Lack of Intrinsic CTLA-4 Expression Has Minimal Effect on Regulation of Antiviral T-Cell Immunity (original) (raw)
Abstract
CTLA-4 is considered one of the most potent negative regulators of T-cell activation. To circumvent experimental limitations due to fatal lymphoproliferative disease associated with genetic ablation of CTLA-4, we have used radiation chimeras reconstituted with a mixture of CTLA-4+/+ and CTLA-4−/− bone marrow that retain a normal phenotype and allow the evaluation of long-term T-cell immunity under conditions of intrinsic CTLA-4 deficiency. Following virus infection, we profiled primary, memory, and secondary CD8+ and CD4+ T-cell responses directed against eight different viral epitopes. Our data demonstrate unaltered antigen-driven proliferation, acquisition of effector functions, distribution of epitope hierarchies, T-cell receptor repertoire selection, functional avidities, and long-term memory maintenance in the absence of CTLA-4. Moreover, regulation of memory T-cell survival and homeostatic proliferation, as well as secondary responses, was equivalent in virus-specific CTLA4+/+ and CTL-A-4−/− T-cell populations. Thus, lack of CTLA-4 expression by antigen-specific T cells can be compensated for by extrinsic factors in the presence of CTLA-4 expression by other cells. These findings have implications for the physiologic, pathological, and therapeutic regulation of costimulation.
The immune system is a complex and dynamic network of tissues, cells, and extracellular factors endowed with remarkable plasticity and the capacity to maintain its overall organization. While the preservation of its own structural features is intimately linked to the integrity of the organism it is part of, perturbation of the immune system by appropriate stimuli will result in a temporary and potentially profound disequilibrium before homeostasis is reinstated. One of the hallmarks of adaptive immunity is the controlled kinetics of specific T-cell populations generated in response to pathogens. By virtue of their preselected T-cell receptor (TCR), naive T cells specifically interact with pathogen-derived peptide fragments displayed by antigen-presenting cells (APC) in the context of the host's major histocompatibility complex (MHC) proteins. The resultant rapid expansion and acquisition of effector function by specific T-cell clones constitute the cellular basis for T-cell-mediated pathogen control and, following the subsequent contraction phase, the establishment of T-cell memory. However, the precise extent and quality of the T-cell response are determined by additional T-cell-APC interactions that are nonspecific and termed costimulatory insofar as they enhance proliferative or functional attributes of responding T-cell populations. Conversely, inhibitory interactions limit the initial expansion of T-cell clones and regulate the return to T-cell homeostasis following successful control of the pathogen.
Due to its potent and opposing effects on developing T-cell responses, the receptor pair CD28 and cytotoxic T-lymphocyte (CTL) antigen 4 (CTLA-4/CD152) has received considerable attention in the past years (2). While CD28, the dominant member for binding the B7.1 (CD80) and B7.2 (CD86) ligands (46), mediates costimulatory signals, CTLA-4 is one of the most powerful negative regulators of T-cell responses (10, 13, 79, 89). A physiological role for CTLA-4 has been documented in peripheral T-cell tolerance and anergy (30, 36, 39, 65, 86, 92), CD4+ T-cell differentiation and CD8+ CTL activity (40, 54, 56, 63, 71, 86), regulation of cell cycle progression (11, 21, 41, 85), and T-cell survival (20, 21, 31, 40, 72), as well as immunological memory and secondary T-cell responses (15, 18, 58). As expected from this functional diversity, CTLA-4-mediated interactions have been implicated in many areas of clinical relevance such as autoimmunity (69) and immune regulation by CD4+ CD25+ regulatory T cells (66, 70, 78), tumor immunity (16), transplantation (69), and host defense (33, 55, 60). Furthermore, costimulatory blockade with CTLA-4 fused to an immunoglobulin (Ig) backbone (CTLA-4 Ig) has been explored in preclinical and clinical settings with the intent of therapeutic reduction of T-cell responses, and the targeted blockade of CTLA-4 itself has gained interest for its potential to enhance T-cell responses to tumors and vaccines. Yet, despite much effort, the details of the molecular mechanism(s) by which CTLA-4 exerts its function remain to be elucidated. CTLA-4 may, in fact, act at different levels of T-cell-APC interactions, including competitive blockade of CD28-B7 interactions (54, 79), disruption of the organizational structure of the immunological synapse (16), modulation of TCR signaling (12, 43, 53, 54), and induction of secondary immunoregulatory factors such as transforming growth factor β (TGF-β) (19), as well as B7-dependent reverse signaling and modulation of tryptophan catabolism in APC (24, 32).
Some of the most compelling evidence for the nonredundant physiological role of CTLA-4 has come from its genetic ablation. CTLA-4-deficient mice develop a severe lymphoproliferative disorder and die within a few weeks after birth (14, 80, 90). The lethal phenotype has hampered the study of long-term T-cell immunity in the absence of CTLA-4 and necessitated the use of experimental systems that minimize the lymphoproliferative pathology of CTLA-4-deficient mice by limiting the available TCR repertoire in crosses to TCR-transgenic mice. However, in the presence of bone marrow-derived CTLA-4-bearing cells, polyclonal CTLA-4-deficient T cells failed to develop an abnormal phenotype in vivo, and prevention of lethal disease was suggested to be achieved by factors produced by wild-type cells (3, 4). Similarly, adoptive transfer of CTLA-4−/− T cells obtained from young CTLA-4−/− donors was shown to cause lethal inflammatory disease in adult Rag2−/− recipients but admixture of CTLA-4+/+ T cells with the pathogenic CTLA-4−/− cells could prevent disease by selective elimination of transferred CTLA-4−/− T cells (81). These findings suggest that loss of CTLA-4 by T-cell populations can be compensated for, to a certain extent, by CTLA-4 expression in trans.
To evaluate the scope and efficiency of CTLA-4 transregulation, we have used mixed CTLA-4 radiation bone marrow chimeras and examined specific T-cell responses with unbiased TCR repertoires generated against a viral pathogen in vivo. Our results confirm previous observations made by Bachmann et al. in a similar model system (3, 4) and provide further evidence that lack of intrinsic CTLA-4 expression does not affect the generation of primary virus-specific CD8+ or CD4+ T-cell responses. In addition, our findings indicate that neither the maintenance nor the recall capacity of virus-specific memory T cells is modulated by absence of T-cell-expressed CTLA-4 and thus demonstrate an unexpected cellular autonomy and systemic flexibility in the intrinsic absence of a major negative regulator of T-cell immunity.
MATERIALS AND METHODS
Mice.
C57BL/6 (B6; DbKbIAb/Thy1.2+ [CD90.2]/CD45.2) mice and congenic B6.PL mice (B6.PL; Ty1.1+ [CD90.1]/CD45.2) or congenic B6.CD45.1 mice (B6.CD45.1; Thy1.2+ [CD90.2]/CD45.1) were obtained from the Rodent Breeding Colony at The Scripps Research Institute. CTLA-4-deficient mice (B6.CTLA-4−/−; DbKbIAb/Thy1.2+ [CD90.2]/CD45.2) have been described previously (90) and were provided by M.-L. Alegre. All mice were housed under specific-pathogen-free conditions.
Generation of mixed CTLA-4+/+ × CTLA-4−/− bone marrow chimeras.
Mixed bone marrow chimeras were generated as described recently (22). Briefly, groups of B6.PL (Thy1.1+) mice were lethally irradiated (1,100 cGy). Bone marrow from young B6.CTLA-4−/− (Thy1.2+) and age-matched B6.PL (Thy1.1+) mice was depleted of T cells with T24 (anti-Thy1.1/2) antibody and guinea pig complement (Sigma, St. Louis, Missouri). Alternatively, T cells were depleted with antibody-coated magnetic beads (7). A mixture of equal numbers (2 × 106 each) of T-cell-depleted bone marrow cell suspensions was then injected intravenously (i.v.) into irradiated hosts. Mixed bone marrow chimeras subsequently reconstituted T-cell populations which consisted of grossly equal numbers of CTLA-4+/+ (Thy1.1+) and −/− (Thy1.2+) peripheral T cells; chimeras that reconstituted the wild-type or CTLA-4-deficient T-cell compartment in excess of 60% were excluded from the study. To verify CTLA-4 deficiency, purified Thy1.2+ cells from these mice were screened for CTLA-4 by PCR according to standard procedures. Control chimeras were reconstituted in an analogous fashion with mixtures of congenic (Thy1.1+ and Thy1.2+), T-cell-depleted CTLA-4+/+ bone marrow cells.
Viruses.
The Armstrong strain of lymphocytic choriomeningitis virus (LCMV), clone 53b, and more-virulent variant clone 13 (73) were used throughout the experiments. LCMV was plaque purified three times on Vero cells and stocks prepared by a single passage on BHK-21 cells. Eight to 12 weeks after bone marrow reconstitution, mixed chimeras were infected with a single intraperitoneal dose of 105 PFU LCMV Armstrong. For secondary challenge, 2 × 106 PFU LCMV clone 13 was administered i.v.
CTL assays.
For evaluation of ex vivo CTL activity, single-cell suspensions obtained from the spleen and lymph nodes 8 days after infection with LCMV were stained for Thy1.1, Thy1.2, and CD4 to avoid blocking of the CD8 coreceptor. CD8+ T cells were then enriched by sorting CTLA-4+/+ (Thy1.1+)/CD4− and CTLA-4−/− (Thy1.2+)/CD4− T cells and used as effectors in a standard 5-h 51Cr release assay on LCMV-infected and uninfected MC57 target cells (35) at effector/target ratios of 10:1, 5:1, 2.5:1, and 1.25:1.
Antibodies and MHC class I tetramers.
The following fluorescein isothiocyanate (FITC)-, phycoerythrin (PE)-, PE-Cy5-, peridinin chlorophyll-a protein (PerCP)-, or allophycocyanin-conjugated and/or biotinylated antibodies (BD PharMingen, La Jolla, California) were used for flow cytometry: CD4 (RM4-5), CD8a (53-6.7), CD44 (IM7), CD25 (7D4), CD45.2 (104), CD69 (H1.2F3), CD62L (MEL-14), Thy1.1 (Ox-7), Thy1.2 (53-2.1), CTLA-4 (UC10-4F10-11), Vβ2 (B20.6), Vβ3 (KJ25), Vβ4 (KT4), Vβ5.1/5.2 (MR9-4), Vβ6 (RR4-7), Vβ7 (TR310), Vβ3 (KJ25), Vβ8.1/8.2 (MR5-2), Vβ8.3 (1B3.3), Vβ9 (MR10-2), Vβ10b (B21.5), Vβ11 (RR3-15), Vβ12 (MR11-15), Vβ13 (MR12-3), Vβ14 (14-2), IL-2 (JES6-5H4), IFN-γ (gamma interferon; XMG1.2), and TNF-α (MP6-XT22). Intracellular detection of Bcl-2 and Bcl-xL was performed with PE-conjugated, Bcl-2-specific (3F11) and isotype control (A19-3) (BDPharMingen), as well as Bcl-xL-specific (7B2.5) and isotype control (B10), antibodies (Southern Biotechnology Associates, Birmingham, Alabama). For five-color flow cytometry, streptavidin-PharRed (BDPharMingen) was used in addition as a secondary reagent. DbGP33, DbNP396, DbGP276, and KbGP34 tetramers were obtained as allophycocyanin and/or PE conjugates or biotinylated monomers from the Tetramer Core Facility, Emory University, Atlanta, Georgia, and used as described previously (34).
Intracellular cytokine staining for flow cytometry.
Single-cell suspensions from lymphatic organs were restimulated for 5 h with 1 μg/ml MHC class I (PeptidoGenic, Livermore, California)-restricted viral peptides or 2 μg/ml MHC class II (Chiron, Clayton Victoria, Australia)-restricted viral peptides in the presence of 10 to 50 U/ml recombinant human interleukin-2 (PharMingen) and 1 μg/ml brefeldin A (Sigma). Staining of cell surface antigen and intracellular antigens was performed as described previously (34). Negative controls were (i) peptide-restimulated cells from uninfected mice, (ii) cells restimulated for 5 h in the absence of viral peptides, and (iii) cells stained with conjugated cytokine-specific antibodies preincubated for 30 min at 4°C with an excess of recombinant cytokine. In some cases, polyclonal stimulation was provided by 5 ng/ml phorbol myristate acetate and 500 ng/ml ionomycin (Sigma) in the presence of brefeldin A. Cells were acquired with a FACSort or FACScalibur flow cytometer (Becton Dickinson & Co., San Jose, California) with Cell Quest software (Becton Dickinson). For five-color analyses, a FACSVantage SE flow cytometer (Becton Dickinson) was used.
In vivo proliferation assays.
A bromodeoxyuridine (BrdU; Sigma) solution of 0.8 mg/ml of sterile water was prepared fresh daily and supplied as drinking water for the first 8 days of virus infection (pulse period). Mice were subsequently switched to regular drinking water (chase period). Intracellular detection of BrdU and cytokines in peptide-restimulated T cells was performed by using the reagents and protocols provided by manufacturer (BrdU flow kit; BDPharMingen). Ki-67, a nuclear cell proliferation-associated antigen expressed in all active stages of the cell cycle, was detected with the FITC-conjugated B56 clone and MOPC 21 isotype control (BDPharMingen). The B56 antibody, specific for human Ki-67, cross-reacts with the murine equivalent.
In vivo IDO blockade.
Slow-release pellets containing 200 mg 1-methyl-dl-tryptophan released over a period of 21 days and placebo pellets were purchased from Innovative Research of America (Sarasota, FL). This dosage was previously shown to effectively inhibit indoleamine 2,3-dioxygenase (IDO) (32). To implant pellets, anesthetized mice were shaved in a small dorsal area above the thoracic vertebrae and an ∼2-cm incision was made. A small subcutaneous pocket was gently enlarged with forceps, the pellet implanted, and the incision closed. One day later, mice were infected with LCMV.
Adoptive transfers.
Approximately 8 months after primary infection, 2 × 107 lymph node cells from mixed CTLA-4 chimeras were transferred i.v. into congenic CD45.1 hosts. Two days later, mice were challenged with 2 × 106 PFU LCMV clone 13 i.v.
Statistical analyses.
Data handling, analysis, and graphic representation were performed with Prism 3 (GraphPad Software, San Diego, California).
RESULTS
Normal phenotype of mixed CTLA-4+/+× CTLA-4−/− chimeras.
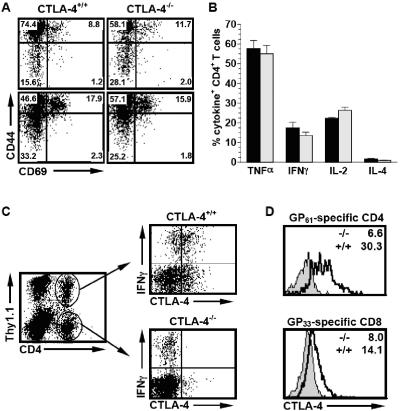
Mixed CTLA-4 bone marrow chimeras were generated by reconstituting lethally irradiated B6.PL mice (H-2b CTLA-4+/+ Thy1.1+) with a 1:1 mixture of bone marrow derived from syngeneic wild-type B6.PL and congenic CTLA-4-deficient B6.CTLA-4−/− mice (H-2b CTLA-4−/− Thy1.2+). This experimental design allows the distinction of CTLA-4+/+ and CTLA-4−/− T cells based on the allelic Thy1 marker and guarantees that residual host T cells (Thy1.1+) are appropriately identifiable as CTLA-4+/+. As reported previously (22) and similar to mixed CTLA-4 chimeras created with Rag-deficient hosts (3, 4), the chimeras showed no signs of abnormal T-cell activation or lymphoproliferative disorder. Over an observation period of >16 months, CTLA-4+/+ and CTLA-4−/− CD8+ and CD4+ T cells gradually upregulated CD44 expression while retaining modest levels of CD69 and CD25 expression, comparable to wild-type mice (Fig. 1A and data not shown). To evaluate the functional potential of T cells, cytokine production was determined after brief polyclonal stimulation. As shown in Fig. 1B, wild-type and CTLA-4-deficient CD4+ T cells had virtually identical cytokine profiles. Furthermore, comparing the fluorescence intensities of specific cytokine stains revealed no differences in the amounts of antigens detected in CTLA-4+/+ and CTLA-4−/− cells (not shown). Similar results were obtained for CD8+ T cells (not shown) and demonstrate that CTLA-4-deficient T cells in a chimeric environment do not exhibit the Th2 skewing observed in CTLA-4−/− mice (40, 63) and some models of CTLA-4 blockade (55, 86).
FIG. 1.
Phenotype and functional profile of T cells from mixed CTLA-4 bone marrow chimeras. (A) Activation and memory marker expression on CTLA-4+/+ and CTLA-4−/− T cells. Splenocytes from aged bone marrow chimeras (∼8 months after reconstitution) were stained for CD8 (top) or CD4 (bottom), as well as Thy1.1, CD44, and CD69. Dot plots are gated on CD8+ or CD4+ T cells among Thy1.1+ CTLA-4+/+ (left) or Thy1.1− CTLA-4−/− (right) populations. (B) Cytokine profiles of CTLA-4+/+ and CTLA-4−/− CD4+ T cells. Peripheral blood lymphocytes were polyclonally stimulated and stained for CD4 and the indicated cytokines as detailed in Materials and Methods. Percentages of cytokine-producing CD4+ T cells among CTLA-4+/+ (black bars) and CTLA-4−/− (gray bars) CD4+ T cells are shown. Values and error bars represent the standard error of the mean of four mice tested (n = 4 per group). (C) IFN-γ production and CTLA-4 expression by virus-specific CD4+ T cells. Eight days after LCMV infection, IFN-γ production and CTLA-4 expression by CTLA4+/+ and CTLA-4−/− CD4+ T cells were analyzed in the same sample (top right, gated on Thy1.1+ [CTLA-4+/+] cells; bottom right, gated on Thy1.1− [CTLA-4−/−] cells). CTLA-4−/− CD4+ T cells also serve as internal negative controls for CTLA-4 staining. (D) CTLA-4 expression levels in virus-specific CD4+ and CD8+ T cells. Values on the right (gated on virus-specific [IFN-γ+] CD4+ or CD8+ T cells) indicate the geometric mean fluorescence intensity of CTLA-4 staining among GP61-specific CD4+ (upper) and GP33-specific CD8+ (lower) T cells (black tracings, Thy1.1+ IFN-γ+ CTLA-4+/+ cells; gray histograms, Thy1.1− IFN-γ+ CTLA-4−/− cells).
CTLA-4 expression by virus-specific T cells in mixed bone marrow chimeras.
The mixed CTLA-4 chimeras are a unique model system to analyze pathogen-specific T-cell responses with unbiased TCR repertoires in the absence of a confounding lymphoproliferative disorder. After peripheral challenge with LCMV Armstrong, the mixed chimeras controlled the virus with normal kinetics (not shown), making this model system suitable for further study of effective antiviral T-cell immunity. In H-2b mice, analysis of six MHC class I-restricted epitopes derived from the LCMV glycoprotein (GP33, GP92, GP118, and GP276) or nucleoprotein (NP205 and NP396) (84) covers nearly the entire virus-specific CD8+ T-cell response (34, 59). Furthermore, analysis of just two MHC class II-restricted epitopes (GP61 and NP309) (64) accounts for approximately 80% of the virus-specific CD4+ T-cell response (34, 38). Here we have used intracellular cytokine staining and MHC tetramers to characterize the generation of a polyclonal, specific T-cell response in its near entirety after LCMV infection of mixed CTLA-4 chimeras. Although CTLA-4 is not readily detectable in naive cells, it is upregulated upon T-cell activation (45, 87). Recent work by Slifka and Whitton has shown that CTLA-4 is also upregulated by LCMV-specific CD8+ T cells in the early phase of the primary immune response but downregulated at the peak of the CD8+ response (76). In agreement with this observation, LCMV-specific CTLA-4+/+ CD8+ T cells generated in mixed chimeras showed only modest CTLA-4 expression 8 days after virus infection compared to the virus-specific CTLA-4-deficient internal controls (Fig. 1D). In contrast, activated virus-specific CD4+ T cells demonstrated clearly upregulated CTLA-4 levels comparable to those in wild-type mice (Fig. 1C and D and data not shown). The differential CTLA-4 expression by virus-specific CD4+ and CD8+ T-cell subsets in our model renders the postulated differential susceptibility to CTLA-4 deficiency (17) testable with polyclonal T-cell populations with defined pathogen specificity.
Scope and focus of the primary T-cell response.
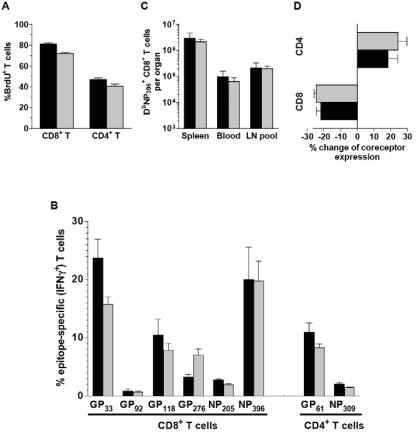
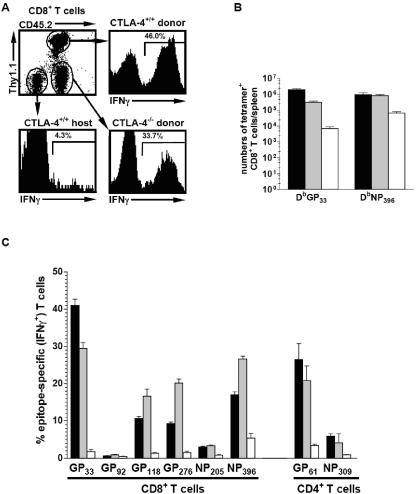
Following LCMV infection, spleen cells in chimeras and wild-type mice expanded alike to counts of ∼1.5 × 108 at the height of the CD8+ T-cell response. To directly visualize the extent of antigen-driven proliferation, chimeras were pulsed with drinking water containing the nucleotide analog BrdU during the first 8 days of infection. Detection of BrdU incorporation by T cells demonstrated that mobilization of ∼90% of CD8+ and ∼50% of CD4+ T cells was comparable among CTLA-4+/+ and CTLA-4−/− subsets (Fig. 2A) and similar to that in LCMV-infected wild-type mice (34). As expected, virtually all epitope-specific CD8+ and CD4+ T cells had acquired BrdU during this period and no significant differences were observed between specific CTLA-4+/+ and CTLA-4−/− T cells (data not shown and references 34 and 59). Interestingly, absence of CTLA-4 had little effect on the overall magnitude of virus-specific CD8+ and CD4+ T-cell responses as there were no gross differences between specific CTLA-4+/+ and CTLA-4−/− T-cell numbers determined in the spleen, lymph nodes, and blood in the course of the primary T-cell response (Fig. 2B and C). Similarly, direct ex vivo CTL activity of LCMV-specific CD8+ effector T cells was comparable among CTLA-4+/+ and CTLA-4−/− CTL populations isolated from the spleen and lymph nodes (data not shown). Although the absolute numbers of specific T cells shown in Fig. 2C are comparable, it should be noted that such an analysis is a function of the relative reconstitution with naive CTLA-4+/+ and CTLA-4−/− T cells in the chimeras. Thus, we focused on specific T-cell frequencies rather than absolute numbers in the majority of our experiments. Here, we did indeed observe some changes in the magnitude of individual epitope-specific CTLA-4−/− and CTLA-4+/+ T-cell populations (i.e., a decrease [GP33 and GP61 specific] or an increase [GP276 specific] in CTLA-4−/− compared to CTLA-4+/+ T-cell frequencies). The significance of these findings remains unclear, as previous work by Bachmann and colleagues found no numerical differences in GP33-specific CTLA-4+/+ and CTLA-4−/− CD8+ T-cell compartments of reconstituted RAG-2−/− recipients (3). Given the immunodominance of GP33-specific CD8+ and GP61-specific CD4+ T cells and their slightly reduced expansions in the CTLA-4−/− compartment in our model system, we selected these populations for further functional evaluation.
FIG. 2.
Analysis of the primary T-cell response in mixed CTLA-4 bone marrow chimeras. (A) Antigen-driven proliferation. The extent of antigen-driven CD8+ and CD4+ T-cell proliferation during the first 8 days of virus infection was determined by BrdU incorporation as detailed in Materials and Methods. Black bars, CTLA-4+/+ cells; gray bars, CTLA-4−/− cells. (B) Magnitude and epitope distribution of the virus-specific T-cell response. The fraction of epitope-specific T cells was determined 8 days after LCMV infection by intracellular cytokine staining as detailed in Materials and Methods. Data (mean ± the standard error of the mean) are representative of two to four mice tested in three independent experiments. (C) Distribution of virus-specific T cells in different organs. Absolute numbers of NP396-specific CD8+ T cells detected with DbNP396 tetramers were calculated by multiplying the fraction of tetramer+ cells among CD8+ T cells by the fraction of CD8+ T cells among live cells, as well as total live spleen cell counts or total pooled (inguinal, mesenteric, axillary, and cervical) lymph node (LN) counts. For an estimate of the total specific CD8+ T cells in blood, the product of the tetramer+ and CD8+ T-cell fractions was multiplied by the white blood cell count per cubic millimeter and a coefficient of 5,850/100 g of body weight. (D) Differential regulation of coreceptor expression. Changes in CD4 and CD8 coreceptor expression on activated T cells 8 days after LCMV is plotted as percent change in the coreceptor geometric mean fluorescence intensity by comparing proliferated (BrdU+) to resting (BrdU−) T cells within the same samples.
Coreceptor regulation, TCR repertoire, and avidities of virus-specific T cells.
It has been shown in several experimental systems, including the LCMV model (76), that activated specific CD8+ effector T cells downregulate CD8 expression. In contrast, CD4 expression by CD4+ T cells is upregulated following antigen encounter (67). While the biological meaning of differential coreceptor regulation remains elusive, altered expression levels are useful for identification and characterization of activated antigen-specific T cells. Since LCMV infection is accompanied by only minimal bystander proliferation (34, 59), we could directly evaluate coreceptor expression by proliferated (BrdU-positive, mostly LCMV-specific) and resting (BrdU-negative, nonspecific) T cells at the height of the primary immune response. As shown in Fig. 2D, activated CTLA-4+/+ and CTLA-4−/− CD8+ T cells downregulated CD8 expression to similar degrees while CD4+ T cells demonstrated a concomitant CD4 upregulation.
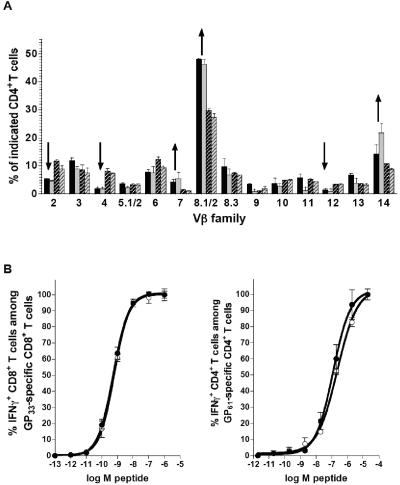
A primary role for CTLA-4 in the regulation of peripheral T-cell homeostasis rather than central tolerance was suggested by the finding of apparently normal thymocyte development in CTLA-4−/− mice (14). Recent analysis of polyclonal T-cell expansions in these mice have furthermore demonstrated a diverse and unbiased peripheral TCR repertoire (28). Our findings demonstrate that virus-specific CTLA-4+/+ and CTLA-4−/− CD4+ T-cell precursors, derived from an unbiased TCR repertoire present among wild-type and CTLA-4-deficient T cells in the mixed chimeras, are selected from all Vβ families. Their differential expansion leads to a degeneration of the TCR Vβ profile indistinguishable between wild-type and deficient T cells (Fig. 3A). As expected, TCR repertoire selection among virus-specific CD8+ T cells, which are thought to be less dependent on CTLA-4-mediated costimulatory regulation, was also identical among CTLA-4+/+ and CTLA-4−/− subsets (data not shown).
FIG. 3.
TCR repertoire and functional avidities of virus-specific T cells. (A) CD4+ T-cell TCR repertoire. TCR Vβ usage by GP61-specific CTLA-4+/+ (black) and CTLA-4−/− (light gray), as well as other CTLA-4+/+ (black hatched) and CTLA-4−/− (gray hatched), CD4+ T cells is shown 8 days after LCMV infection. Arrows indicate narrowing (degeneration) of the TCR repertoire among GP61-specific T cells compared to other CD4+ T cells. (B) Functional avidities of virus-specific effector T cells. Effector T-cell populations were incubated with graded concentrations of MHC class I (GP33) or II (GP61) peptides and subsequently stained for intracellular IFN-γ. Functional avidities were measured as peptide concentrations that elicited detectable IFN-γ production in 50% of a specific T-cell population. The 50% effective concentration was 5.3 × 10−10 M for CTLA-4+/+ GP33-specific CD8+ T cells, 6.1 × 10−10 M for CTLA-4−/− GP33-specific CD8+ T cells, 6.0 × 10−8 M for CTLA-4+/+ GP61-specific CD4+ T cells, and 1.1 × 10−7 M for CTLA-4−/− GP61-specific CD4+ T cells. Closed symbols, CTLA-4+/+; open symbols, CTLA-4−/−.
A recent study has suggested that engagement of CTLA-4 may promote diversity of T-cell responses by preferential regulation of high-avidity T cells (42). We evaluated functional avidities of epitope-specific CD4+ and CD8+ T-cell populations by measuring the fraction of IFN-γ-producing T cells as a function of the peptide concentration required to induce cytokine synthesis. Functional avidities determined in this manner were virtually identical between virus-specific CTLA-4+/+ and CTLA-4−/− T cells (Fig. 3B) and reproduced the difference between CD8+ (high avidities) and CD4+ (lower avidities) T cells found in wild-type mice (34). Furthermore, NP396- and NP205-specific CD8+ T-cell populations, which have respective ∼5- and ∼10-fold higher avidities than GP33-specific CD8+ T cells (D.H., unpublished observation), are not preferentially expanded in the CTLA-4−/− compartment (Fig. 2C). Thus, lack of CTLA-4 expression by virus-specific T cells does not alter the coreceptor regulation, selection of a TCR repertoire, functional responsiveness, or population size of specific high- and low-avidity T-cell responders.
Memory maintenance and homeostatic proliferation.
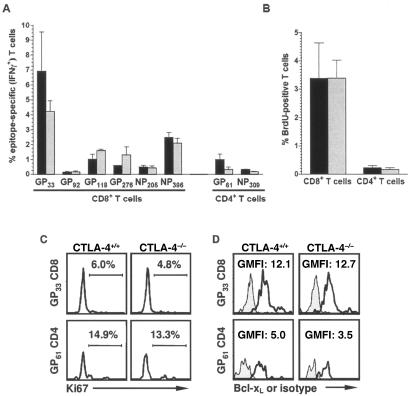
To investigate whether CTLA-4 was involved in the maintenance of virus-specific T-cell memory, frequencies of LCMV-specific T cells were determined at various time points after infection. Up to 8 months after infection, epitope-specific CD8+ and CD4+ memory T-cell populations were maintained equally well irrespective of their CTLA-4 genotype (Fig. 4A). In parallel, mice that received a BrdU pulse during the first 8 days of infection (resulting in BrdU incorporation by all specific T cells) were subsequently switched to regular drinking water and the loss of BrdU among virus-specific T cells was monitored during this chase phase (25, 82). No differential kinetics of BrdU loss were observed among CTLA-4+/+ and CTLA-4−/− virus-specific T cells. Interestingly, even ∼8 months after infection, a fraction of cells among all epitope-specific populations retained detectable BrdU (∼10% of specific CD8+ and ∼5% of specific CD4+ T cells), indicating minimal or no turnover in a subpopulation of memory T cells (Fig. 4B). In addition, cell cycle analysis with an antibody specific for the proliferation-associated antigen Ki-67 confirmed similar proliferative activity among virus-specific memory T-cell populations irrespective of their capacity to express CTLA-4 (Fig. 4C). It should be noted, however, that the fraction of Ki-67+ CD4+ memory T cells in the mixed chimeras is clearly higher than that found in wild-type mice (44), indicating that reduced systemic availability of CTLA-4 in the mixed chimeras may facilitate homeostatic proliferation of CD4+ memory T cells.
FIG. 4.
Survival and homeostatic proliferation of memory T cells. (A) Maintenance of T-cell memory. The extent and distribution of LCMV-specific T-cell memory were evaluated among CTLA-4+/+ (black bars) and CTLA-4−/− (gray bars) T cells 238 days after infection by intracellular cytokine staining. Representative data (mean ± the standard error of the mean) for two or three mice tested in two independent experiments are shown. (B) Determining proliferation by BrdU chase. After pulsing CD8+ and CD4+ T cells during the first 8 days of virus infection, mice were switched to regular drinking water and loss of BrdU by T cells was monitored. Approximately 8 months after infection, only a fraction of the CD8+ and CD4+ T cells retained detectable BrdU. Black bars, CTLA-4+/+; gray bars, CTLA-4−/−. (C) Proliferation of specific memory T cells. Expression of the nuclear cell proliferation-associated antigen Ki-67, expressed in all active stages of the cell cycle, is shown 238 days after virus infection in GP33-specific CD8+ (top) and GP61-specific CD4+ (bottom) T cells identified by intracellular IFN-γ staining. (D) Bcl-xL expression by virus-specific T cells. Bcl-xL (black tracings) was detected in GP33-specific CD8+ (top) and GP61-specific CD4+ (bottom) T cells identified by intracellular IFN-γ staining 238 days after virus infection as detailed in Materials and Methods. Gray histograms, isotype staining. Values indicate the normalized geometric mean of fluorescence intensity derived by dividing the geometric mean fluorescence intensity of Bcl-xL staining by the geometric mean fluorescence intensity of corresponding isotype control staining.
Regulation of memory T-cell survival.
The potential role of CTLA-4 in the regulation of T-cell survival and apoptosis remains controversial (16). Both induction and inhibition of apoptosis via CTLA-4 have been described previously (20, 31, 72). Furthermore, while upregulation of the survival factor Bcl-xL via CD28 engagement is not impaired by CTLA-4 ligation (9), recent reports have demonstrated that CD28-mediated survival signals can be antagonized by CTLA-4 in vitro (21), and an apoptosis-resistant phenotype of CTLA-4-deficient T cells was associated with increased Bcl-xL expression in vivo (40). Analysis of Bcl-xL expression by virus-specific memory T cells in our experimental system revealed equivalent levels of Bcl-xL in both CTLA-4+/+ and CTLA-4−/− compartments (Fig. 4D). Furthermore, the differential Bcl-xL expression among specific CD8+ and CD4+ T cells, which we reported to be associated with impaired survival of CD4+ memory T cells (34), was also observed in CTLA-4-deficient memory T cells. Expression of another survival factor, Bcl-2, is downregulated in effector T cells but significantly elevated in memory T cells (29, 34). In the mixed CTLA-4 chimeras, regulation of Bcl-2 expression followed identical patterns in wild-type and deficient LCMV-specific T cells (not shown). These findings suggest that T-cell survival is not differentially regulated in the absence of T-cell endogenous CTLA-4 expression.
Secondary T-cell responses.
The biological importance of antigen-specific memory T cells maintained at elevated frequencies and committed to defined effector functions lies in their role for facilitated and accelerated control of pathogens upon reinfection. To evaluate the capacity of CTLA-4+/+ and CTLA-4−/− memory T cells to respond to a secondary challenge in vivo, memory T cells obtained ∼8 months after primary challenge were adoptively transferred into congenic CD45.1 mice, which were challenged 2 days later with a high dose of LCMV clone 13. In the absence of functional LCMV-specific memory, infection with more-virulent clone 13 causes an abortive T-cell response and results in virus persistence (73). Six days following rechallenge, mice were analyzed for expansion of virus-specific CTLA-4+/+ and CTLA-4−/− memory T cells. As shown in Fig. 5, memory T cells of both wild-type and deficient compartments comprising all epitope specificities expanded preferentially and dominated the accelerated immune response. The extents of the CTLA-4+/+ and CTLA-4−/− T-cell responses were comparable, and the distribution of epitope hierarchies was largely preserved. Nevertheless, some differences in the magnitude of individual CTLA-4+/+ and CTLA-4−/− epitope-specific T-cell populations were apparent, a likely reflection of similar differences observed in memory populations (Fig. 4A). Finally, the presence of high numbers of memory T cells did not preclude the generation of a concurrent primary response, as demonstrated by small but distinct virus-specific T-cell populations among the CD45.1+ host cells, an observation that is in agreement with studies utilizing similar adoptive transfer systems for the visualization of secondary T-cell responses (6, 83).
FIG. 5.
Secondary T-cell responses in the absence of intrinsic CTLA-4 expression. Memory T cells obtained ∼8 months after primary infection of mixed CTLA-4 chimeras were transferred into congenic (CD45.1) hosts that were subsequently challenged with high-dose LCMV. (A) Epitope-specific CD8+ T-cell responses. GP33-specific CD8+ T-cell responses by donor CTLA-4+/+ (CD45.2+ Thy1.1+), donor CTLA-4−/− (CD45.2+ Thy1.1−), and host CTLA-4+/+ (CD45.2− Thy1.2−) CD8+ T cells 6 days after rechallenge are shown. (B) Quantitation of secondary T-cell responses. Absolute numbers of splenic epitope-specific CD8+ T cells identified by MHC class I tetramer staining are shown (black, CTLA-4+/+ donor; gray, CTLA-4−/− donor; white, CTLA-4+/+ host). (C) Extent and distribution of LCMV-specific secondary T-cell responses. Epitope-specific T cells identified 6 days after secondary challenge by intracellular cytokine staining are shown. CTLA-4+/+ donor, black bars; CTLA-4−/− donor, gray bars; CTLA-4+/+ host, white bars. Values represent the mean ± the standard error of the mean of four mice per group.
Role of tryptophan catabolism in the primary T-cell response.
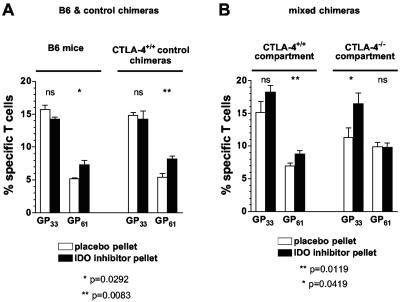
Work by P. Puccetti's group has made the intriguing proposal that CTLA-4-expressing cells, including CD4+ CD25+ regulatory T cells, modulate tryptophan catabolism in APC by engagement of B7 (reverse signaling) and induction of the immunomodulatory enzyme IDO (24, 32). As this mechanism may explain, at least in part, the effectiveness of CTLA-4 transregulation, we employed pharmacological blockade of IDO in wild-type mice, as well as control and mixed CTLA-4 chimeras. We hypothesized that in mixed CTLA-4 chimeras, induction of IDO by CTLA-4+/+ T cells may be sufficient to allow the development of normal T-cell responses but additional blockade of IDO may render the CTLA-4−/− compartment susceptible to facilitated expansion. In wild-type mice and control chimeras, specific CD8+ T-cell responses were not affected by IDO inhibition but in experimental chimeras a preferential increase in the CTLA-4−/− subset was noted (Fig. 6). The observation that CTLA-4+/+ CD8+ T-cell responses were also slightly (though not significantly) enhanced is compatible with the notion that CTLA-4-dependent regulation of IDO in the mixed chimeras is a direct function of the reduced numbers of CTLA-4+/+ cells and therefore pharmacological IDO blockade is more effective in mixed than control chimeras or wild-type mice. However, while the facilitated CD4+ T-cell expansion in B6 mice treated with IDO blockade and reproduced in control chimeras (Fig. 6A) conforms to the established view that functional incapacitation of CTLA-4 facilitates T-cell responses preferentially in the CD4 compartment, a corresponding increase was found only in the CTLA-4+/+ CD4 compartment of mixed chimeras and not among CTLA-4−/− CD4+ T-cell populations (Fig. 6B). In summary, primary antiviral T-cell responses are only slightly affected by IDO blockade. Nevertheless, it appears that IDO functions preferentially as a negative regulator of specific CD4+ T-cell responses while a similar role for CD8+ T-cell responses is unmasked only in the mixed CTLA-4 chimeras, i.e., under conditions of reduced systemically available CTLA-4.
FIG. 6.
Effect of IDO blockade on primary virus-specific T-cell responses. B6 mice, as well as control and mixed CTLA-4 chimeras, were implanted with slow-release pellets containing the IDO inhibitor 1-methyl-tryptophan or placebo pellets. One day after implantation, mice were infected with LCMV and frequencies of specific T cells (GP33-specific CD8+ and GP61-specific CD4+ T cells) were determined 8 days later. (A) IDO blockade in B6 mice and control chimeras. The congenic CTLA-4+/+ Thy1.1+ and Thy1.2+ populations in control chimeras gave equivalent results and are therefore displayed with combined values. Statistically significant differences between CTLA-4+/+ and CTLA-4−/− T-cell populations were calculated by Student's t test (ns, not significant; *, P = 0.0292; **, P = 0.0083). (B) IDO blockade in mixed CTLA-4 chimeras indicates differential responsiveness in CTLA-4+/+ and CTLA-4−/− compartments. Values represent the mean ± the standard error of the mean of three mice per group in one of two independent experiments.
DISCUSSION
The present study was conceived to evaluate polyclonal, pathogen-specific T-cell responses under conditions of CTLA-4 deficiency. By choosing an experimental system that allows identification of nearly all specific CD8+ and CD4+ T cells generated against a viral pathogen by its natural murine host, we aimed at a comprehensive approach suitable to understand complex interactions between host and pathogen in vivo. Since genetic disruption of CTLA-4 results in lethal disease, we used radiation bone marrow chimeras to study long-term T-cell immunity. This experimental design also enabled us to simultaneously evaluate the potentially differential impact of intrinsic CTLA-4 deficiency on CD8+ and CD4+ T-cell immunity. CD4+ T cells have been shown to initiate the lymphoproliferative pathology in CTLA-4-deficient mice and both primary and secondary proliferative CD4+ T-cell responses are increased in CTLA-4−/− TCR-transgenic mice (15, 17). Similarly, CTLA-4 blockade during initiation of parasite-specific T-cell responses amplified Th2- or Th1-type responses (33, 55, 60, 68). In contrast, although CTLA-4 blockade could enhance antigen-specific CD8+ T-cell immunity induced by peptide-pulsed dendritic cells (56), generation of primary CD8+ T-cell responses was not affected when tested in CTLA-4−/− TCR-transgenic mice (5, 18). The latter results have recently been confirmed in mixed CTLA-4 chimeras and extended to pathogen-specific primary CD4+ T-cell responses, which were found to be comparable in CTLA-4+/+ and CTLA-4−/− compartments (3). However, a detailed analysis of antigen-driven proliferation, effector function acquisition, distribution of epitope hierarchies, TCR repertoire selection, and functional avidities, as well as memory maintenance, survival, and secondary responses, of specific CD8+ and CD4+ T cells in vivo has not yet been provided. Our observation that virtually all aspects of primary, memory, and secondary CD8+ and CD4+ T-cell responses are largely independent of autonomous CTLA-4 expression raises one major question: how can these findings be reconciled with the abundance of experimental evidence that CTLA-4 is, in fact, a nonredundant negative regulator of T-cell immunity?
In the majority of in vivo studies with CTLA-4-deficient mice or treatment with specific antibodies, lack or blockade of CTLA-4 was systemic by design. Given the nonredundant functions of CTLA-4 (14, 80, 90), these studies have successfully identified multiple parameters of T-cell activation dependent on functional expression of CTLA-4. Although the molecular basis of CTLA-4-mediated inhibition of T-cell activation is not understood completely, five distinct and non-mutually exclusive levels of activity have been proposed. CTLA-4 may inhibit T-cell responses via (i) competition with CD28, (ii) interference with TCR- or CD28-mediated signaling events, (iii) production of secondary factors such as immunosuppressive cytokines, or (iv) induction of IDO expression and modulation of tryptophan catabolism (1, 24, 32, 74). In contrast to systemic CTLA-4 deficiency or blockade, mixed CTLA-4-deficient bone marrow chimeras retained CTLA-4 expression in radioresistant cells and about half of the hematopoietically derived cell subsets (4, 22). In this respect, the chimeras resembled experimental systems where the specific response of CTLA-4-deficient TCR-transgenic T cells was evaluated after adoptive transfer into wild-type hosts (5). Since the absence of altered T-cell immunity in the CTLA-4−/− compartment of the mixed chimeras is a function of the presence of CTLA-4-expressing cells in the same local environment (22), the mechanism(s) for effective CTLA-4-mediated regulation of specific T-cell immunity may be operative at any of the four levels outlined above.
Several events proximal to CTLA-4 signaling may contribute to the normal phenotype of mixed CTLA-4-deficient chimeras. Recent studies have documented alternatively spliced CTLA-4 mRNA in hematopoietic tissues of mice and humans and found a native soluble form of CTLA-4 in human sera (49, 61, 62). Since the pathology associated with CTLA-4 deficiency is dependent on the presence of CD80 or CD86 (51), binding of secreted CTLA-4 to CD80/CD86 in mixed CTLA-4 chimeras may participate in effective maintenance of normal T-cell homeostasis. In support of this hypothesis, administration of a soluble CTLA-4 Ig to CTLA-4−/− mice from birth or breeding to a transgenic strain expressing high serum levels of CTLA-4 Ig was shown to delay or prevent the onset of lymphoproliferative disease (40, 80). Interestingly, soluble CTLA-4, apparently produced by monocytes rather than T cells, is upregulated by IFN-β (27, 88). Thus, viral infections, via early induction of type I interferons (8), may directly promote the secretion of biologically active CTLA-4 and regulate the extent of costimulatory activity mediated by CD80-CD86 interactions. It should be noted that the complexity of CTLA-4 function is further increased by the recent description of mRNA isoforms for soluble CD28, CD80, and CD86 (23, 48, 91) and the discovery of secreted CD86 in human sera at levels of biologically relevant costimulatory activity (37).
The dynamic architecture of the interface between T cells and APC or target cells, i.e., the immunological synapse, not only implies the simultaneous engagement of multiple costimulatory and inhibitory interactions, but spatial and temporal considerations suggest the interaction of a multiplicity of cells. Accordingly, it has been proposed that T cells, rather than being dependent on a specific obligatory costimulus (historically referred to as signal 2), integrate a complex of costimulatory and inhibitory interactions that translate minimal kinetic alterations into profound modifications of the T-cell response (26). Recent in vivo studies have provided evidence for some aspects of this scenario. Rapid cardiac allograft rejection was observed in an experimental system that only allowed for costimulation in trans; i.e., TCR-transduced and CD28-mediated signals were provided by ligands expressed on distinct cells. Concurrent treatment with CTLA-4 Ig (blockade in trans) significantly prolonged graft acceptance or prevented rejection (50). Furthermore, direct visualization of the immunologic synapse in vivo has demonstrated that specific effector T cells can engage several target cells simultaneously (57). In light of these findings, it is possible that the inhibitory effect of CTLA-4 on T-cell activation in the mixed chimeras can be effectively contributed by CTLA-4-bearing T cells that provide inhibition in trans. By scavenging of CD80 and CD86, CTLA-4-expressing T cells may thus limit the binding of CD28 to CD80/CD86, required to drive the lymphoproliferative disorder that occurs in the absence of CTLA-4 (51, 52), and actively maintain physiological conditions for T-cell activation and homeostasis.
The original observation by Bachmann et al. that mixed CTLA-4-deficient chimeras exhibit a normal phenotype suggested the possibility that active regulation of T-cell homeostasis is mediated by unidentified factors produced by CTLA-4-bearing cells (4). Due to phenotypic similarities among CTLA-4- and TGF-β-deficient mice (75), as well as the observation of TGF-β induction by CTLA-4 cross-linking in vitro (19), TGF-β was proposed to mediate the suppressive activity of CTLA-4. However, CTLA-4+/+ and CTLA-4−/− T cells recovered from mixed chimeras carried comparable levels of TGF-β message (4), and a recent study provided evidence that CTLA-4-mediated inhibition occurs independently of TGF-β (77). Yet the possibility remains that the suppressive activity of an unidentified factor secreted by CTLA-4-bearing cells as a consequence of CTLA-4 engagement modulates effective T-cell homeostasis. A related and novel concept is the engagement of B7 by CTLA-4 and induction of IDO activity by reverse signaling (24, 32). While our results support a negative regulatory role for IDO, in particular for CD4+ T-cell responses in wild-type mice, this effect was only accentuated among CTLA-4−/− CD8+ but not CD4+ T-cell populations in the mixed chimeras and indicates that other mechanisms must be operative in effective regulation of CTLA-4−/− T-cell responses in the presence of other CTLA-4-expressing cells. An alternative interpretation of our data suggests that increased tryptophan catabolism may unmask a stimulatory function of CTLA-4 (i.e., B7-CTLA-4 interactions lead to activation rather than inhibition of T-cell responses). Such stimulatory effects of CTLA-4 engagement have been postulated in other model systems, but the interpretation of the experimental results has remained controversial (47).
Finally, it is conceivable that the presence of CTLA-4 in the mixed chimeras confers lasting changes during the ontogeny of CTLA-4−/− cells. We are currently testing this hypothesis in experiments where the pathogenic potential of T cells from CTLA-4−/− mice transferred into Rag−/− recipients (81) is compared to that of CTLA-4−/− cells purified from mixed chimeras. Regardless of these speculations, however, the mixed bone marrow chimeras used in our study should provide an excellent tool to identify any factors involved in the transmission of the profound CTLA-4-dependent immunomodulatory effects. It is possible that the identification of such a factor or factors will provide novel targets for therapeutic interventions that specifically inhibit events downstream of CTLA-4 signaling without affecting the competitive blockade of CD28-CD80/86 interactions by membrane-bound or soluble CTLA-4. In this respect, genetic disruption of CTLA-4 signaling (achieved by breeding mice transgenic for a tailless form of CTLA-4 to the CTLA-4−/− background) was associated with facilitated activation of T cells in the absence of multiorgan lymphocytic infiltration or a reduced life span (54).
In conclusion, our study identifies an unexpected independence of intrinsic CTLA-4 expression in all stages of the adaptive T-cell response, as well as an intercellular and cooperative nature of CTLA-4-mediated immunomodulation. It furthermore suggests the potential for targeting therapeutic intervention based on specific blockade of currently unidentified factors operative in the CTLA-4 signaling pathway.
Acknowledgments
This work was supported by NIH training grant AG-00080, Juvenile Diabetes Foundation international fellowship 3-1999-629 (D.H.), Deutsche Forschungsgemeinschaft grant DFG Du-333/1 (W.D.), and U.S. Public Health Service grants AI09484 and DK58541 (M.B.A.O.), as well as DK51091 and U19 AI 51973 (M.V.).
We thank Ellie M. Ling and Christophe M. Fillipi for help with experiments, Maria-Luisa Alegre for providing CTLA-4-deficient mice, and James Allison and Cynthia Chambers for critical readings of the manuscript and insightful discussions.
REFERENCES
- 1.Alegre, M.-L., K. A. Frauwirth, and C. B. Thompson. 2001. T cell regulation by CD28 and CTLA-4. Nat. Rev. Immunol. 1**:**220-228. [DOI] [PubMed] [Google Scholar]
- 2.Allison, J. P., and M. F. Krummel. 1995. The Yin and Yang of T cell costimulation. Science 270**:**932-933. [DOI] [PubMed] [Google Scholar]
- 3.Bachmann, M. F., A. Gallimore, E. Jones, B. Ecabert, H. Acha-Orbea, and M. Kopf. 2001. Normal pathogen-specific immune responses mounted by CTLA-4-deficient T cells: a paradigm reconsidered. Eur. J. Immunol. 31**:**450-458. [DOI] [PubMed] [Google Scholar]
- 4.Bachmann, M. F., G. Kohler, B. Ecabert, T. W. Mak, and M. Kopf. 1999. Cutting edge: lymphoproliferative disease in the absence of CTLA-4 is not T cell autonomous. J. Immunol. 163**:**1128-1131. [PubMed] [Google Scholar]
- 5.Bachmann, M. F., P. Waterhouse, D. E. Speiser, K. McKall-Faienza, T. W. Mak, and P. S. Ohashi. 1998. Normal responsiveness of CTLA-4-deficient anti-viral cytotoxic T cells. J. Immunol. 160**:**95-100. [PubMed] [Google Scholar]
- 6.Badovinac, V. P., K. A. Messingham, S. E. Hamilton, and J. T. Harty. 2003. Regulation of CD8+ T cells undergoing primary and secondary responses to infection in the same host. J. Immunol. 170**:**4933-4942. [DOI] [PubMed] [Google Scholar]
- 7.Berger, D. P., D. Homann, and M. B. A. Oldstone. 2000. Defining parameters for successful immunocytotherapy of persistent viral infection. Virology 266**:**257-263. [DOI] [PubMed] [Google Scholar]
- 8.Biron, C. A. 1999. Initial and innate responses to viral infections—pattern setting in immunity or disease. Curr. Opin. Microbiol. 2**:**374-381. [DOI] [PubMed] [Google Scholar]
- 9.Blair, P. J., J. L. Riley, B. L. Levine, K. P. Lee, N. Craighead, T. Francomano, S. J. Perfetto, G. S. Gray, B. M. Carreno, and C. H. June. 1998. CTLA-4 ligation delivers a unique signal to resting human CD4 T cells that inhibits interleukin-2 secretion but allows Bcl-XL induction. J. Immunol. 160**:**12-15. [PubMed] [Google Scholar]
- 10.Bluestone, J. A. 1997. Is CTLA-4 a master switch for peripheral T cell tolerance? J. Immunol. 158**:**1989-1993. [PubMed] [Google Scholar]
- 11.Brunner, M. C., C. A. Chambers, F. K. Chan, J. Hanke, A. Winoto, and J. P. Allison. 1999. CTLA-4-mediated inhibition of early events of T cell proliferation. J. Immunol. 162**:**5813-5820. [PubMed] [Google Scholar]
- 12.Calvo, C. R., D. Amsen, and A. M. Kruisbeek. 1997. Cytotoxic T lymphocyte antigen 4 (CTLA-4) interferes with extracellular signal-regulated kinase (ERK) and Jun NH2-terminal kinase (JNK) activation, but does not affect phosphorylation of T cell receptor zeta and ZAP70. J. Exp. Med. 186**:**1645-1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chambers, C. A., and J. P. Allison. 1997. Co-stimulation in T cell responses. Curr. Opin. Immunol. 9**:**396-404. [DOI] [PubMed] [Google Scholar]
- 14.Chambers, C. A., D. Cado, T. Truong, and J. P. Allison. 1997. Thymocyte development is normal in CTLA-4-deficient mice. Proc. Natl. Acad. Sci. USA 94**:**9296-9301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chambers, C. A., M. S. Kuhns, and J. P. Allison. 1999. Cytotoxic T lymphocyte antigen-4 (CTLA-4) regulates primary and secondary peptide-specific CD4+ T cell responses. Proc. Natl. Acad. Sci. USA 96**:**8603-8608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chambers, C. A., M. S. Kuhns, J. G. Egen, and J. P. Allison. 2001. CTLA-4-mediated inhibition in regulation of T cell responses: mechanisms and manipulation in tumor immunotherapy. Annu. Rev. Immunol. 19**:**565-594. [DOI] [PubMed] [Google Scholar]
- 17.Chambers, C. A., T. J. Sullivan, and J. P. Allison. 1997. Lymphoproliferation in CTLA-4-deficient mice is mediated by costimulation-dependent activation of CD4+ T cells. Immunity 7**:**885-895. [DOI] [PubMed] [Google Scholar]
- 18.Chambers, C. A., T. J. Sullivan, T. Truong, and J. P. Allison. 1998. Secondary but not primary T cell responses are enhanced in CTLA-4-deficient CD8+ T cells. Eur. J. Immunol. 28**:**3137-3143. [DOI] [PubMed] [Google Scholar]
- 19.Chen, W., W. Jin, and S. M. Wahl. 1998. Engagement of cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) induces transforming growth factor beta (TGF-β) production by murine CD4+ T cells. J. Exp. Med. 188**:**1849-1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.da Rocha Dias, S., and C. E. Rudd. 2001. CTLA-4 blockade of antigen-induced cell death. Blood 97**:**1134-1137. [DOI] [PubMed] [Google Scholar]
- 21.Doyle, A. M., A. C. Mullen, A. V. Villarino, A. S. Hutchins, F. A. High, H. W. Lee, C. B. Thompson, and S. L. Reiner. 2001. Induction of cytotoxic T lymphocyte antigen 4 (CTLA-4) restricts clonal expansion of helper T cells. J. Exp. Med. 194**:**893-902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dummer, W., B. Ernst, E. LeRoy, D. Lee, and C. Surh. 2001. Autologous regulation of naive T cell homeostasis within the T cell compartment. J. Immunol. 166**:**2460-2468. [DOI] [PubMed] [Google Scholar]
- 23.Faas, S. J., M. A. Giannoni, A. P. Mickle, C. L. Kiesecker, D. J. Reed, D. Wu, W. L. Fodor, J. P. Mueller, L. A. Matis, and R. P. Rother. 2000. Primary structure and functional characterization of a soluble, alternatively spliced form of B7-1. J. Immunol. 164**:**6340-6348. [DOI] [PubMed] [Google Scholar]
- 24.Fallarino, F., U. Grohmann, K. W. Hwang, C. Orabona, C. Vacca, R. Bianchi, M. L. Belladonna, M. C. Fioretti, M.-L. Alegre, and P. Puccetti. 2003. Modulation of tryptophan catabolism by regulatory T cells. Nat. Immunol. 4**:**206-212. [DOI] [PubMed] [Google Scholar]
- 25.Flynn, K. J., J. M. Riberdy, J. P. Christensen, J. D. Altman, and P. C. Doherty. 1999. In vivo proliferation of naive and memory influenza-specific CD8+ T cells. Proc. Natl. Acad. Sci. USA 96**:**8597-8602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gett, A. V., and P. D. Hodgkin. 2000. A cellular calculus for signal integration by T cells. Nat. Immunol. 1**:**239-244. [DOI] [PubMed] [Google Scholar]
- 27.Giorelli, M., P. Livrea, G. Defazio, F. Ricchiuti, E. Pagano, and M. Trojano. 2001. IFN-β1a modulates the expression of CTLA-4 and CD28 splice variants in human mononuclear cells: induction of soluble isoforms. J. Interferon Cytokine Res. 21**:**809-812. [DOI] [PubMed] [Google Scholar]
- 28.Gozalo-Sanmillan, S., J. M. McNally, M. Y. Lin, C. A. Chambers, and L. J. Berg. 2001. Cutting edge: two distinct mechanisms lead to impaired T cell homeostasis in Janus kinase 3- and CTLA-4-deficient mice. J. Immunol. 166**:**727-730. [DOI] [PubMed] [Google Scholar]
- 29.Grayson, J. M., A. J. Zajac, J. D. Altman, and R. Ahmed. 2000. Cutting edge: increased expression of Bcl-2 in antigen-specific memory CD8+ T cells. J. Immunol. 164**:**3950-3954. [DOI] [PubMed] [Google Scholar]
- 30.Greenwald, R. J., V. A. Boussiotis, R. B. Lorsbach, A. K. Abbas, and A. H. Sharpe. 2001. CTLA-4 regulates induction of anergy in vivo. Immunity 14**:**145-155. [DOI] [PubMed] [Google Scholar]
- 31.Gribben, J. G., G. J. Freeman, V. A. Boussiotis, P. Rennert, C. L. Jellis, E. Greenfield, M. Barber, V. A. Restivo, Jr., X. Ke, G. S. Gray, et al. 1995. CTLA4 mediates antigen-specific apoptosis of human T cells. Proc. Natl. Acad. Sci. USA 92**:**811-815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grohmann, U., C. Orabona, F. Fallarino, C. Vacca, F. Calcinaro, A. Falorni, P. Candeloro, M. L. Belladonna, R. Bianchi, M. C. Fioretti, and P. Puccetti. 2002. CTLA-4-Ig regulates tryptophan catabolism in vivo. Nat. Immunol. 3**:**1097-1101. [DOI] [PubMed] [Google Scholar]
- 33.Heinzel, F. P., and R. A. Maier, Jr. 1999. Interleukin-4-independent acceleration of cutaneous leishmaniasis in susceptible BALB/c mice following treatment with anti-CTLA4 antibody. Infect. Immun. 67**:**6454-6460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Homann, D., L. Teyton, and M. B. A. Oldstone. 2001. Differential regulation of antiviral T-cell immunity results in stable CD8+ but declining CD4+ T-cell memory. Nat. Med. 7**:**913-919. [DOI] [PubMed] [Google Scholar]
- 35.Homann, D., A. Tishon, D. P. Berger, W. O. Weigle, M. G. von Herrath, and M. B. A. Oldstone. 1998. Evidence for an underlying CD4 helper and CD8 T-cell defect in B-cell-deficient mice: failure to clear persistent virus infection after adoptive immunotherapy with virus-specific memory cells from muMT/muMT mice. J. Virol. 72**:**9208-9216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Issazadeh, S., M. Zhang, M. H. Sayegh, and S. J. Khoury. 1999. Acquired thymic tolerance: role of CTLA4 in the initiation and maintenance of tolerance in a clinically relevant autoimmune disease model. J. Immunol. 162**:**761-765. [PubMed] [Google Scholar]
- 37.Jeannin, P., G. Magistrelli, J. P. Aubry, G. Caron, J. F. Gauchat, T. Renno, N. Herbault, L. Goetsch, A. Blaecke, P. Y. Dietrich, J. Y. Bonnefoy, and Y. Delneste. 2000. Soluble CD86 is a costimulatory molecule for human T lymphocytes. Immunity 13**:**303-312. [DOI] [PubMed] [Google Scholar]
- 38.Kamperschroer, C., and D. G. Quinn. 1999. Quantification of epitope-specific MHC class-II-restricted T cells following lymphocytic choriomeningitis virus infection. Cell. Immunol. 193**:**134-146. [DOI] [PubMed] [Google Scholar]
- 39.Kearney, E. R., T. L. Walunas, R. W. Karr, P. A. Morton, D. Y. Loh, J. A. Bluestone, and M. K. Jenkins. 1995. Antigen-dependent clonal expansion of a trace population of antigen-specific CD4+ T cells in vivo is dependent on CD28 costimulation and inhibited by CTLA-4. J. Immunol. 155**:**1032-1036. [PubMed] [Google Scholar]
- 40.Khattri, R., J. A. Auger, M. D. Griffin, A. H. Sharpe, and J. A. Bluestone. 1999. Lymphoproliferative disorder in CTLA-4 knockout mice is characterized by CD28-regulated activation of Th2 responses. J. Immunol. 162**:**5784-5791. [PubMed] [Google Scholar]
- 41.Krummel, M. F., and J. P. Allison. 1996. CTLA-4 engagement inhibits IL-2 accumulation and cell cycle progression upon activation of resting T cells. J. Exp. Med. 183**:**2533-2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kuhns, M. S., V. Epshteyn, R. A. Sobel, and J. P. Allison. 2000. Cytotoxic T lymphocyte antigen-4 (CTLA-4) regulates the size, reactivity, and function of a primed pool of CD4+ T cells. Proc. Natl. Acad. Sci. USA 97**:**12711-12716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee, K. M., E. Chuang, M. Griffin, R. Khattri, D. K. Hong, W. Zhang, D. Straus, L. E. Samelson, C. B. Thompson, and J. A. Bluestone. 1998. Molecular basis of T cell inactivation by CTLA-4. Science 282**:**2263-2266. [DOI] [PubMed] [Google Scholar]
- 44.Lenz, D. C., S. K. Kurz, E. Lemmens, S. P. Schoenberger, J. Sprent, M. B. A. Oldstone, and D. Homann. 2004. IL-7 regulates basal homeostatic proliferation of antiviral CD4+ T cell memory. Proc. Natl. Acad. Sci. USA 101**:**9357-9362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lindsten, T., K. P. Lee, E. S. Harris, B. Petryniak, N. Craighead, P. J. Reynolds, D. B. Lombard, G. J. Freeman, L. M. Nadler, G. S. Gray, et al. 1993. Characterization of CTLA-4 structure and expression on human T cells. J. Immunol. 151**:**3489-3499. [PubMed] [Google Scholar]
- 46.Linsley, P. S., J. L. Greene, W. Brady, J. Bajorath, J. A. Ledbetter, and R. Peach. 1994. Human B7-1 (CD80) and B7-2 (CD86) bind with similar avidities but distinct kinetics to CD28 and CTLA-4 receptors. Immunity 1**:**793-801. [DOI] [PubMed] [Google Scholar]
- 47.Liu, Y. 1997. Is CTLA-4 a negative regulator for T-cell activation? Immunol. Today 18**:**569-572. [DOI] [PubMed] [Google Scholar]
- 48.Magistrelli, G., P. Jeannin, G. Elson, J. F. Gauchat, T. N. Nguyen, J. Y. Bonnefoy, and Y. Delneste. 1999. Identification of three alternatively spliced variants of human CD28 mRNA. Biochem. Biophys. Res. Commun. 259**:**34-37. [DOI] [PubMed] [Google Scholar]
- 49.Magistrelli, G., P. Jeannin, N. Herbault, A. Benoit De Coignac, J. F. Gauchat, J. Y. Bonnefoy, and Y. Delneste. 1999. A soluble form of CTLA-4 generated by alternative splicing is expressed by nonstimulated human T cells. Eur. J. Immunol. 29**:**3596-3602. [DOI] [PubMed] [Google Scholar]
- 50.Mandelbrot, D. A., K. Kishimoto, H. Auchincloss, Jr., A. H. Sharpe, and M. H. Sayegh. 2001. Rejection of mouse cardiac allografts by costimulation in trans. J. Immunol. 167**:**1174-1178. [DOI] [PubMed] [Google Scholar]
- 51.Mandelbrot, D. A., A. J. McAdam, and A. H. Sharpe. 1999. B7-1 or B7-2 is required to produce the lymphoproliferative phenotype in mice lacking cytotoxic T lymphocyte-associated antigen 4 (CTLA-4). J. Exp. Med. 189**:**435-440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mandelbrot, D. A., M. A. Oosterwegel, K. Shimizu, A. Yamada, G. J. Freeman, R. N. Mitchell, M. H. Sayegh, and A. H. Sharpe. 2001. B7-dependent T-cell costimulation in mice lacking CD28 and CTLA4. J. Clin. Investig. 107**:**881-887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Marengere, L. E., P. Waterhouse, G. S. Duncan, H. W. Mittrucker, G. S. Feng, and T. W. Mak. 1996. Regulation of T cell receptor signaling by tyrosine phosphatase SYP association with CTLA-4. Science 272**:**1170-1173. [DOI] [PubMed] [Google Scholar]
- 54.Masteller, E. L., E. Chuang, A. C. Mullen, S. L. Reiner, and C. B. Thompson. 2000. Structural analysis of CTLA-4 function in vivo. J. Immunol. 164**:**5319-5327. [DOI] [PubMed] [Google Scholar]
- 55.McCoy, K., M. Camberis, and G. L. Gros. 1997. Protective immunity to nematode infection is induced by CTLA-4 blockade. J. Exp. Med. 186**:**183-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McCoy, K. D., I. F. Hermans, J. H. Fraser, G. Le Gros, and F. Ronchese. 1999. Cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) can regulate dendritic cell-induced activation and cytotoxicity of CD8+ T cells independently of CD4+ T cell help. J. Exp. Med. 189**:**1157-1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McGavern, D. B., U. Christen, and M. B. A. Oldstone. 2002. Molecular anatomy of antigen-specific CD8+ T cell engagement and synapse formation in vivo. Nat. Immunol. 3**:**918-925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Metz, D. P., D. L. Farber, T. Taylor, and K. Bottomly. 1998. Differential role of CTLA-4 in regulation of resting memory versus naive CD4 T cell activation. J. Immunol. 161**:**5855-5861. [PubMed] [Google Scholar]
- 59.Murali-Krishna, K., J. D. Altman, M. Suresh, D. J. Sourdive, A. J. Zajac, J. D. Miller, J. Slansky, and R. Ahmed. 1998. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity 8**:**177-187. [DOI] [PubMed] [Google Scholar]
- 60.Murphy, M. L., S. E. Cotterell, P. M. Gorak, C. R. Engwerda, and P. M. Kaye. 1998. Blockade of CTLA-4 enhances host resistance to the intracellular pathogen, Leishmania donovani. J. Immunol. 161**:**4153-4160. [PubMed] [Google Scholar]
- 61.Oaks, M. K., and K. M. Hallett. 2000. Cutting edge: a soluble form of CTLA-4 in patients with autoimmune thyroid disease. J. Immunol. 164**:**5015-5018. [DOI] [PubMed] [Google Scholar]
- 62.Oaks, M. K., K. M. Hallett, R. T. Penwell, E. C. Stauber, S. J. Warren, and A. J. Tector. 2000. A native soluble form of CTLA-4. Cell. Immunol. 201**:**144-153. [DOI] [PubMed] [Google Scholar]
- 63.Oosterwegel, M. A., D. A. Mandelbrot, S. D. Boyd, R. B. Lorsbach, D. Y. Jarrett, A. K. Abbas, and A. H. Sharpe. 1999. The role of CTLA-4 in regulating Th2 differentiation. J. Immunol. 163**:**2634-2639. [PubMed] [Google Scholar]
- 64.Oxenius, A., M. F. Bachmann, P. G. Ashton-Rickardt, S. Tonegawa, R. M. Zinkernagel, and H. Hengartner. 1995. Presentation of endogenous viral proteins in association with major histocompatibility complex class II: on the role of intracellular compartmentalization, invariant chain and the TAP transporter system. Eur. J. Immunol. 25**:**3402-3411. [DOI] [PubMed] [Google Scholar]
- 65.Perez, V. L., L. Van Parijs, A. Biuckians, X. X. Zheng, T. B. Strom, and A. K. Abbas. 1997. Induction of peripheral T cell tolerance in vivo requires CTLA-4 engagement. Immunity 6**:**411-417. [DOI] [PubMed] [Google Scholar]
- 66.Read, S., V. Malmstrom, and F. Powrie. 2000. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25+ CD4+ regulatory cells that control intestinal inflammation. J. Exp. Med. 192**:**295-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ridgway, W., M. Fasso, and C. G. Fathman. 1998. Following antigen challenge, T cells up-regulate cell surface expression of CD4 in vitro and in vivo. J. Immunol. 161**:**714-720. [PubMed] [Google Scholar]
- 68.Saha, B., S. Chattopadhyay, R. Germond, D. M. Harlan, and P. J. Perrin. 1998. CTLA4 (CD152) modulates the Th subset response and alters the course of experimental Leishmania major infection. Eur. J. Immunol. 28**:**4213-4220. [DOI] [PubMed] [Google Scholar]
- 69.Salomon, B., and J. A. Bluestone. 2001. Complexities of CD28/B7: CTLA-4 costimulatory pathways in autoimmunity and transplantation. Annu. Rev. Immunol. 19**:**225-252. [DOI] [PubMed] [Google Scholar]
- 70.Salomon, B., D. J. Lenschow, L. Rhee, N. Ashourian, B. Singh, A. Sharpe, and J. A. Bluestone. 2000. B7/CD28 costimulation is essential for the homeostasis of the CD4+ CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity 12**:**431-440. [DOI] [PubMed] [Google Scholar]
- 71.Saverino, D., C. Tenca, D. Zarcone, A. Merlo, A. M. Megiovanni, M. T. Valle, F. Manca, C. E. Grossi, and E. Ciccone. 1999. CTLA-4 (CD152) inhibits the specific lysis mediated by human cytolytic T lymphocytes in a clonally distributed fashion. J. Immunol. 162**:**651-658. [PubMed] [Google Scholar]
- 72.Scheipers, P., and H. Reiser. 1998. Fas-independent death of activated CD4+ T lymphocytes induced by CTLA-4 crosslinking. Proc. Natl. Acad. Sci. USA 95**:**10083-10088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sevilla, N., S. Kunz, A. Holz, H. Lewicki, D. Homann, H. Yamada, K. P. Campbell, J. C. de La Torre, and M. B. A. Oldstone. 2000. Immunosuppression and resultant viral persistence by specific viral targeting of dendritic cells. J. Exp. Med. 192**:**1249-1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sharpe, A., and G. J. Freeman. 2002. The B7-CD28 superfamily. Nat. Rev. Immunol. 2**:**116-126. [DOI] [PubMed] [Google Scholar]
- 75.Shull, M. M., I. Ormsby, A. B. Kier, S. Pawlowski, R. J. Diebold, M. Yin, R. Allen, C. Sidman, G. Proetzel, D. Calvin, et al. 1992. Targeted disruption of the mouse transforming growth factor-beta 1 gene results in multifocal inflammatory disease. Nature 359**:**693-699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Slifka, M. K., and J. L. Whitton. 2000. Activated and memory CD8+ T cells can be distinguished by their cytokine profiles and phenotypic markers. J. Immunol. 164**:**208-216. [DOI] [PubMed] [Google Scholar]
- 77.Sullivan, T. J., J. J. Letterio, A. van Elsas, M. Mamura, J. van Amelsfort, S. Sharpe, B. Metzler, C. A. Chambers, and J. P. Allison. 2001. Lack of a role for transforming growth factor-beta in cytotoxic T lymphocyte antigen-4-mediated inhibition of T cell activation. Proc. Natl. Acad. Sci. USA 98**:**2587-2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Takahashi, T., T. Tagami, S. Yamazaki, T. Uede, J. Shimizu, N. Sakaguchi, T. W. Mak, and S. Sakaguchi. 2000. Immunologic self-tolerance maintained by CD25+ CD4+ regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J. Exp. Med. 192**:**303-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Thompson, C. B., and J. P. Allison. 1997. The emerging role of CTLA-4 as an immune attenuator. Immunity 7**:**445-450. [DOI] [PubMed] [Google Scholar]
- 80.Tivol, E. A., F. Borriello, A. N. Schweitzer, W. P. Lynch, J. A. Bluestone, and A. H. Sharpe. 1995. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity 3**:**541-547. [DOI] [PubMed] [Google Scholar]
- 81.Tivol, E. A., and J. Gorski. 2002. Re-establishing peripheral tolerance in the absence of CTLA-4: complementation by wild-type T cells points to an indirect role for CTLA-4. J. Immunol. 169**:**1852-1858. [DOI] [PubMed] [Google Scholar]
- 82.Tough, D. F., and J. Sprent. 1994. Turnover of naive- and memory-phenotype T cells. J. Exp. Med. 179**:**1127-1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Turner, S. J., R. Cross, W. Xie, and P. C. Doherty. 2001. Concurrent naive and memory CD8+ T cell responses to an influenza A virus. J. Immunol. 167**:**2753-2758. [DOI] [PubMed] [Google Scholar]
- 84.van der Most, R. G., K. Murali-Krishna, J. L. Whitton, C. Oseroff, J. Alexander, S. Southwood, J. Sidney, R. W. Chesnut, A. Sette, and R. Ahmed. 1998. Identification of Db- and Kb-restricted subdominant cytotoxic T-cell responses in lymphocytic choriomeningitis virus-infected mice. Virology 240**:**158-167. [DOI] [PubMed] [Google Scholar]
- 85.Walunas, T. L., C. Y. Bakker, and J. A. Bluestone. 1996. CTLA-4 ligation blocks CD28-dependent T cell activation. J. Exp. Med. 183**:**2541-2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Walunas, T. L., and J. A. Bluestone. 1998. CTLA-4 regulates tolerance induction and T cell differentiation in vivo. J. Immunol. 160**:**3855-3860. [PubMed] [Google Scholar]
- 87.Walunas, T. L., D. J. Lenschow, C. Y. Bakker, P. S. Linsley, G. J. Freeman, J. M. Green, C. B. Thompson, and J. A. Bluestone. 1994. CTLA-4 can function as a negative regulator of T cell activation. Immunity 1**:**405-413. [DOI] [PubMed] [Google Scholar]
- 88.Wang, X. B., R. Giscombe, Z. Yan, T. Heiden, D. Xu, and A. K. Lefvert. 2002. Expression of CTLA-4 by human monocytes. Scand. J. Immunol. 55**:**53-60. [DOI] [PubMed] [Google Scholar]
- 89.Waterhouse, P., L. E. Marengere, H. W. Mittrucker, and T. W. Mak. 1996. CTLA-4, a negative regulator of T-lymphocyte activation. Immunol. Rev. 153**:**183-207. [DOI] [PubMed] [Google Scholar]
- 90.Waterhouse, P., J. M. Penninger, E. Timms, A. Wakeham, A. Shahinian, K. P. Lee, C. B. Thompson, H. Griesser, and T. W. Mak. 1995. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science 270**:**985-988. [DOI] [PubMed] [Google Scholar]
- 91.Yang, S., and G. K. Sim. 1999. New forms of dog CD80 and CD86 transcripts that encode secreted B7 molecules. Immunogenetics 50**:**349-353. [DOI] [PubMed] [Google Scholar]
- 92.Zheng, X. X., T. G. Markees, W. W. Hancock, Y. Li, D. L. Greiner, X. C. Li, J. P. Mordes, M. H. Sayegh, A. A. Rossini, and T. B. Strom. 1999. CTLA4 signals are required to optimally induce allograft tolerance with combined donor-specific transfusion and anti-CD154 monoclonal antibody treatment. J. Immunol. 162**:**4983-4990. [PubMed] [Google Scholar]