Loss of the limbic mineralocorticoid receptor impairs behavioral plasticity (original) (raw)
Abstract
Corticosteroid action in the brain is mediated by the mineralocorticoid (MR) and the glucocorticoid (GR) receptor. Disturbances in MR- and GR-mediated effects are thought to impair cognition, behavior, and endocrine control. To assess the function of the limbic MR in these processes, we inactivated the MR gene in the forebrain of the mouse using the Cre/loxP-recombination system. We screened the mice with a limbic MR deficiency in various learning and exploration tests. The mutant mice show impaired learning of the water-maze task and deficits in measures of working memory on the radial maze due to behavioral perseverance and stereotypy. They exhibit a hyperreactivity toward a novel object but normal anxiety-like behavior. The behavioral changes are associated with abnormalities of the mossy fiber projection and an up-regulation of GR expression in the hippocampus. Adult mutant mice show normal corticosterone levels at circadian trough and peak. This genetic model provides important information about the consequences of a permanently altered balance between limbic MR and GR, with implications for stress-related neuroendocrine and neuropsychiatric diseases.
Keywords: behavior, neuroendocrine, synaptic plasticity
Corticosteroids influence neuronal excitability and plasticity, neurogenesis and neuronal death, and neuroendocrine control and behavioral responses (1, 2). Their actions are mediated by two ligand-activated transcription factors, the mineralocorticoid (MR) and the glucocorticoid (GR) receptors. The MR binds corticosterone with a 10-fold higher affinity than the GR and, in addition, binds the mineralocorticoid aldosterone with equal affinity (3). The GR is expressed throughout the brain, in neurons and glial cells, and is most abundant in hypothalamic neurons producing corticotropin-releasing hormone. The MR is expressed in neurons only, with highest abundance in the limbic system, in particular within the hippocampus (4). The limbic MR strongly responds to corticosterone, because the prereceptor protection mechanism achieving aldosterone selectivity in tight epithelia is not present (5). Thus, MR and GR, whose DNA binding domains are almost identical, form a binary response system for corticosterone that is able to regulate differentially two overlapping networks of target genes (3). The adrenal release of corticosterone is controlled by the hypothalamic–pituitary–adrenal (HPA) axis that shows circadian activity, with low activity at the onset of the resting phase and high activity at the onset of the activity phase, and a strong increase in activity after stress. The high-affinity MR is always substantially occupied, even at basal levels of HPA-axis activity. High concentrations of corticosterone, at circadian peak or after stress, progressively saturate the GR (3).
Lesioning and electrical stimulation studies suggest an overall inhibitory influence of the hippocampus on HPA-axis activity (6), whereas the amygdala appears to have an excitatory influence (7). Intracerebroventricular or intrahippocampal administration of a MR antagonist in rats elevates basal trough levels and the diurnal rise of plasma corticosterone (8–10) suggesting that the hippocampal MR is involved in the maintenance of basal HPA-axis activity throughout the circadian cycle. Intrahippocampal application of a GR antagonist suppresses adrenal corticosterone release under conditions in which MR antagonists enhance HPA-axis activity (10). Such effects are in agreement with electrophysiological data showing opposite effects of MR and GR activation on excitability and excitatory outflow of the hippocampus (11). Electrophysiological studies at the cellular level in the hippocampus on Ca2+ influx and responsiveness to transmitters revealed that conditions of predominant MR activation are implicated in the maintenance of excitability, so that steady excitatory input to the hippocampal CA1 area results in considerable excitatory hippocampal output (11). Synaptic strength is enhanced [long-term potentiation (LTP)] when MR is almost completely activated and GR is only partially activated. Subsequent complete occupation of GR (and MR), e.g., after stress, decreases the hippocampal output and the synaptic efficacy in the CA1 region (12).
Chronic absence or chronic overexposure to corticosterone results in structural changes of the hippocampus, indicating that corticosterone-dependent gene expression is crucial for hippocampal integrity (1). The dentate gyrus shows neurogenesis also during adulthood, which can be blocked by chronic stress and adrenal steroids (13).
The use of receptor-specific antagonists suggests that MR- and GR-mediated effects are important for cognitive and behavioral processes as well. Memory in spatial and avoidance tasks is impaired in the absence of GR activation after the learning task (14–16), whereas MR modulates ongoing behavioral activity. MR antagonists are only effective during, but not after, the training session (15, 16). Thus, activation of MR is thought to be implicated in memory acquisition (appraisal of information and response selection), whereas activation of GR is thought to be related to consolidation of acquired information (17).
The role of the limbic MR in neuronal excitability and plasticity as well as in HPA-axis regulation and behavior has so far been investigated by pharmacological means only; that is, by changing ligand accessibility using MR antagonists or adrenal-ectomy in combination with low corticosterone substitution that should predominantly occupy MR. The conclusions drawn from such studies depend on antagonist specificity and application of appropriate corticosterone doses. We used a genetic approach to inactivate the MR gene in the forebrain of the mouse to investigate whether a permanently altered balance between MR and GR will disturb basal circadian HPA-axis activity and behavior. Indeed, mice lacking limbic MR were impaired in hippocampus-dependent learning procedures and showed massively increased object exploration. However, they showed normal anxiety-like behavior and normal basal circadian HPA-axis activity.
Materials and Methods
Generation of Conditional Mutant Mice. To flank exon 3 of the mouse Nr3c2 gene encoding the MR by loxP sites, we used homologous recombination in embryonic stem cells. For more details, see Supporting Methods, which is published as supporting information on the PNAS web site. For the conditional inactivation of the MR in the forebrain, we used CaMKCre-transgenic mice (18). The MRflox/floxCaMKCre mice (MRCaMKCre) and their control (MRflox/flox) littermates were obtained by breeding MRflox/flox with MRflox/wtCaMKCre mice. The genetic background comprised a mix of C57BL/6, 129Ola, and FVB/N. The use of Cre deleter mice (19) to recombine the MRflox allele in the germ line and the breeding of the obtained MRnull allele to homozygosity (MRnull/null mice) resulted in postnatal lethality and a phenotype identical to MR knockout mice (data not shown).
Immunohistochemistry and Timm Staining. Adult (4 months old) and 12-day-old mice were transcardially perfused with 4% paraformaldehyde. The dissected brains were postfixed overnight at 4°C in 4% paraformaldehyde and cut at a thickness of 50 μm on a vibratome (Leica). At postnatal day (P) 0 and P6 the brains were dissected without prior paraformaldehyde perfusion. The floating sections were processed for immunohistochemical detection by using the VECTASTAIN ABC system (Vector Laboratories) and diaminobenzidine (Sigma) incubation. We used the following primary antibodies: polyclonal anti-MR (4), polyclonal anti-Cre (20), polyclonal anti-GR (Santa Cruz Biotechnology), and polyclonal anti-CRH (Abcam).
Hippocampal mossy fiber synaptic fields were visualized by Timm staining (21). Adult mice (4 months old) were perfused transcardially with phosphate-buffered 1% sodium sulfide and 3% glutaraldehyde. After postfixation and cryoprotection, 40-μm cryosections were obtained and developed in a solution containing Arabic gum, hydroquinone, citric acid, and silver nitrate.
Corticosterone Measurement. One week before the experiment, adult mice (4 months old) were separated and housed individually (light on 08:00–20:00). To determine the plasma corticosterone level at circadian trough and peak, blood sampling was performed in the morning (09:00–10:00) and the evening (19:00–20:00) by bleeding after decapitation, with the time from first handling of the animal to completion of bleeding not exceeding 45 s (n = 7–8 per genotype, gender, and time point). For evaluation of the stress release of corticosterone, blood samples were taken in the morning (09:00–10:00) immediately after 40 min of restraint stress for which animals were placed in 50-ml conical tubes (plastic conical tube with the bottom removed) (n = 10–12 per genotype and gender). Plasma corticosterone was measured by using a commercially available RIA kit (MP Biomedicals). Results are expressed as mean ± SEM. The measurements were analyzed by using unpaired, two-tailed Student's t test.
Behavioral Studies. One week before the experimental period, male and female mice (4–7 months old) were transferred to standard single-mouse cages, maintained at a 12:12-h inverted cycle (light on 20:00–08:00), and tested during the dark period. The home cage rack was brought to the test room at least 30 min before each experiment. The behavioral tests were performed as described in refs. 22 and 23. During all of the tests, except the conditioned taste aversion, animals were video-tracked by using the EthoVision system (Noldus Information Technology) at an image frequency of 4.2 per s. Raw data were transferred to wintrack 2.4 (www.dpwolfer.ch/wintrack) for off-line analysis. For statistical analysis, see Supporting Methods.
Results
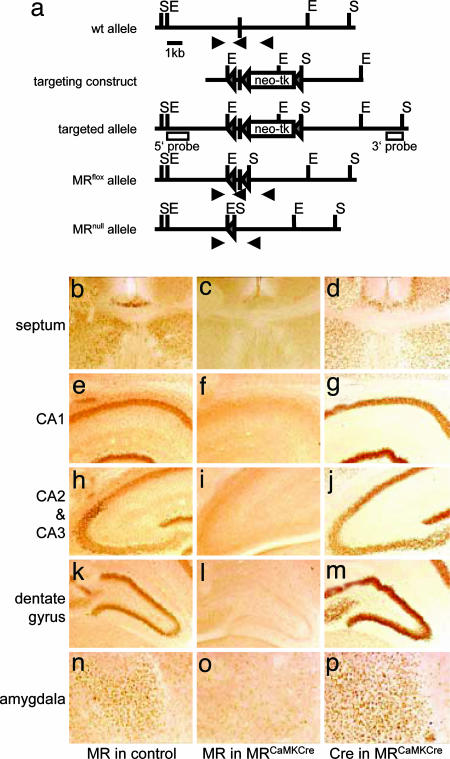
Generation of Mice Lacking MR in the Forebrain. Using homologous recombination in mouse embryonic stem cells, we generated a modified MR allele (MRflox allele), in which exon 3, encoding the first zinc finger of the MR DNA binding domain, was flanked by loxP sites (Fig. 1_a_). To generate mice lacking MR in the forebrain, we crossed mice harboring the MRflox allele to BAC-transgenic mice expressing Cre recombinase under the control of the regulatory elements of the mouse CaMKIIα gene (18) to obtain MRflox/floxCaMKCre (MRCaMKCre) mice. The MR protein is detectable as early as embryonic day 16.5. During pre- and postnatal development, as well as during adulthood, MR protein detection in the forebrain of the mouse is restricted to the limbic system (4). We found that the Cre transgene we used is already expressed in the limbic system at embryonic day 16.5 (see Fig. 6_a_, which is published as supporting information on the PNAS web site). Therefore, we checked the loss of MR immunoreactivity in MRCaMKCre mice at P0, P6, and P12. This analysis revealed that MR protein expression is already reduced at P0 and is almost lost at P6 (see Figs. 6_b_ and 7, which are published as supporting information on the PNAS web site). No immunoreactivity is found in the limbic system of MRCaMKCre mice neither at P12 (Fig. 1 b_–_p) nor in the adult (data not shown). The careful examination of the tissue sections on high power did not reveal any MR expressing neurons that might have escaped from recombination.
Fig. 1.
Generation of mice lacking MR in the forebrain. (a) Organization of the mouse MR wild-type locus around exon 3. This exon was flanked with loxP sites by homologous recombination in embryonic stem cells. Transient expression of Cre recombinase in embryonic stem cells resulted in removal of the selection cassette (neomycin resistance–thymidine kinase), generating the MRflox allele. This allele encodes an active MR protein but is recombined in any cell expressing Cre recombinase. Recombination results in deletion of exon 3 and thereby in inactivation of this allele (MRnull allele). The scheme shown depicts the wild-type locus, the targeting vector, and the resulting alleles. Triangles represent loxP sites, the filled box represents exon 3, open boxes represent the probes used for Southern blot analysis, and the arrows represent primers used for genotyping by PCR. E, EcoRV; S, SpeI. (b_–_p) MR protein expression in control animals (b, e, h, k, and n) and Cre expression (d, g, j, m, and p) and the corresponding loss of MR expression (c, f, i, l, and o) in MRCaMKCre mice as revealed by immunohistochemistry on vibratome sections of 12-day-old animals. Depicted are the regions that show highest expression of MR in controls: lateral septum and indusium griseum (b_–_d), CA1 (e_–_g), CA2 and CA3 (h_–_j), dentate gyrus (k_–_m), and central amygdala (n_–_p).
In rats, expression of MR is also found in the hypothalamus (24). Because the nucleus paraventricularis (PVN) is an important site for feedback inhibition of the HPA axis, we analyzed this region for expression of MR. We could detect neither the MR protein nor the MR mRNA in the PVN of the mouse (see Fig. 8, which is published as supporting information on the PNAS web site). Nevertheless, the PVN shows high expression of Cre recombinase and therefore recombination of the MR gene is expected.
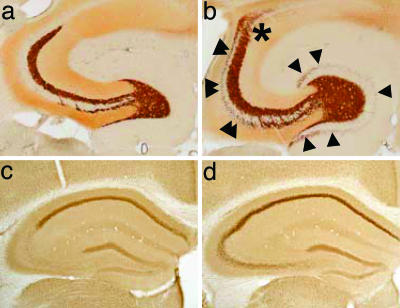
Inactivation of the MR gene in the forebrain did not impair survival. MRCaMKCre mice were visually indistinguishable from control littermates and performed normally when tested for sensory (acoustic startle profile and prepulse inhibition) and motor (rotarod) function (data not shown). In adult MRCaMKCre mice, a gross survey of the cornu ammonis and the dentate gyrus of the hippocampus by using Nissl staining showed no conspicuous change in cell number and density (data not shown). However, analysis of the projection of granule cell axons to the CA3 field of the hippocampus revealed an aberrant layout of the mossy fibers in MRCaMKCre mice compared with control animals (Fig. 2 a and b). Determination of the hippocampal GR expression by immunohistochemistry revealed an up-regulation of GR in the cornu ammonis of MRCaMKCre mice compared with controls (Fig. 2 c and d). In the PVN, a site without detectable MR expression, the expression of GR is not changed (Fig. 8).
Fig. 2.
Altered mossy fiber projections and hippocampal GR up-regulation in adult MRCaMKCre mice. (a and b) Timm stain labeling of the Zn2+-containing mossy fiber terminals. Compared with control animals (a), MRCaMKCre mice showed an aberrant layout of mossy fiber projections (b): variable excess of supragranular boutons in the dentate gyrus (single arrowheads), extension of the infrapyramidal mossy fiber field to the tip of CA3 and beyond into CA2 (double arrowheads), and a fuzzy distal boundary of the suprapyramidal field with boutons spreading across the pyramidal cell layer into CA2 and CA1 (asterisk). (c and d) Hippocampal GR expression as revealed by immunohistochemistry. GR is up-regulated in the cornu ammonis of adult MRCaMKCre mice (d) compared with controls (c), most apparently in CA2 and CA3.
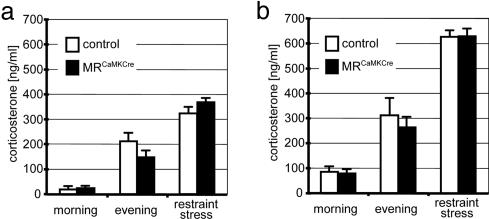
Analysis of HPA-Axis Activity and Hippocampal Synaptic Plasticity. Lesions of the hippocampus raise resting levels of glucocorticoids and stress-induced responses in glucocorticoid secretion (6). Studies using MR antagonists suggest that the hippocampal MR is involved in the maintenance of basal circadian HPA-axis activity (8–10). Therefore, we determined the basal HPA-axis activity in MRCaMKCre mice by measuring the corticosterone levels at the diurnal trough in the morning and at the diurnal peak in the evening. In addition, we determined the release of corticosterone after restraint stress. Males and females were analyzed separately, because female mice display higher plasma corticosterone levels than male mice. The corticosterone measurements in adult mice revealed no significant differences between MRCaMKCre mice and littermate control animals, neither at diurnal trough and peak nor after restraint stress (Fig. 3 a and b). In the PVN, an important site for feedback inhibition of the HPA-axis activity after stress, MRCaMKCre mice showed under basal conditions no change in CRH mRNA (Fig. 8).
Fig. 3.
Normal basal circadian HPA-axis activity in MRCaMKCre mice. (a and b) Plasma corticosterone levels of adult male (a) and female (b) MRCaMKCre mice and their control littermates at diurnal trough (morning) and peak (evening), as well as after 40 min of restraint stress, showed no significant differences between both genotypes.
The CA1 region is the major source of hippocampal output conferring inhibitory constraints on HPA-axis activity (11). The analysis of the basic properties of synaptic transmission in the CA1 region of hippocampal slices from MRCaMKCre and control mice revealed no significant differences in either stimulus input/output relations of synaptic responses or paired pulse facilitation between both groups (see Fig. 9 a and b, which is published as supporting information on the PNAS web site). High- and low-frequency stimulation of the Schaffer collateral axons in the CA1 region of hippocampal slices from MRCaMKCre mice reliably induced LTP and long-term depression, respectively, of synaptic transmission not significantly different from that obtained from control mice (Fig. 9 c and d).
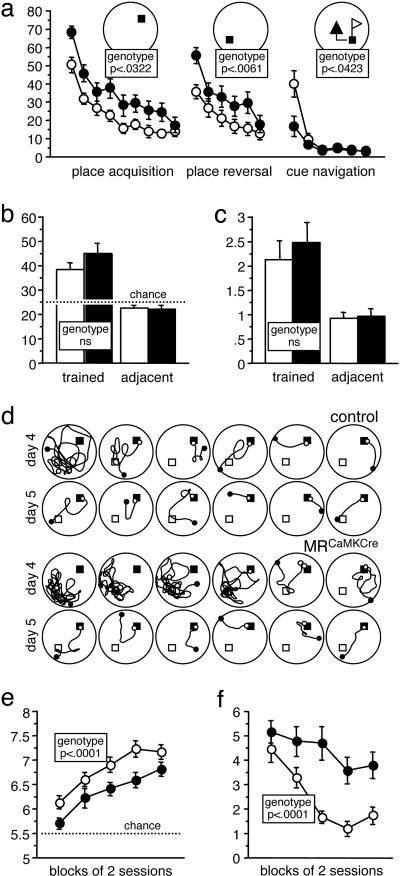
Impaired Learning Behavior of MRCaMKCre Mice. To investigate the learning abilities of the MRCaMKCre mice, we tested two independent cohorts of animals matched for genotype, gender, and age (in total 64 animals) in several learning paradigms. We assessed the spatial learning of MRCaMKCre mice in the Morris water maze in which mice must use visual cues to find a platform that is hidden in a constant location beneath the surface of opaque water. Mice were trained for 18 trials for a first platform location (place acquisition) followed by 12 trials with a new platform location in the opposite quadrant (place reversal), with trial 19 serving as probe trial. In this water-maze task, the MRCaMKCre mice showed a clear acquisition deficit. They showed reduced swim speed and an increased use of inappropriate strategies, such as passive floating and aimless swimming. Overall escape performance was impaired in MRCaMKCre mice according to all evaluated measures (see Table 1, which is published as supporting information on the PNAS web site). Despite reduced average performance, they reached the same performance as controls at the very end of training (Fig. 4_a_). Upon relocation of the platform, both groups displayed a transient increase of escape latencies, indicating that spatial learning had occurred. MRCaMKCre mice showed normal retention of spatial memory in the probe trial, also according to the annulus crossings of the trained quadrant, the most stringent measure of spatial selectivity (Fig. 4 b and c). During the reversal phase, MRCaMKCre mice were more strongly affected by the relocation of the platform and again performed inferior to controls, reaching performance levels similar to controls only at the very end (Fig. 4_a_ and Table 1). During reversal training, a few MRCaMKCre mice showed such intense perseverative searching for the old goal site that this was directly evident to the observer (Fig. 4_d_).
Fig. 4.
Impaired learning of MRCaMKCre mice in a water-maze place navigation task with reversal and in a working memory procedure on the radial maze. (a) Place navigation: Training performance (six trials per day, blocks of two trials, cumulative search error in m·s). Despite reduced average performance, MRCaMKCre mice (n = 33, controls = 31) showed significant learning at indistinguishable rates compared with controls during place acquisition as well as during place reversal. During subsequent cue navigation, the tested subset of MRCaMKCre mice (n = 16, controls = 16) outperformed the control mice (which showed a transient wall-hugging response) during the first trial block, but the performances became indistinguishable during the second block. (b) Place navigation: Probe trial (% time in quadrant). Both groups spend significantly more time in the trained quadrant. (c) Place navigation: Probe trial. Both groups crossed the trained goal annulus significantly more often than control annuli in the adjacent quadrants. (d) Place navigation: Path plots of trials 19–30 (days 4 and 5, place reversal). A MRCaMKCre mouse showing perseverative searching for the old goal site. Plots of a representative control mouse are shown for comparison. Filled square, actual goal; open square, previous goal; filled dot, begin; open dot, end of path. (e) Radial maze: Number of correct choices in the first eight. Both groups (n = 27, controls = 30) showed indistinguishable learning rates and performed above chance, but the average performance of MRCaMKCre mice was clearly inferior. (f) Radial maze: Reentry errors. Whereas controls showed significant learning, no significant reduction of errors could be observed in MRCaMKCre mice. Filled circles/columns, MRCaMKCre; open circles/columns, control.
Next, we tested the MRCaMKCre mice on the eight-arm radial maze. At the beginning of a working memory procedure all arms were baited and the mice were permitted to collect and consume all baits. Reentering a previously visited arm was counted as an error. Aborted choices and bait neglect errors were as infrequent in MRCaMKCre mice as in controls, indicating normal adaptation to the test situation. As judged by the number of correct choices during the first eight arm visits, the average performance of MRCaMKCre mice was significantly impaired (Fig. 4_e_). Counting reentry errors during the entire session revealed that unlike controls, MRCaMKCre mice were unable to overcome their tendency to visit the same arms over time (Fig. 4_f_). As in the water maze, MRCaMKCre mice showed an increased tendency to be passive. Control animals made, as typically observed in mice, strong use of regular response patterns to increase their efficiency. MRCaMKCre mice did this to a lesser degree and showed less consistent choice patterns (Table 1).
We analyzed the performance of MRCaMKCre mice in associative learning tests involving aversive stimuli. In the two-way avoidance test, mice are placed in a two-chamber box and taught to avoid an electric shock preceded by a warning light by running into the opposite compartment. In this test, MRCaMKCre mice were indistinguishable from control animals and showed the same response latency and locomotor activity (data not shown). In the conditioned taste aversion test, mice learn to associate a novel taste with nausea, and as a consequence avoid drinking fluid with this specific taste. During the saccharin-water choice tests after conditioning, MRCaMKCre and control mice showed indistinguishable strong saccharin avoidance and similar subsequent extinction (data not shown).
MRCaMKCre Mice Show Increased Reactivity to a Novel Object. We assessed the exploratory behavior of MRCaMKCre mice in five tests differing in the overall degree of novelty and aversiveness of the environment. In all tests, zones can be defined within the arenas that allow the quantification of exploratory activity and fear-related behavior.
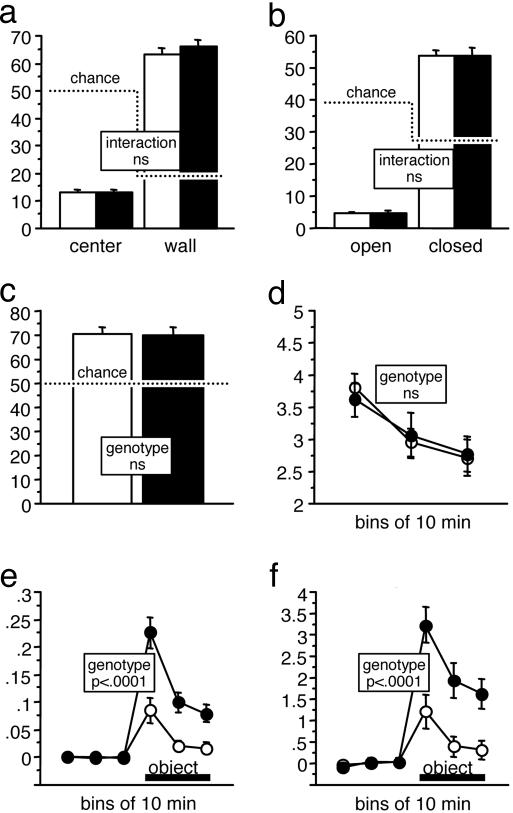
In the open field, MRCaMKCre mice and controls showed identical levels of activity and rates of habituation within and across days (data not shown). Both groups strongly avoided the center field and preferred the wall zone to the same degree (Fig. 5_a_). On the elevated O maze, MRCaMKCre and control mice strongly avoided the open sectors. Again, sector preferences were not affected by the genotype (Fig. 5_b_). In the light–dark box, both groups preferred the dark to the lit compartment to the same degree (Fig. 5_c_). Although MRCaMKCre mice were slightly less active than controls on the O maze and in the light-dark box test (Table 2, which is published as supporting information on the PNAS web site), both groups showed identical levels of activity and rates of habituation in the emergence test (Fig. 5_d_). In an object exploration test, both groups showed a clear approach response toward a novel object that was introduced into an otherwise familiar arena, but MRCaMKCre mice displayed increased horizontal and vertical exploratory activity toward the object compared to controls (Fig. 5 e and f). This strong increase of exploratory activity toward a novel object was not associated with a general increase of locomotor activity (Table 2).
Fig. 5.
MRCaMKCre mice (n = 31, controls = 33) were hyperreactive in an object exploration task but showed normal anxiety- and exploration-related behaviors in other tests. (a) Open-field. Zone preferences were not affected by the genotype. Chance levels for percent time depended on relative zone size and are shown by a dotted line. (b) Elevated O maze. Sector preferences were not affected by the genotype. Chance levels for percent time depended on relative zone size and are shown by a dotted line. (c) Light–dark box (% time in dark). Both groups preferred the dark box over the lit compartment to the same degree. (d) Emergence test (distance moved in m/min). The level of activity and the rate of habituation were not affected by the genotype. (e) Object exploration test. MRCaMKCre mice displayed more than two times greater horizontal exploratory activity toward the object (m/min). (f) Object exploration test. MRCaMKCre mice showed nearly three times as much estimated vertical exploratory activity toward the novel object (x/min) as did controls.
To find an association between the massively increased exploration of a novel object by MRCaMKCre mice and their performance deficit in the water maze and the radial maze, we performed a factor analysis of behavioral variables. For this analysis, two representative factorial variables were chosen from the object exploration task: the place navigation test in the water maze, and the radial maze working memory procedure (Table 3, which is published as supporting information on the PNAS web site). The analysis revealed a strong association between the mutation effect on reentry errors in the radial maze task and spatial perseverance during place reversal in the water maze. In another subset of animals, radial maze reentry errors were more associated with the hyperreactivity in the novel object test.
To rule out that the Cre-transgene accounts for the behavioral phenotype of the MRCaMKCre mice, we compared MRwt/wtCaMKCre mice and their respective littermate controls (MRwt/wt mice) in the water maze, the radial maze, and the object exploration task. This analysis revealed that the Cre-transgene has no effect on its own in these behavioral tasks (Table 4, which is published as supporting information on the PNAS web site).
Discussion
We report the generation of a forebrain-specific inactivation of the mouse MR gene that allows the analysis of MR function in neurons of the limbic system. Whereas the germ line inactivation (knockout) of the mouse MR gene results in postnatal lethality due to renal loss of sodium and water (25), the forebrain-specific inactivation did not impair survival. Therefore, we were able to characterize the role of limbic MR in hippocampal development, HPA-axis regulation, cognitive and behavioral processes, and synaptic plasticity in the CA1 region.
Loss of Limbic MR Results in Aberrant Hippocampal Mossy Fiber Layout and GR Up-Regulation. In contrast to an earlier study showing that the loss of CREB protein starts at P6 and is complete 2 weeks after birth (18), we show by using the same Cre transgenic mouse line that the MR protein is already reduced at P0, and the loss of MR is almost complete at P6. We suspect that this discrepancy reflects a different turnover of the two proteins and not a different timing of the recombination of the two floxed alleles.
The early perinatal onset of the absence of MR induces adaptive changes, like the observed hippocampal GR up-regulation and sprouting of mossy fibers. Thus, the phenotype we describe is showing the consequences of a permanently disturbed balance of GR and MR signaling. The power of the genetic approach we used, in contrast to pharmacological approaches using receptor antagonists, is the high selectivity of interference with and the completeness of inactivation of the respective receptor-mediated signaling pathway. The continuous absence in a developing organism allows separating redundant from nonredundant functions. To determine whether the actual behavioral phenotype described here is the consequence of the altered hippocampal development or the lack of MR in the adult brain, we currently develop transgenic mice expressing an inducible Cre-fusion protein (26) in the forebrain that allows the inactivation of the MR gene in the adult brain only.
The early postnatal period was shown to be critical for the development of the hippocampal mossy fiber layout (27). The early perinatal onset of the MR deficiency in MRCaMKCre mice results in an aberrant mossy fiber layout that is comparable with the altered mossy fiber projection in adult salt-rescued MR knockout mice (28), in which the MR gene is inactivated in all cells from gestation on. However, in contrast to the salt-rescued MR knockout mice, the altered mossy fiber projection in MR-CaMKCre mice was not associated with a conspicuous decreased density of granule cells in the upper blade of the dentate gyrus.
Limbic MR Deficiency Changes Neither Basal Circadian HPA-Axis Activity Nor Basic Synaptic Properties in the CA1 Region. Adult MRCaMKCre mice showed normal basal corticosterone levels as well as normal CRH mRNA and GR protein expression in the PVN. In addition, we could not detect MR expression in the PVN. Therefore, we conclude that the limbic MR is either dispensable for the maintenance of the basal HPA-axis activity or can be completely substituted. The hippocampal GR up-regulation might reflect such a compensatory mechanism.
Electrophysiological analysis of the basic synaptic properties and LTP induced by high-frequency stimulation revealed no differences between MRCaMKCre mice and controls. Whereas some studies have shown that the induction of LTP can be affected by stress and hormonal manipulations (12, 29), others indicate that induction of LTP is less sensitive than primed-burst potentiation (PBP) to stress and hormonal manipulations (30, 31). A more extensive electrophysiological evaluation of LTP and PBP in the CA1 region and other hippocampal subregions, e.g., mossy fiber–CA3 pathway, in combination with hormonal manipulations, is required to precisely define the electrophysiological correlates of the behavioral phenotype delineated in this study.
Loss of Limbic MR Impairs Learning and Induces Behavioral Stereotypy. The behavioral screen of MRCaMKCre mice in various learning and exploration tests revealed that the genetic inactivation of MR in the limbic system causes a mixed behavioral syndrome with distinct cognitive deficits. Several but not all behavioral changes correspond to a septo-hippocampal malfunction syndrome. Among them are the impaired learning of the water-maze task and deficits in measures of working memory on the radial maze. Changes that are not related to learning include the hyperreactivity toward a novel object and the behavioral perseverance, two typical signs of septal and/or hippocampal lesions in rats (32).
On the other hand, the MRCaMKCre mice showed no improvement of two-way avoidance, another classic hippocampal lesion syndrome in rats (33), and showed clearly intact spatial memory. Conditioned taste aversion, a learning task less dependent on hippocampal integrity but dependent on amygdalar modulation (34), was not affected in MRCaMKCre mice. Learning deficits were also reported in studies using MR antagonists in rats. In contrast to our study, the acute and continuous intracerebroventricular administration of a MR antagonist led to impaired retention of spatial memory (16, 35), whereas on the eight-arm radial maze, MR antagonists only led to an increase in reentry errors when applied in combination with GR antagonists (36).
The loss of MR in MRCaMKCre mice is not restricted to the septum and hippocampus. It is possible that the loss of MR in the amygdala contributes to the observed behavioral phenotype of MRCaMKCre mice.
Limbic MR Deficiency Spares Anxiety-Related Behavior but Induces Abnormal Exploratory Behavior Toward a Novel Object. Studies in rats using MR antagonists suggested that MR is involved in the regulation of anxiety-like behavior (37, 38). This involvement was not observed in the MRCaMKCre mice, because they were indistinguishable from controls in the commonly used paradigms for assessing the balance between exploratory and fear-related behavior, i.e., open-field, light–dark box, and elevated O maze. On the other hand, the MRCaMKCre mice showed a hyperreactivity toward a novel object introduced into a test box to which the mice were already habituated. This hyperreactivity was not associated with a general increase of locomotor activity. Taking all of this into account, the altered behavior in the novel object test most likely represents an impulsive reaction to a salient stimulus or could be seen as evidence for an increased exploratory drive that is detectable in a familiar, but not in a novel, test environment. Interestingly, a hyperreactive locomotor activity toward a novel object like we observed in MRCaMKCre mice was also reported in rats receiving an acute intracerebroventricular injection of a MR antagonist (39).
Taken together, loss of limbic MR in MRCaMKCre mice appears to primarily cause a defect in behavioral flexibility combined with lacking control of appropriate responses, reflected in various learning-related and -unrelated deficits. These deficits are associated with adaptive and probably also functional changes, such as sprouting of mossy fibers and up-regulation of GR expression in the hippocampus. In addition, our results indicate that limbic MR is dispensable for the maintenance of basal circadian HPA-axis activity, spatial and gustatory memory, and anxiety-related mechanisms. Our observations provide insights in limbic MR function with implications for stress-related neuroendocrine and neuropsychiatric diseases.
Supplementary Material
Supporting Information
Acknowledgments
We thank Inger Drescher, Heidrun Kern, Nadine Sold, and Magdalena Westphal for their expert technical assistance. This work was supported by the Deutsche Forschungsgemeinschaft through Sonderforschungsbereich 636, “Learning, Memory and Brain Plasticity: Implications for Psychopathology,” the Fonds der Chemischen Industrie, the European Community, the Bundesministerium für Forschung und Bildung, the Alexander von Humboldt-Stiftung, the Swiss National Science Foundation, and the NCCR, “Neural Plasticity and Repair.”
Author contributions: S.B., D.P.W., O.S., H.M.R., H.W., H.L.H., H.-P.L., and G.S. designed research; S.B., D.P.W., O.S., H.A., G.E., H.M.R., A.N.C., and H.W. performed research; S.B., D.P.W., O.S., H.A., G.E., H.M.R., A.N.C., H.W., H.L.H., and H.-P.L. analyzed data; and S.B., D.P.W., O.S., H.L.H., H.-P.L., and G.S. wrote the paper.
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: GR, glucocorticoid receptor; HPA, hypothalamic–pituitary–adrenal; LTP, long-term potentiation; MR, mineralocorticoid receptor; P_n_, postnatal day n; PVN, nucleus paraventricularis.
References
- 1.McEwen, B. S. & Sapolsky, R. M. (1995) Curr. Opin. Neurobiol. 5**,** 205–216. [DOI] [PubMed] [Google Scholar]
- 2.De Kloet, E. R., Vreugdenhil, E., Oitzl, M. S. & Joels, M. (1998) Endocr. Rev. 19**,** 269–301. [DOI] [PubMed] [Google Scholar]
- 3.Arriza, J. L., Simerly, R. B., Swanson, L. W. & Evans, R. M. (1988) Neuron 1**,** 887–900. [DOI] [PubMed] [Google Scholar]
- 4.Kretz, O., Schmid, W., Berger, S. & Gass, P. (2001) NeuroReport 12**,** 1133–1137. [DOI] [PubMed] [Google Scholar]
- 5.Robson, A. C., Leckie, C. M., Seckl, J. R. & Holmes, M. C. (1998) Brain Res. Mol. Brain Res. 61**,** 1–10. [DOI] [PubMed] [Google Scholar]
- 6.Jacobson, L. & Sapolsky, R. (1991) Endocr. Rev. 12**,** 118–134. [DOI] [PubMed] [Google Scholar]
- 7.Herman, J. P. & Cullinan, W. E. (1997) Trends Neurosci. 20**,** 78–84. [DOI] [PubMed] [Google Scholar]
- 8.Ratka, A., Sutanto, W., Bloemers, M. & de Kloet, E. R. (1989) Neuroendocrinology 50**,** 117–123. [DOI] [PubMed] [Google Scholar]
- 9.Oitzl, M. S., van Haarst, A. D., Sutanto, W. & de Kloet, E. R. (1995) Psychoneuroendocrinology 20**,** 655–675. [DOI] [PubMed] [Google Scholar]
- 10.van Haarst, A. D., Oitzl, M. S. & de Kloet, E. R. (1997) Neurochem. Res. 22**,** 1323–1328. [DOI] [PubMed] [Google Scholar]
- 11.Joels, M. (2001) J. Neuroendocrinol. 13**,** 657–669. [DOI] [PubMed] [Google Scholar]
- 12.Pavlides, C., Ogawa, S., Kimura, A. & McEwen, B. S. (1996) Brain Res. 738**,** 229–235. [DOI] [PubMed] [Google Scholar]
- 13.Gould, E., Cameron, H. A., Daniels, D. C., Woolley, C. S. & McEwen, B. S. (1992) J. Neurosci. 12**,** 3642–3650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roozendaal, B., Portillo-Marquez, G. & McGaugh, J. L. (1996) Behav. Neurosci. 110**,** 1074–1083. [DOI] [PubMed] [Google Scholar]
- 15.Sandi, C. & Rose, S. P. (1994) Eur. J. Neurosci. 6**,** 1292–1297. [DOI] [PubMed] [Google Scholar]
- 16.Oitzl, M. S. & de Kloet, E. R. (1992) Behav. Neurosci. 106**,** 62–71. [DOI] [PubMed] [Google Scholar]
- 17.Lupien, S. J. & McEwen, B. S. (1997) Brain Res. Brain Res. Rev. 24**,** 1–27. [DOI] [PubMed] [Google Scholar]
- 18.Casanova, E., Fehsenfeld, S., Mantamadiotis, T., Lemberger, T., Greiner, E., Stewart, A. F. & Schutz, G. (2001) Genesis 31**,** 37–42. [DOI] [PubMed] [Google Scholar]
- 19.Schwenk, F., Baron, U. & Rajewsky, K. (1995) Nucleic Acids Res. 23**,** 5080–5081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kellendonk, C., Tronche, F., Casanova, E., Anlag, K., Opherk, C. & Schutz, G. (1999) J. Mol. Biol. 285**,** 175–182. [DOI] [PubMed] [Google Scholar]
- 21.Schwegler, H. & Lipp, H. P. (1983) Behav. Brain Res. 7**,** 1–38. [DOI] [PubMed] [Google Scholar]
- 22.Madani, R., Kozlov, S., Akhmedov, A., Cinelli, P., Kinter, J., Lipp, H. P., Sonderegger, P. & Wolfer, D. P. (2003) Mol. Cell. Neurosci. 23**,** 473–494. [DOI] [PubMed] [Google Scholar]
- 23.Minichiello, L., Korte, M., Wolfer, D., Kuhn, R., Unsicker, K., Cestari, V., Rossi-Arnaud, C., Lipp, H. P., Bonhoeffer, T. & Klein, R. (1999) Neuron 24**,** 401–414. [DOI] [PubMed] [Google Scholar]
- 24.Han, F., Ozawa, H., Matsuda, K., Nishi, M. & Kawata, M. (2005) Neurosci. Res. 51**,** 371–381. [DOI] [PubMed] [Google Scholar]
- 25.Berger, S., Bleich, M., Schmid, W., Cole, T. J., Peters, J., Watanabe, H., Kriz, W., Warth, R., Greger, R. & Schutz, G. (1998) Proc. Natl. Acad. Sci. USA 95**,** 9424–9429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Indra, A. K., Warot, X., Brocard, J., Bornert, J. M., Xiao, J. H., Chambon, P. & Metzger, D. (1999) Nucleic Acids Res. 27**,** 4324–4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crusio, W. E. & Schwegler, H. (1991) Physiol. Behav. 50**,** 259–261. [DOI] [PubMed] [Google Scholar]
- 28.Gass, P., Kretz, O., Wolfer, D. P., Berger, S., Tronche, F., Reichardt, H. M., Kellendonk, C., Lipp, H. P., Schmid, W. & Schutz, G. (2000) EMBO Rep. 1**,** 447–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim, J. J., Foy, M. R. & Thompson, R. F. (1996) Proc. Natl. Acad. Sci. USA 93**,** 4750–4753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alfarez, D. N., Wiegert, O., Joels, M. & Krugers, H. J. (2002) Neuroscience 115**,** 1119–1126. [DOI] [PubMed] [Google Scholar]
- 31.Mesches, M. H., Fleshner, M., Heman, K. L., Rose, G. M. & Diamond, D. M. (1999) J. Neuroscience 19**,** 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Isaacson, R. L. & Kimble, D. P. (1972) Behav. Biol. 7**,** 767–793. [DOI] [PubMed] [Google Scholar]
- 33.Jarrard, L. E. (1976) J. Comp. Physiol. Psychol. 90**,** 1035–1050. [DOI] [PubMed] [Google Scholar]
- 34.Welzl, H., D'Adamo, P. & Lipp, H. P. (2001) Behav. Brain Res. 125**,** 205–213. [DOI] [PubMed] [Google Scholar]
- 35.Yau, J. L., Noble, J. & Seckl, J. R. (1999) Neurosci. Lett. 277**,** 45–48. [DOI] [PubMed] [Google Scholar]
- 36.Douma, B. R., Korte, S. M., Buwalda, B., la Fleur, S. E., Bohus, B. & Luiten, P. G. (1998) Psychoneuroendocrinology 23**,** 33–44. [DOI] [PubMed] [Google Scholar]
- 37.Smythe, J. W., Murphy, D., Timothy, C. & Costall, B. (1997) Pharmacol. Biochem. Behav. 56**,** 507–513. [DOI] [PubMed] [Google Scholar]
- 38.Korte, S. M., de Boer, S. F., de Kloet, E. R. & Bohus, B. (1995) Psychoneuroendocrinology 20**,** 385–394. [DOI] [PubMed] [Google Scholar]
- 39.Oitzl, M. S., Fluttert, M. & de Kloet, E. R. (1994) Eur. J. Neurosci. 6**,** 1072–1079. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information