Phagocytosis and Killing of Bacteria by Professional Phagocytes and Dendritic Cells (original) (raw)
Abstract
Dendritic cells (DC) represent a class of professional antigen-presenting cells whose primary function is to alert the immune system, not to clear invading microorganisms. The objective of our study was to compare the abilities of polymorphonuclear neutrophilic leukocytes (PMN), monocytes, monocyte-derived macrophages (MDM), monocyte-derived immature DC (imDC), and mature DC (maDC) to ingest and destroy Staphylococcus aureus and Escherichia coli. Acridine orange staining and fluorescence microscopy demonstrated that MDM, followed by monocytes, imDC, and PMN, internalized bacteria well but that maDC exhibited less pronounced phagocytic activity. PMN, monocytes, and MDM exhibited a much higher capacity to kill ingested bacteria than both imDC and maDC. In summary, these data are in agreement with the generally accepted idea that different types of leukocytes fulfill specialized tasks in antigen presentation and killing of pathogens.
Dendritic cells (DC) represent a unique system of cells that induce, sustain, and regulate immune responses. Immature DC (imDC) precursors exit the bone marrow and circulate via the bloodstream to reach their target tissues, taking up residence at sites of potential pathogen entry in a physiological stage that is specialized for antigen capture (17). In this phase, imDC efficiently and continuously sample the antigenic content of their environment by phagocytosis of particulates, high-volume fluid phase macropinocytosis, receptor-mediated endocytosis, or direct contact with apoptotic or infected cells (1, 3). Unlike professional phagocytes, their major task is not to clear pathogens but to enable the presentation of antigens to the immune system. After receiving appropriate signals either directly from pathogens or via inflammatory stimuli, DC switch the expression of chemokine receptors, allowing them to leave the peripheral tissue and to migrate to draining lymphoid organs. Here, they undergo a process of maturation, becoming mature DC (maDC), and acquire the ability to activate naïve T cells (2).
Professional phagocytes have evolved to internalize and kill as many bacteria as possible. By contrast, DC limit their phagocytic activity after ingestion of living bacteria by both entering the maturation program and enhancing migratory activity (5, 13). Since the capacities of professional phagocytes and professional antigen-presenting cells (APC) to ingest and destroy bacteria have not been compared to date, in the present study we investigated the phagocytosis and killing of Staphylococcus aureus and Escherichia coli by polymorphonuclear neutrophilic leukocytes (PMN), monocytes, monocyte-derived macrophages (MDM), imDC, and maDC in vitro. This comparative analysis provides new insights into the cooperation between phagocytic cells and APC during the process of antigen presentation.
MATERIALS AND METHODS
Reagents.
All chemicals were obtained from Sigma (St. Louis, Mo.) unless otherwise indicated. Ficoll and Dextran T-500 were purchased from Pharmacia (Pistacataway, N.J.).
Monocytes and MDM.
Monocytes were separated from buffy coats of healthy human donors by adherence, as described previously (8). Briefly, peripheral blood mononuclear cells were prepared by centrifugation on a Ficoll density gradient. Ten-milliliter aliquots of a suspension of peripheral blood mononuclear cells were incubated on gelatin-coated petri dishes for 40 min at 37°C. Nonadherent cells were aspirated, while adherent cells were washed and detached with 5 mM EDTA. Detached cells (>93% CD14+ monocytes) were washed twice and resuspended (106 cells/ml) in Hanks balanced salt solution (HBSS). MDM were monocytes cultivated for 5 days in the presence of RPMI 1640-5% pooled normal human AB serum (NHS). Thereafter, MDM were detached with EDTA, washed, and resuspended in HBSS (106 cells/ml) as described above.
Generation of monocyte-derived imDC and maDC.
CD14+ cells were cultured at a cell density of 106 cells/ml in six-well plates in RPMI 1640-10% fetal calf serum medium supplemented with 1,000 U of interleukin-4 (IL-4; PromoCell, Heidelberg, Germany) per ml and 800 U of granulocyte-macrophage colony-stimulating factor (GM-CSF) (Peprotech, Rocky Hill, N.J.) per ml. The cultures were fed fresh IL-4 and GM-CSF every third day, and the cells were monitored by light microscopy. On day 6, the cells were harvested, washed, and transferred to new six-well plates. Fresh medium with cytokines was added, and the cells were cultivated for two additional days in the presence or absence of stimuli for maturation.
DC differentiation was assessed by indirect immunofluorescence staining followed by flow cytometry analysis. Primary monoclonal antibodies used included anti-HLA-DR (clone DK22; Dako, Glostrup, Denmark); immunoglobulin G1 (IgG1) and an IgG2a isotype control (clones 1B7.11 and S-S.1, respectively; American Type Culture Collection, Manassas, Va.); anti-CD1a, anti-CD11a, anti-CD11b, and anti-CD18 (clones OKT-6, LFA-1α, OKM-1, and TS1/188, respectively; American Type Culture Collection); anti-CD83 (clone HB15; kindly provided by T. F. Tedder, Durham, N.C.); and anti-CD4, anti-CD11c, and anti-CD86 (clones RPA-T4, B-ly6, and 2331 [FUN-1]; PharMingen, San Diego, Calif.). Primary monoclonal antibodies were detected with fluorescein isothiocyanate-labeled sheep [F(ab′)2 polyclonal antibodies to mouse IgG plus IgM] (An der Grub, Kaumberg, Austria).
Cells cultivated for 8 days in the presence of IL-4 and GM-CSF without other stimuli showed the imDC phenotype and did not express CD83. Full differentiation was achieved by the addition of monocyte-conditioned medium (20%) for the last 48 h of the culture period, as described previously (14). Flow cytometry analysis revealed that these cells expressed CD83, CD86, and large amounts of HLA class II molecules and were, therefore, defined as maDC. On the basis of data from previous studies, the exclusion criteria for each DC preparation used in our experiments have been defined as follows: >8% of contaminating lymphocytes, >10% of CD83+ DC within imDC, and <90% of CD83+ DC in maDC preparation.
PMN.
Pellets from Ficoll centrifugation, containing erythrocytes and PMN, were mixed with 8 ml of 6% dextran T-500 in phosphate-buffered saline and allowed to sediment for 1 h at room temperature. After centrifugation of PMN-rich supernatants (400 × g, 10 min, room temperature), the remaining erythrocytes were removed by hypotonic lysis. Purified PMN suspensions regularly contained >90% of PMN, as determined by cytofluorometric analysis.
Bacteria.
Deep-frozen S. aureus ATCC 25923 and E. coli ATCC 11129 were grown overnight on tryptic soy agar (Merck, Darmstadt, Germany). Colonies from agar were grown in tryptic soy broth (Merck) for 6 h at 37°C to 2 × 108 to 4 × 109 CFU/ml, as confirmed by quantitative cultures (see below for technique), and washed twice in 0.9% saline. For opsonization, bacteria were diluted 100-fold in fresh NHS and incubated for 30 min at 37°C under continuous rotation.
Phagocytosis assay.
Washed bacteria (ca. 109 CFU/ml) were suspended in fresh NHS for 30 min at 37°C under continuous rotation for opsonization. Ten-microliter volumes of these suspensions were added to 1-ml volumes of various phagocytic cells (106 cells/ml) in HBSS and incubated for 30 min at 37°C under rotation. Subsequently, the cells were centrifuged for 4 min at 110 × g and the supernatant was removed. The pellet was stained by the addition of 200 μl of acridine orange (15 mg/liter) for 1 min, centrifuged at 110 × g for 4 min, and washed twice with HBSS (21). The phagocytes were placed on object slides by using a cytocentrifuge, dried, and fixed with a solution containing Mowiol (0.13 g/ml; Calbiochem Inc., Bad Soden, Germany) (10), glycerol (0.33 g/ml), and 1,4-diazobicyclo-(2,22)-octane (0.37 g/ml). The number of phagocytosed bacteria per cell was counted under a fluorescence microscope.
Killing assay.
Opsonized bacteria (1.5 μl; final count, 8.9 × 104 to 1.1 × 106 CFU/ml) were added to 150 μl of cells (5 × 105/ml) in HBSS enriched with 10% heat-inactivated human serum and incubated at 37°C for 30 min under continuous rotation. For some experiments, the killing assays were done in the presence of 100 ng of phorbol 12-myristate 13-acetate (PMA) per ml. Thereafter, all samples were centrifuged for 4 min at 110 × g. Supernatants were used for evaluation of the count of extracellular, nonphagocytized bacteria. Ten- and 100-fold dilutions in saline (50 μl of each) were plated on tryptic soy agar with an automatic spiral plater (model WASP; Don Whitley Scientific Limited, West Yorkshire, United Kingdom), and CFU were counted after incubation for 24 h at 37°C.
To evaluate the count of intracellular bacteria, the pellet was washed twice in saline, and samples were resuspended in 0.5% NP-40 for 10 min at room temperature to lyse the phagocytic cells. Further determinations of extra- and intracellular bacterial counts were performed 2, 4, and 16 h later. Cells pelleted after 30 min were resuspended in HBSS plus 10% inactive serum and further incubated at 37°C under rotation. Subsequently, the samples were centrifuged for 4 min at 110 × g again and quantitative cultures from both pellets and supernatants were performed.
Statistics.
One-way analysis of variance (Graphpad Software Inc.) was applied for evaluation of differences in the CFU counts of the killing assays and the numbers of ingested bacteria of the phagocytosis assays. P values of <0.05 were considered significant.
RESULTS
Phagocytosis assays.
Fluorescence microscopy of samples stained with acridine orange revealed that all investigated cell types internalized bacteria (Table 1). The highest number of bacteria per cell was ingested by MDM (P < 0.01). Monocytes, PMN, and imDC phagocytosed similar amounts of bacteria (_P_ > 0.05), while maDC ingested less than all other types of tested leukocytes (P < 0.01). There was a tendency for the uptake of S. aureus to be higher than that of E. coli; however, this difference was significant only for imDC (P < 0.01).
TABLE 1.
Phagocytosis by various phagocytic cells
| Cell type | Mean no. of ingested bacteria (SD)a | |
|---|---|---|
| S. aureus | E. coli | |
| PMN | 12.8 (8.8) | 11.1 (5.6) |
| Monocytes | 14.5 (6.0) | 13.0 (7.6) |
| MDM | 21.9 (8.7) | 19.0 (10.4) |
| imDC | 15.8 (7.7) | 11.5 (4.8) |
| maDC | 8.8 (3.3) | 8.5 (3.4) |
Killing of bacteria.
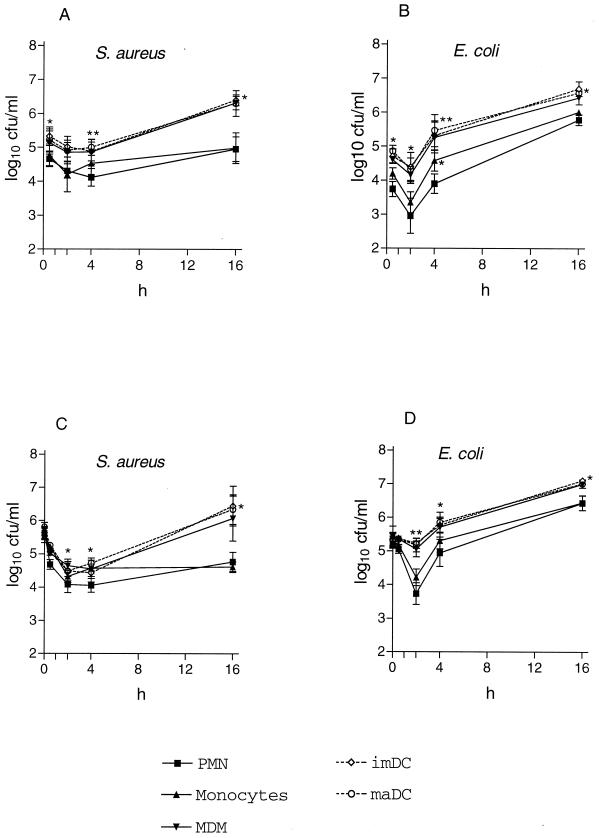
After 30 min of phagocytosis, intracellular counts of viable S. aureus cells phagocytosed by PMN and monocytes decreased to markedly lower levels from 0.5 to 4 h than those ingested by MDM, imDC, and maDC (P < 0.05) (Fig. 1). Moreover, counts for the latter subsequently increased. Similarly, E. coli CFU decreased in cultures of all tested types of leukocytes during the first 2 h of incubation, though again, to a lesser extent in the presence of MDM, imDC, and maDC (P < 0.05) (Fig. 1). Both bacterial strains grew within DC and MDM during the following hours. In contrast to S. aureus, E. coli multiplied in PMN and monocytes after 2 h but to a lesser extent than in the other cell types (P < 0.05). In experiments using cells stimulated with PMA, the killing properties of MDM became comparable to those of PMN and monocytes, while DC still demonstrated only minimal bactericidal activity (data not shown).
FIG. 1.
Intracellular (A and B) and extracellular (C and D) counts of viable bacteria after phagocytosis. Opsonized bacteria (1.9 × 105 to 1.1 × 106 CFU/ml for S. aureus and 8.9 × 104 to 4.0 × 105 CFU/ml for E. coli) were incubated with cells (1 × 106/ml) at 37°C under continuous rotation. Subsequent to centrifugation for 4 min at 110 × g, quantitative cultures of the supernatant were performed to evaluate the extracellular number of bacteria. Intracellular counts were done after two washing steps and lysis of the phagocytes in the pellet with 0.5% NP-40 for 10 min. Results are the means ± standard errors of the means of results from three independent experiments. Symbols: ▪, PMN; ▴, monocytes; ▾, MDM; ◊, imDC; ○, maDC; *, P < 0.05 compared to results for PMN; **, P < 0.01 compared to results for PMN.
In the presence of PMN and monocytes, extracellular viable counts of both strains decreased to a greater extent during the first 2 h of incubation (especially in E. coli) and subsequently remained lower than in the presence of MDM and DC, which probably confirms the superior killing activity of PMN and monocytes (Fig. 1). When MDM were stimulated with PMA, their effect on extracellular counts matched that of PMN.
DISCUSSION
Despite enormous progress in the field of antigen presentation, only limited data are available about the killing capacity of professional APC (7, 13). In this report, the levels of phagocytosis as well as the killing of bacteria by both professional phagocytes and MDM were compared. As expected, MDM ingested the highest number of pathogens per cell, and their ability to kill bacteria increased in the presence of PMA as an additional stimulus (4, 6). The phagocytotic activity of imDC was equivalent to that of PMN and monocytes, while that of maDC was significantly lower. A previous study demonstrated that imDC can efficiently phagocytose a variety of particular antigens, i.e., Saccharomyces cerevisiae, Corynebacterium parvum, S. aureus, zymosan particles, and latex beads, ranging in diameter from 0.5 to 6 μm (12). Moreover, Listeria monocytogenes and a recombinant Streptococcus gordonii strain induce DC maturation and reduce phagocytic activity upon ingestion (5, 9). The regrowth of extracellular bacteria following the initial decrease in the presence of imDC in the present study may also be explained by maturation of these cells after ingestion of bacteria.
Since the intracellular bacterial count in both imDC and maDC decreased slightly during the first 2 h of phagocytosis, and the extracellular counts decreased markedly, DC obviously destroy ingested pathogens. However, their killing capacity is limited in both extent and duration. Another study reported >95% killing of ingested E. coli and >75% killing of Salmonella enterica serovar Typhimurium by imDC after 6 h of incubation (15). Although we did not evaluate the intracellular count at incubation times shorter than 30 min, since killing activity might then be underestimated, these values seem high compared to our data, with which only a 50 to 70% reduction in the number of E. coli CFU and a 50% reduction in the number of S. aureus CFU were observed. However, a 50-μg/ml concentration of gentamicin was used to inactivate extracellular bacteria after phagocytosis and before evaluation of intracellular CFU counts in the previous study (15). Although it has been reported that this antibiotic does not accumulate within macrophages (18), others found a significant intracellular bactericidal activity when it was added to the extracellular fluid at concentrations exceeding 1 to 5 μg/ml (11, 19). Therefore, we used only washing steps in the standard assay to avoid any potential influence of antimicrobial agents (20). In accordance with our results, Mycobacterium tuberculosis has been shown to be readily phagocytosed by DC, but unlike with MDM, accelerated intracellular growth was observed (16).
No difference in levels of intracellular killing by imDC and maDC was detected, suggesting a minimal killing activity in both DC subtypes. Of note, the typical killing mechanisms used by professional phagocytes, such as superoxide radicals and myeloperoxidase activity, have not been detected in DC. By contrast, professional phagocytes demonstrated the well-known high and prolonged intracellular toxicity against pathogens. Although the in vivo relevance of the monocyte-derived DC used in our experiments remains to be cleared, this study confirms that the function of DC is mainly the presentation of antigens. imDC internalized more bacteria than maDC; this finding is in accordance with the physiological function of imDC as sentinels for incoming pathogens, while antigen presentation to naïve T cells is the specialty of the maDC. Thus, reported dissimilarities in phagocytic and killing activity correspond well with the present knowledge of function and cooperation between different leukocyte populations in immune response.
Acknowledgments
This work was supported by the Austrian Science Fund (grants P12298-MED and P15240), the BMWV, the Jubiläumsfonds of the Austrian National Bank (grants 6801/1 and 8366), the Ludwig-Boltzmann-Society, and the State of Tyrol.
References
- 1.Albert, M. L., S. F. Pearce, L. M. Francisco, B. Sauter, P. Roy, R. L. Silverstein, and N. Bhardwaj. 1998. Immature dendritic cells phagocytose apoptotic cells via αvβ5 and CD36, and cross-present antigens to cytotoxic T lymphocytes. J. Exp. Med. 188**:**1359-1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banchereau, J., F. Briere, C. Caux, J. Davoust, S. Lebecque, Y. J. Liu, B. Pulendran, and K. Palucka. 2000. Immunobiology of dendritic cells. Annu. Rev. Immunol. 18**:**767-811. [DOI] [PubMed] [Google Scholar]
- 3.Banchereau, J., and R. M. Steinman. 1998. Dendritic cells and the control of immunity. Nature 392**:**245-252. [DOI] [PubMed] [Google Scholar]
- 4.Bergamini, A., F. Bolacchi, B. Bongiovanni, M. Cepparulo, L. Ventura, M. Capozzi, C. Sarrecchia, and G. Rocchi. 2000. Granulocyte-macrophage colony-stimulating factor regulates cytokine production in cultured macrophages through CD14-dependent and -independent mechanisms. Immunology 101**:**254-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corinti, S., D. Medaglini, A. Cavani, M. Rescigno, G. Pozzi, P. Ricciardi Castagnoli, and G. Girolomoni. 1999. Human dendritic cells very efficiently present a heterologous antigen expressed on the surface of recombinant gram-positive bacteria to CD4+ T lymphocytes. J. Immunol. 163**:**3029-3036. [PubMed] [Google Scholar]
- 6.Gordon, S. 1999. Macrophages and the immune response, p. 533-545. In W. E. Paul (ed.), Fundamental immunology. Lippincott-Raven, Philadelphia, Pa.
- 7.Grage-Griebenow, E., H. D. Flad, M. Ernst, M. Bzowska, J. Skrzeczynska, and J. Pryjma. 2000. Human Mo subsets as defined by expression of CD64 and CD16 differ in phagocytic activity and generation of oxygen intermediates. Immunobiology 202**:**42-50. [DOI] [PubMed] [Google Scholar]
- 8.Kacani, L., I. Frank, M. Spruth, M. G. Schwendinger, B. Mullauer, G. M. Sprinzl, F. Steindl, and M. P. Dierich. 1998. Dendritic cells transmit human immunodeficiency virus type 1 to monocytes and monocyte-derived macrophages. J. Virol. 72**:**6671-6677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kolb-Mäurer, A., I. Gentschev, H.-W. Fries, F. Fiedler, E.-B. Bröcker, E. Kämpgen, and W. Goebel. 2000. _Listeria monocytogenes_-infected human dendritic cells: uptake and host cell response. Infect. Immun. 68**:**3680-3688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Longin, A., C. Souchier, M. Ffrench, and P. A. Bryon. 1993. Comparison of anti-fading agents used in fluorescence microscopy: image analysis and laser confocal microscopy study. J. Histochem. Cytochem. 41**:**1833-1840. [DOI] [PubMed] [Google Scholar]
- 11.Ohya, S., H. Xiong, Y. Tanabe, M. Arakawa, and M. Mitsuyama. 1998. Killing mechanism of Listeria monocytogenes in activated macrophages as determined by an improved assay system. J. Med. Microbiol. 47**:**211-215. [DOI] [PubMed] [Google Scholar]
- 12.Reise Sousa, C., P. D. Stahl, and J. M. Austyn. 1993. Phagocytosis of antigens by Langerhans cells in vitro. J. Exp. Med. 178**:**509-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rescigno, M., F. Granucci, S. Citterio, M. Foti, and P. Ricciardi Castagnoli. 1999. Coordinated events during bacteria-induced DC maturation. Immunol. Today 20**:**200-203. [DOI] [PubMed] [Google Scholar]
- 14.Romani, N., D. Reider, M. Heuer, S. Ebner, E. Kampgen, B. Eibl, D. Niederwieser, and G. Schuler. 1996. Generation of mature dendritic cells from human blood. An improved method with special regard to clinical applicability. J. Immunol. Methods 196**:**137-151. [DOI] [PubMed] [Google Scholar]
- 15.Schoppet, M., H. I. Huppertz, A. Simm, and A. Bubert. 2000. Infection of dendritic cells by enterobacteriaceae. Med. Microbiol. Immunol. 188**:**191-196. [DOI] [PubMed] [Google Scholar]
- 16.Steffen, S. 2000. Role of dendritic cells in host defence against M. tuberculosis. Immunol. Lett. 73**:**87. [Google Scholar]
- 17.Steinman, R. M. 1991. The dendritic cell system and its role in immunogenicity. Annu. Rev. Immunol. 9**:**271-296. [DOI] [PubMed] [Google Scholar]
- 18.Tabrizi, S. N., and R. M. Robins Browne. 1993. Elimination of extracellular bacteria by antibiotics in quantitative assays of bacterial ingestion and killing by phagocytes. J. Immunol. Methods 158**:**201-206. [DOI] [PubMed] [Google Scholar]
- 19.Utili, R., L. E. Adinolfi, M. Dilillo, M. F. Tripodi, A. Marrone, and G. Ruggiero. 1991. Activity of aminoglycosides against phagocytosed bacteria. J. Antimicrob. Chemother. 28**:**897-904. [DOI] [PubMed] [Google Scholar]
- 20.van den Broek, P. J., F. A. Dehue, P. C. Leijh, M. T. van den Barselaar, and R. van Furth. 1982. The use of lysostaphin in in vitro assays of phagocyte function: adherence to and penetration into granulocytes. Scand. J. Immunol. 15**:**467-473. [DOI] [PubMed] [Google Scholar]
- 21.Wenisch, C., S. Patruta, F. Daxbock, R. Krause, and W. Horl. 2000. Effect of age on human neutrophil function. J. Leukoc. Biol. 67**:**40-45. [DOI] [PubMed] [Google Scholar]