure 9A). However, as seen previously with perinatal-derived cells, Jagged1-deficient adult neurospheres showed a highly significant reduction in the number of secondary neurospheres (42.6±6.8% of control; Figure 9B). Furthermore, and also in agreement with the perinatal cell data, Notch1-deficient adult neurospheres had almost completely lost self-renewing, spherogenic NSCs (4.6±2.1% of control; Figure 9B).
Figure 9.
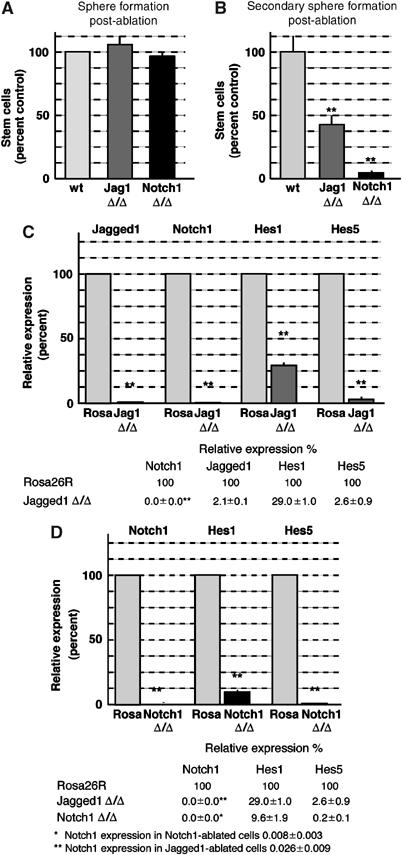
Jagged1 and Notch1 are required by adult-derived NSCs. (A) Adult Jagged1-deficient (Jag1 Δ/Δ: 105.9±7.0%) and Notch1-deficient (Notch1 Δ/Δ: 96.5±2.9%) NSCs formed neurospheres as efficiently as Adeno-Cre-infected Rosa26R (wt) NSCs 5 days post-ablation. (B) Adult Jagged1-deficient (Jag1 Δ/Δ: 42.6±6.8%) and Notch1-deficient (Notch1 Δ/Δ: 4.6±2.1%) NSCs have a highly reduced self-renewal capacity. Graph depicts the relative number of spheres formed from _Jagged1_- and _Notch1_-ablated NSCs compared to Adeno-Cre-infected neurospheres (**Student's _t_-test P<0.001). Each experiment was repeated in triplicate with three independent cultures. (C) Graphic representation and numerical values of real-time quantitative RT–PCR analysis of canonical Notch signaling components after Jagged1 ablation compared to control-infected Rosa26R neurospheres. (D) Real-time PCR analysis of Notch1 and its targets after Notch1 ablation compared to control-infected Rosa26R neurospheres. The values in (C) and (D) are averages of three individual samples performed in duplicate. The cDNA levels were standardized using GAPDH quantitative PCR.
We then addressed whether Notch signaling was affected in these adult NSCs by real-time PCR. At 5 days post-Jagged1 ablation, we found an almost complete loss of Jagged1 mRNA (2.1±0.1% of control; Figure 9C). Interestingly, Notch1 mRNA was completely lost, and Hes1 (down to 26.9±1.0%) and Hes5 (down to 2.6±0.9%) mRNA levels were highly reduced in adult _Jagged1_-deficient spheres compared to control-infected adult Rosa26R spheres (Figure 9C). Similarly, real-time PCR confirmed the ablation of Notch1 from infected adult floxed Notch1 NSCs 5 days post-infection, and a concomitant reduction in Hes1 (down to 9.6±1.9% of control) and Hes5 (down to 0.2±0.1% of control) expression (Figure 9D). These results are comparable to the changes in the expression of Notch target genes observed after Jagged1 and Notch1 ablation from perinatal NSCs, supporting the view that EGF-dependent stem cells isolated from perinatal and adult brains have similar requirements for Jagged1 and Notch1.
Jagged1 activity can replace growth factors to maintain adult-type NSCs in vitro
Our ablation experiments suggested that Jagged1 is required for NSC maintenance in vivo and in vitro; therefore, we addressed whether activation of Notch signaling is sufficient to increase NSC numbers. We treated wt EGF-dependent NSC cultures for 5 days with soluble Jagged1–Fc fusion protein (Figure 10A) (Shimizu et al, 1999) in EGF-containing, defined neurosphere medium. Subsequently, the number of self-replicating spherogenic cells in the cultures was assessed by dissociation. In the presence of EGF, Jagged1–Fc did not induce a significant increase in the number of NSCs in the cultures (data not shown). We then assessed whether Jagged1 could maintain neurosphere-forming cells in the absence of EGF. NSCs were dissociated and cultured in neurosphere medium containing increasing concentrations of Jagged1–Fc, but lacking EGF. Cells cultured in medium lacking both EGF and Jagged1–Fc (see Supplementary data) generated only few neurospheres (Figure 10B). However, Jagged1–Fc treatment resulted in a dose-dependent rescue of spherogenic cells almost to the levels seen in EGF-containing medium (Figure 10B). Hence, Jagged1 is able and sufficient to maintain NSCs in the absence of EGF.
Figure 10.
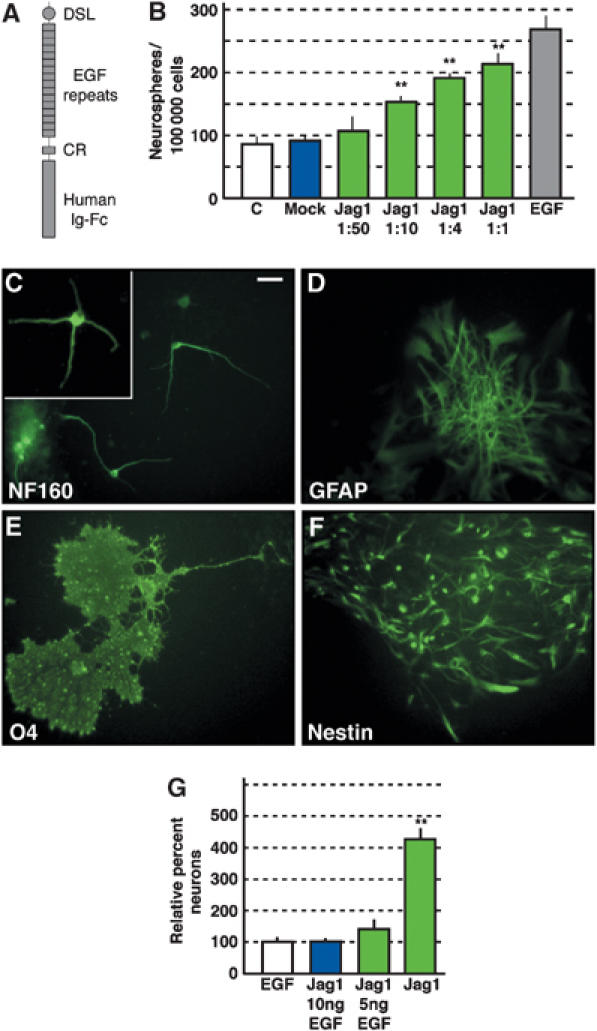
Jagged1 can maintain NSC self-renewal in vitro in the absence of trophic factors and increases the neurogenic potential. (A) NSCs dissociated from primary wt neurospheres were treated with increasing concentrations of recombinant Jagged1 extracellular domain–fhuman IgFc fusion protein. DSL—delta/serrate/lag domain, EGF—epidermal growth factor repeats, CR—cysteine-rich domain. (B) Quantification of sphere formation revealed a highly significant, dose-dependent increase in the number of spheres rescued by Jagged1–Fc treatment compared to cultures treated with mock medium (see Supplementary data) and cells grown in neurosphere medium containing 10 ng/ml EGF or lacking EGF (C) (**Student's _t_-test P<0.001). Jagged1–Fc containing neurosphere medium lacking EGF was diluted 1:50, 1:10, 1:4, and 1:1 with neurosphere medium lacking EGF. (C–F) Jagged1–Fc-treated NSCs remain multipotent and can differentiate into (C) neurons (neurofilament160), (D) astrocytes (GFAP), (E) oligodendrocytes (O4), and remain as Nestin-positive progenitors (F). (G) Treatment of NSCs with soluble Jagged1 during neurosphere formation results in a highly significant (**Student's _t_-test P<0.001) increase in neurons compared to EGF-treated cultures upon differentiation. EGF treatment reduced the Jagged1 effect on neurogenic potential. Scale bar in (C)=10 μm for panels C–F.
Jagged1 treatment increases the neurogenic potential of NSCs in vitro
Increased Notch activity has been shown to induce gliogenesis in the nervous system (Furukawa et al, 2000; Gaiano et al, 2000; Morrison et al, 2000; Scheer et al, 2001). Thus, we assessed the multipotency of Jagged1–Fc-treated NSCs. Neurospheres treated for 5 days with soluble Jagged1–Fc were plated at clonal density on substrate-coated dishes to induce differentiation. Jagged1–Fc-treated NSCs retained the ability to generate neurons, astrocytes, and oligodendroglia, indicating multipotency and that activation of endogenous Notch signaling by Jagged1 does not result in lineage commitment (Figure 10C–F).
Although there were no obvious differences between the differentiation potential of EGF and Jagged1–Fc-treated neurospheres on a clonal basis, clones derived from NSCs grown in the presence of exogenous Jagged1 seemed to contain more neurons. Hence, we quantified the proportion of _β_-TubulinIII/neurofilament 160-positive cells in the differentiation assays. In three independent experiments, the proportion of neurons generated upon differentiation increased three-fold (Student's _t_-test, P<0.001) as a result of Jagged1–Fc treatment during sphere formation (Figure 10G). Interestingly, the increased neurogenic potential caused by Jagged1 treatment was abolished by the presence of EGF in the medium (Figure 10G).
Discussion
We show that active Jagged1 is required for the maintenance of multipotent adult-type NSCs in vitro and mitotic cells in the postnatal SVZ. This is the first demonstration of a function for Jagged1 in maintaining progenitors of the CNS, which we propose is through activation of Notch1. Reduced Jagged1/Notch1 signaling results in a reduction in proliferating cells in the neurogenic SVZ and RMS. Due to the absence of specific markers for NSCs in vivo, we could not exclude that not only NSCs but also transient amplifying cells and neuroblasts are affected by the reduction in Jagged1 and Notch1. However, using self-replication and sphere-forming potential as a reporter for NSCs in vitro, we can clearly show that Jagged1 is required for maintaining EGF-dependent NSCs isolated from perinatal and adult mice. Thus, we believe that the effects of reducing Jagged1/Notch1 signaling in vivo are due, at least in part, to a reduction in NSC numbers.
Notch signaling is activated by ligands of the Dll and Jagged families, as well as by noncanonical ligands such as Contactin and NB-3 (Hu et al, 2003; Cui et al, 2004). Although multiple putative ligands are present in the neurosphere cultures, Jagged1 is the only ligand required for multipotency and EGF-dependent NSC maintenance in vitro, in agreement with its localization in the SVZ in vivo. This is also supported by previous in vitro data reporting that an absence of the Notch ligand Dll1 does not affect neurosphere formation and NSC maintenance, but reduces oligodendrocyte and astrocyte differentiation (Grandbarbe et al, 2003). In contrast, we show that the loss of Jagged1 and Notch1 does not significantly alter neurogenic or gliogenic differentiation of NSC. These differences may be due to the source of EGF-dependent NSCs as Grandbarbe et al isolated embryonic-derived NSCs and we isolated NSCs from postnatal and adult brain.
The mechanism by which Jagged1 and Dll1 induce different effects on EGF-dependent progenitor cells is unclear. Changes in the _O_-gylcosylation of Notch molecules by glucosaminyltransferases of the Fringe family modulate signaling responses to different classes of ligand (Fleming et al, 1997; Bruckner et al, 2000; Hicks et al, 2000). Thus, individual Notch ligands may act through specific receptors at different stages to regulate maintenance and differentiation. Furthermore, as loss of Notch1 affected predominantly maintenance of self-renewal and not lineage determination, one might speculate that Dll acts through another mechanism or Notch family member to regulate cell fate.
Although Notch1 ablation in vitro resulted in a reduction in Nestin-expressing clones, which is in agreement with a role for Notch1 in maintaining progenitors, Jagged1 ablation did not. It is possible that Notch1 plays a role in Nestin-expressing transient intermediate cells. Thus, loss of Notch1 may have resulted in the differentiation of both NSCs and transient amplifying cells. In addition, loss of Jagged1 had a less pronounced effect on the loss of NSCs compared to ablation of Notch1, which may also reflect the maintenance of Nestin expression.
We show that endogenous Notch1 is not required for the formation of any neural lineage from NSCs (Figure 8). Furthermore, we show that the activation of endogenous Notch signaling by treatment with Jagged1 promotes multipotency rather than gliogenesis, resulting in increased neuronal differentiation potential (Figure 10). Our data and those of others fit with a model whereby Notch1 maintains NSCs in an undifferentiated and competent state to respond, potentially in combination with Notch signaling, to additional differentiation cues. Hence, we propose that direct activation of Notch signaling by Jagged1 does not result in glial differentiation but, in combination with other signals such as EGF or cytokine signaling downstream of the gp130 receptor, may potentiate gliogenesis (Chojnacki et al, 2003; Kamakura et al, 2004). This is supported indirectly by our observation that Jagged1-induced neurogenic potential is abolished in the presence of EGF. Similarly, ectopic activation of Notch signaling by intracellular domain overexpression may induce targets downstream of multiple Notch pathways, including those activated by Dll1.
The signals that modulate NSC self-replication and differentiation are likely to be encoded in the SVZ niche. Recent evidence suggests that blood vessel endothelium may constitute at least part of the NSC niche both in the embryo and adult (Shen et al, 2004). We find that Notch1-expressing progenitors are in close contact with Jagged1-positive cells within the neurogenic regions of the adult brain and that both Notch1 and Jagged1 are required for NSC maintenance. The Jagged1-expressing cells in the SVZ of adult mice also express GFAP and are located in a position consistent with them being SVZ astrocytes. Hence, both endothelial cells and SVZ astrocytes may provide Notch1 ligands to NSCs in the SVZ. It is interesting to note that, in vitro, endothelial cells induce expression of the Notch target Hes1 but not Hes5 (Shen et al, 2004). Ablation of Jagged1, on the other hand, results in reductions in Hes1, but predominantly Hes5 expression. Hence, it remains to be shown whether there are two niches in the adult SVZ, potentially for two populations of NSCs.
Jagged1 expression by a subpopulation of astroglia in neurospheres and in the adult SVZ is intriguing, based on the accumulating data that some SVZ astrocytes (B cells) have stem cell-like properties (reviewed by Doetsch, 2003). Although some of these data may be explained by GFAP-positive cells in the niche, providing signals to the NSC (Song et al, 2002), it is likely that multipotent progenitors in the SVZ activate, at some point, the GFAP promoter. Therefore, it remains possible that Jagged1 expressed by SVZ astrocytes signals to neighboring cells and promotes the multipotent NSC state. Thus, some SVZ astrocytes may represent the niche and others the multipotent progenitors. Notch1-expressing cells in the SVZ and in neurospheres do not express high levels of GFAP, and most are found in the caudal SVZ, where they do not express the neuroblast marker Dcx. Conversely, the distal RMS is comprised mainly of Dcx-positive cells, suggesting that the Notch-dependent NSCs are mainly located in the caudal SVZ, where few Dcx cells are found. Interestingly, neuroblasts in the medial RMS do express Notch1, suggesting a potential function outside the neural progenitors.
We propose that Jagged1-expressing cells form the niche for a population of NSCs in the SVZ. Mechanistically, Jagged1 likely activates Notch1 on NSCs, preventing differentiation and, in a positive feedback loop, increasing Notch1 gene transcription. Our findings demonstrate that Jagged1 forms an important part of the stem cell maintenance pathway in the postnatal brain and acts through Notch1, whereas other ligands and Notchs do not seem to play a major role in maintenance.
Materials and methods
Production of ‘floxed' Jagged1 locus
The floxed Jagged1 allele was generated by inserting loxP sites into the Jagged1 gene, flanking the first and second coding exons and the sequences between (for detailed description, see Supplementary data).
Isolation of NSCs and culture of neurospheres
NSCs were isolated from early postnatal mice and cultured in defined medium supplemented with B27 and EGF (for detailed description, see Supplementary data). Quantification, immunofluorescence and cell fate analysis are described in detail in the Supplementary data.
RNA isolation and RT–PCR analysis
Total RNA was isolated from neurosphere cultures using Trizol Reagent (Roche) and purified using RNeasy extraction kit according to the manufacturer's instructions (Qiagen). RT–PCR reactions were performed using gene-specific primers and conditions described in the Supplementary data.
Antibodies and immunostaining of cells and sections
Cells were fixed and processed for immunostaining as described in Supplementary data. Brain sections for immunostaining were either fresh frozen or paraffin embedded. Details of the staining proceedure and antibodies are given in Supplementary data.
Soluble Jagged1–Fc treatment of NSCs
Soluble Jagged1–Fc was generated as described previously (Shimizu et al, 1999). Neurospheres were dissociated and cultured in defined medium supplemented with Jagged1–Fc in the presence or absence of EGF. Subsequently, the number of neurospheres generated in the cultures, the differentiation potential and the maintenance of self-replicating spherogenic cells were analyzed (see Supplementary data).
Supplementary Material
Supplementary information
Supplementary Figure 1
Supplementary Figure 2
Supplementary Figure 3
Acknowledgments
We thank Drs P Soriano, M Giovannini and H Hirai for supplying the reagents used in this study. We thank Dr S Lutolf for help with stem cell cultures, Dr Ivan Radovanovic for help with the Adenovirus, Mr F Sager for excellent technical assistance and Dr M Aguet for continued support. We thank Drs R Kemler, M Hammerschmidt, M Oelgeschlager, D Junghans, and R Cassada for critical reading of the manuscript and helpful discussions. The Rat-401 anti-Nestin antibody developed by Dr S Hockfield was obtained from the Developmental Studies Hybridoma Bank maintained by The University of Iowa, Department of Biological Sciences, Iowa City, IA 52242, under the contract NO1-HD-7-3263 from the NICHD. This work was supported by the Swiss National Science Foundation via the NCCR grant ‘Neural Plasticity and Repair' and the Deutsche Forschungsgemeinschaft (SPP1109; TA-310).
References
- Alvarez-Buylla A, Lim DA (2004) For the long run: maintaining germinal niches in the adult brain. Neuron 41: 683–686 [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Temple S (1998) Stem cells in the developing and adult nervous system. J Neurobiol 36: 105–110 [PubMed] [Google Scholar]
- Blau HM, Brazelton TR, Weimann JM (2001) The evolving concept of a stem cell: entity or function? Cell 105: 829–841 [DOI] [PubMed] [Google Scholar]
- Bruckner K, Perez L, Clausen H, Cohen S (2000) Glycosyltransferase activity of Fringe modulates Notch–Delta interactions. Nature 406: 411–415 [DOI] [PubMed] [Google Scholar]
- Chojnacki A, Shimazaki T, Gregg C, Weinmaster G, Weiss S (2003) Glycoprotein 130 signaling regulates Notch1 expression and activation in the self-renewal of mammalian forebrain neural stem cells. J Neurosci 23: 1730–1741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu VT, Gage FH (2001) Chipping away at stem cells. Proc Natl Acad Sci USA 98: 7652–7653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui XY, Hu QD, Tekaya M, Shimoda Y, Ang BT, Nie DY, Sun L, Hu WP, Karsak M, Duka T, Takeda Y, Ou LY, Dawe GS, Yu FG, Ahmed S, Jin LH, Schachner M, Watanabe K, Arsenijevic Y, Xiao ZC (2004) NB-3/Notch1 pathway via Deltex1 promotes neural progenitor cell differentiation into oligodendrocytes. J Biol Chem 279: 25858–25865 [DOI] [PubMed] [Google Scholar]
- de la Pompa JL, Wakeham A, Correia KM, Samper E, Brown S, Aguilera RJ, Nakano T, Honjo T, Mak TW, Rossant J, Conlon RA (1997) Conservation of the Notch signalling pathway in mammalian neurogenesis. Development 124: 1139–1148 [DOI] [PubMed] [Google Scholar]
- Doetsch F (2003) The glial identity of neural stem cells. Nat Neurosci 6: 1127–1134 [DOI] [PubMed] [Google Scholar]
- Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A (1999) Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell 97: 703–716 [DOI] [PubMed] [Google Scholar]
- Doetsch F, Petreanu L, Caille I, Garcia-Verdugo JM, Alvarez-Buylla A (2002) EGF converts transit-amplifying neurogenic precursors in the adult brain into multipotent stem cells. Neuron 36: 1021–1034 [DOI] [PubMed] [Google Scholar]
- Fleming RJ, Gu Y, Hukriede NA (1997) Serrate-mediated activation of Notch is specifically blocked by the product of the gene fringe in the dorsal compartment of the Drosophila wing imaginal disc. Development 124: 2973–2981 [DOI] [PubMed] [Google Scholar]
- Furukawa T, Mukherjee S, Bao ZZ, Morrow EM, Cepko CL (2000) rax, Hes1, and notch1 promote the formation of Muller glia by postnatal retinal progenitor cells. Neuron 26: 383–394 [DOI] [PubMed] [Google Scholar]
- Gaiano N, Nye JS, Fishell G (2000) Radial glial identity is promoted by Notch1 signaling in the murine forebrain. Neuron 26: 395–404 [DOI] [PubMed] [Google Scholar]
- Genoud S, Lappe-Siefke C, Goebbels S, Radtke F, Aguet M, Scherer SS, Suter U, Nave KA, Mantei N (2002) Notch1 control of oligodendrocyte differentiation in the spinal cord. J Cell Biol 158: 709–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandbarbe L, Bouissac J, Rand M, Hrabe de Angelis M, Artavanis-Tsakonas S, Mohier E (2003) Delta-Notch signaling controls the generation of neurons/glia from neural stem cells in a stepwise process. Development 130: 1391–1402 [DOI] [PubMed] [Google Scholar]
- Hicks C, Johnston SH, diSibio G, Collazo A, Vogt TF, Weinmaster G (2000) Fringe differentially modulates Jagged1 and Delta1 signalling through Notch1 and Notch2. Nat Cell Biol 2: 515–520 [DOI] [PubMed] [Google Scholar]
- Hitoshi S, Alexson T, Tropepe V, Donoviel D, Elia AJ, Nye JS, Conlon RA, Mak TW, Bernstein A, van der Kooy D (2002) Notch pathway molecules are essential for the maintenance, but not the generation, of mammalian neural stem cells. Genes Dev 16: 846–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu QD, Ang BT, Karsak M, Hu WP, Cui XY, Duka T, Takeda Y, Chia W, Sankar N, Ng YK, Ling EA, Maciag T, Small D, Trifonova R, Kopan R, Okano H, Nakafuku M, Chiba S, Hirai H, Aster JC, Schachner M, Pallen CJ, Watanabe K, Xiao ZC (2003) F3/contactin acts as a functional ligand for Notch during oligodendrocyte maturation. Cell 115: 163–175 [DOI] [PubMed] [Google Scholar]
- Irvin DK, Nakano I, Paucar A, Kornblum HI (2004) Patterns of Jagged1, Jagged2, Delta-like 1 and Delta-like 3 expression during late embryonic and postnatal brain development suggest multiple functional roles in progenitors and differentiated cells. J Neurosci Res 75: 330–343 [DOI] [PubMed] [Google Scholar]
- Kalamarides M, Niwa-Kawakita M, Leblois H, Abramowski V, Perricaudet M, Janin A, Thomas G, Gutmann DH, Giovannini M (2002) Nf2 gene inactivation in arachnoidal cells is rate-limiting for meningioma development in the mouse. Genes Dev 16: 1060–1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamakura S, Oishi K, Yoshimatsu T, Nakafuku M, Masuyama N, Gotoh Y (2004) Hes binding to STAT3 mediates crosstalk between Notch and JAK-STAT signalling. Nat Cell Biol 6: 547–554 [DOI] [PubMed] [Google Scholar]
- Lardelli M, Williams R, Mitsiadis T, Lendahl U (1996) Expression of the Notch 3 intracellular domain in mouse central nervous system progenitor cells is lethal and leads to disturbed neural tube development. Mech Dev 59: 177–190 [DOI] [PubMed] [Google Scholar]
- Lindsell CE, Boulter J, diSibio G, Gossler A, Weinmaster G (1996) Expression patterns of Jagged, Delta1, Notch1, Notch2, and Notch3 genes identify ligand–receptor pairs that may function in neural development. Mol Cell Neurosci 8: 14–27 [DOI] [PubMed] [Google Scholar]
- Lindsell CE, Shawber CJ, Boulter J, Weinmaster G (1995) Jagged: a mammalian ligand that activates Notch1. Cell 80: 909–917 [DOI] [PubMed] [Google Scholar]
- Lutolf S, Radtke F, Aguet M, Suter U, Taylor V (2002) Notch1 is required for neuronal and glial differentiation in the cerebellum. Development 129: 373–385 [DOI] [PubMed] [Google Scholar]
- Malatesta P, Hartfuss E, Gotz M (2000) Isolation of radial glial cells by fluorescent-activated cell sorting reveals a neuronal lineage. Development 127: 5253–5263 [DOI] [PubMed] [Google Scholar]
- McCright B, Lozier J, Gridley T (2002) A mouse model of Alagille syndrome: Notch2 as a genetic modifier of Jag1 haploinsufficiency. Development 129: 1075–1082 [DOI] [PubMed] [Google Scholar]
- Morrison SJ, Perez SE, Qiao Z, Verdi JM, Hicks C, Weinmaster G, Anderson DJ (2000) Transient Notch activation initiates an irreversible switch from neurogenesis to gliogenesis by neural crest stem cells. Cell 101: 499–510 [DOI] [PubMed] [Google Scholar]
- Morshead CM, Reynolds BA, Craig CG, McBurney MW, Staines WA, Morassutti D, Weiss S, van der Kooy D (1994) Neural stem cells in the adult mammalian forebrain: a relatively quiescent subpopulation of subependymal cells. Neuron 13: 1071–1082 [DOI] [PubMed] [Google Scholar]
- Noctor SC, Flint AC, Weissman TA, Dammerman RS, Kriegstein AR (2001) Neurons derived from radial glial cells establish radial units in neocortex. Nature 409: 714–720 [DOI] [PubMed] [Google Scholar]
- Price J, Williams BP (2001) Neural stem cells. Curr Opin Neurobiol 11: 564–567 [DOI] [PubMed] [Google Scholar]
- Radtke F, Wilson A, Stark G, Bauer M, van Meerwijk J, MacDonald HR, Aguet M (1999) Deficient T cell fate specification in mice with an induced inactivation of Notch1. Immunity 10: 547–558 [DOI] [PubMed] [Google Scholar]
- Reynolds BA, Weiss S (1992) Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science 255: 1707–1710 [DOI] [PubMed] [Google Scholar]
- Scheer N, Groth A, Hans S, Campos-Ortega JA (2001) An instructive function for Notch in promoting gliogenesis in the zebrafish retina. Development 128: 1099–1107 [DOI] [PubMed] [Google Scholar]
- Shen Q, Goderie SK, Jin L, Karanth N, Sun Y, Abramova N, Vincent P, Pumiglia K, Temple S (2004) Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science 304: 1338–1340 [DOI] [PubMed] [Google Scholar]
- Shimizu K, Chiba S, Kumano K, Hosoya N, Takahashi T, Kanda Y, Hamada Y, Yazaki Y, Hirai H (1999) Mouse jagged1 physically interacts with notch2 and other notch receptors. Assessment by quantitative methods. J Biol Chem 274: 32961–32969 [DOI] [PubMed] [Google Scholar]
- Song H, Stevens CF, Gage FH (2002) Astroglia induce neurogenesis from adult neural stem cells. Nature 417: 39–44 [DOI] [PubMed] [Google Scholar]
- Stidworthy MF, Genoud S, Li WW, Leone DP, Mantei N, Suter U, Franklin RJ (2004) Notch1 and Jagged1 are expressed after CNS demyelination, but are not a major rate-determining factor during remyelination. Brain 127: 1928–1941 [DOI] [PubMed] [Google Scholar]
- Stump G, Durrer A, Klein A, Lutolf S, Suter U, Taylor V (2002) Notch1 and its ligands Delta-like and Jagged are expressed and active in distinct cell populations in the postnatal mouse brain. Mech Dev 114: 153–159 [DOI] [PubMed] [Google Scholar]
- Temple S, Alvarez-Buylla A (1999) Stem cells in the adult mammalian central nervous system. Curr Opin Neurobiol 9: 135–141 [DOI] [PubMed] [Google Scholar]
- Toma JG, Akhavan M, Fernandes KJ, Barnabe-Heider F, Sadikot A, Kaplan DR, Miller FD (2001) Isolation of multipotent adult stem cells from the dermis of mammalian skin. Nat Cell Biol 3: 778–784 [DOI] [PubMed] [Google Scholar]
- Tropepe V, Sibilia M, Ciruna BG, Rossant J, Wagner EF, van der Kooy D (1999) Distinct neural stem cells proliferate in response to EGF and FGF in the developing mouse telencephalon. Dev Biol 208: 166–188 [DOI] [PubMed] [Google Scholar]
- van der Kooy D, Weiss S (2000) Why stem cells? Science 287: 1439–1441 [DOI] [PubMed] [Google Scholar]
- Wagers AJ, Sherwood RI, Christensen JL, Weissman IL (2002) Little evidence for developmental plasticity of adult hematopoietic stem cells. Science 297: 2256–2259 [DOI] [PubMed] [Google Scholar]
- Wang S, Sdrulla AD, diSibio G, Bush G, Nofziger D, Hicks C, Weinmaster G, Barres BA (1998) Notch receptor activation inhibits oligodendrocyte differentiation. Neuron 21: 63–75 [DOI] [PubMed] [Google Scholar]
- Watt FM, Hogan BL (2000) Out of Eden: stem cells and their niches. Science 287: 1427–1430 [DOI] [PubMed] [Google Scholar]
- Weissman IL, Anderson DJ, Gage F (2001) Stem and progenitor cells: origins, phenotypes, lineage commitments, and transdifferentiations. Annu Rev Cell Dev Biol 17: 387–403 [DOI] [PubMed] [Google Scholar]
- Xue Y, Gao X, Lindsell CE, Norton CR, Chang B, Hicks C, Gendron-Maguire M, Rand EB, Weinmaster G, Gridley T (1999) Embryonic lethality and vascular defects in mice lacking the Notch ligand Jagged1. Hum Mol Genet 8: 723–730 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information
Supplementary Figure 1
Supplementary Figure 2
Supplementary Figure 3