Analysis of Sequence Upstream of the Endogenous H19 Gene Reveals Elements Both Essential and Dispensable for Imprinting (original) (raw)
Abstract
Imprinting of the linked and oppositely expressed mouse H19 and Igf2 genes requires a 2-kb differentially methylated domain (DMD) that is located 2 kb upstream of H19. This element is postulated to function as a methylation-sensitive insulator. Here we test whether an additional sequence 5′ of H19 is required for H19 and Igf2 imprinting. Because repetitive elements have been suggested to be important for genomic imprinting, the requirement of a G-rich repetitive element that is located immediately 3′ to the DMD was first tested in two targeted deletions: a 2.9-kb deletion (ΔDMDΔG) that removes the DMD and G-rich repeat and a 1.3-kb deletion (ΔG) removing only the latter. There are also four 21-bp GC-rich repetitive elements within the DMD that bind the insulator-associated CTCF (CCCTC-binding factor) protein and are implicated in mediating methylation-sensitive insulator activity. As three of the four repeats of the 2-kb DMD were deleted in the initial 1.6-kb ΔDMD allele, we analyzed a 3.8-kb targeted allele (Δ3.8kb-5′H19), which deletes the entire DMD, to test the function of the fourth repeat. Comparative analysis of the 5′ deletion alleles reveals that (i) the G-rich repeat element is dispensable for imprinting, (ii) the ΔDMD and ΔDMDΔG alleles exhibit slightly more methylation upon paternal transmission, (iii) removal of the 5′ CTCF site does not further perturb H19 and Igf2 imprinting, suggesting that one CTCF-binding site is insufficient to generate insulator activity in vivo, (iv) the DMD sequence is required for full activation of H19 and Igf2, and (v) deletion of the DMD disrupts H19 and Igf2 expression in a tissue-specific manner.
Genomic imprinting is an epigenetic modification to differentiate the maternal and paternal alleles of a gene, the consequence of which is parent-specific gene expression. Differential DNA methylation, allele-specific nuclease hypersensitivity, and repetitive elements have been proposed to be important for the regulation of imprinted gene expression (5, 6, 43). The imprinted mouse H19 gene, which is expressed from the maternal allele, possesses each of these characteristics. A 2-kb sequence between −4 and −2 kb relative to the H19 transcription start site (designated as the differentially methylated domain [DMD] or the imprinting control region) is exclusively methylated on the paternal allele throughout development (40, 52, 54). Maternally specific hypersensitive sites have been mapped to this 2-kb region in embryonic and neonatal tissues (28, 34). Coincident with these hypersensitive sites are four 21-bp GC-rich repeats that are conserved in mouse, rat, and human tissue (25, 48). Finally, a 461-bp sequence harboring 32 copies of a G-rich element (GGGGTATA) resides immediately 3′ to the DMD (48, 53).
The mouse H19 gene is located 90 kb 3′ to the oppositely imprinted insulin-like growth factor 2 (Igf2) gene. The expression of the H19 and Igf2 genes is mediated through shared enhancers that are located 3′ to H19 (1, 3, 32, 37). Perhaps the most critical part of the joint regulation of H19 and Igf2 is the DMD, which has been the focus of multiple targeted deletion studies at the endogenous locus (32, 47, 51). While these studies are consistent with a role for the DMD in the silencing of H19 on the paternal allele and Igf2 on the maternal allele, the DMD is also hypothesized to function as a methylation-sensitive insulator. Insulators isolate a gene from enhancers, thereby inhibiting enhancer-induced expression of the gene (10). According to the insulator model, the DMD insulator is active on the maternal allele and prevents the expression of Igf2 by blocking its access to downstream enhancers. On the paternal allele, hypermethylation of the DMD is postulated to inactivate insulator function by inhibiting critical protein-DNA interactions, as described for the ubiquitous zinc finger protein CCCTC-binding factor (CTCF) (9, 27, 39). The DMD insulator activity has been demonstrated by using in vitro enhancer blocking assays, and this activity is dependent upon the 21-bp repeats of the DMD sequence (9, 27, 29, 32, 33, 50). CTCF binds to these repeat elements in vitro in a methylation-sensitive manner.
It was previously reported that a 1.6-kb deletion of the 2-kb DMD, which is designated ΔDMD and removes three of the four CTCF-binding 21-bp conserved repeats, results in the loss of H19 and Igf2 imprinted expression and perturbations in allele-specific methylation (51). To further define the cis elements required for H19 and Igf2 imprinting at the endogenous locus, we have constructed three additional mutations 5′ of H19 and compared methylation and allelic expression of the mutant alleles to the original 1.6-kb ΔDMD allele (Fig. 1). To test the role of the G-rich repeat sequence in H19 and Igf2 imprinting, we generated the ΔG deletion that removes 1.3 kb of sequence, including the 461-bp G-rich repetitive element. In addition, to determine whether there is a synergistic effect of the DMD and G-rich repeat sequence on H19 and Igf2 imprinting, the ΔDMDΔG deletion was generated by removal of 2.9 kb of sequence, including part of the DMD and all of the G-rich repeat element. We also tested the role of the remaining seven CpG dinucleotides and the CTCF-binding site within the DMD in directing H19 and Igf2 imprinted expression and H19 allele-specific methylation in the Δ3.8kb-5′H19 deletion. This deletion removes a region that encompasses all of the DMD, including the fourth 21-bp conserved element that remained in the initial ΔDMD allele, and the G-rich repeat sequence. Finally, we tested the tissue-specific effects on H19 and Igf2 expression that these deletions confer. While the ΔG deletion had no effect on expression or methylation, the ΔDMD and ΔDMDΔG deletions similarly disrupted H19 and Igf2 expression and H19 parent-specific methylation. These perturbations were tissue specific, suggesting that the DMD interacts differentially with enhancers and other regulatory elements. Finally, the Δ3.8kb-5′H19 allele, from which the entire DMD and the last CTCF-binding site were removed, exhibited tissue-specific disruption of imprinting similar to that which was detected from the ΔDMD and ΔDMDΔG alleles. This result demonstrated that deletion of the 1.6-kb DMD sequence was sufficient to perturb fully the insulator and silencing activity conferred by the 2-kb DMD. Our findings complement those of other studies which assay the DMD function either in vitro by transfection assays or in vivo by deleting the DMD together with several additional kilobases of 5′ H19 sequence from the endogenous locus (9, 27, 29, 32, 33, 47, 50). Taken together, these experiments show that the DMD is a complex element that exhibits methylation-dependent silencing and methylation-sensitive insulator activities that are required for allele-specific silencing of H19 and Igf2, respectively, in all tissues.
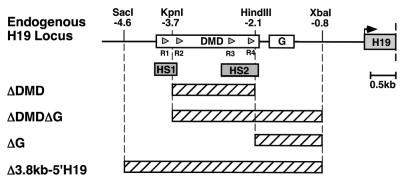
FIG. 1.
DMD and G-rich repeat sequence deletions generated at the H19 endogenous locus. The top line shows the 5′ H19 sequence with boxes indicating the 2-kb DMD, the 461-bp G-rich repeat element (G), and the first H19 exon. The gray triangles within the DMD depict the 21-bp repeats (R1 to R4) that are conserved in mouse, rat, and human 5′ H19 sequences. These sites also function as CTCF-binding sites. The maternal allele-specific hypersensitivity sites (HS1 and HS2) are indicated. The crosshatched boxes correspond to sequences that are removed in each of the targeted deletions. The deleted sequences are as follows: a 1.6-kb _Kpn_I-_Hin_dIII fragment in the ΔDMD deletion, a 2.9-kb _Kpn_I-_Xba_I fragment in the ΔDMDΔG deletion, a 1.3-kb _Hin_dIII-_Xba_I fragment in the ΔG deletion, and a 3.8-kb _Sac_I-_Xba_I fragment in the Δ3.8kb-5′H19 deletion.
MATERIALS AND METHODS
Targeting vectors.
The ΔDMD targeting vector was previously described (51). The other vectors were designed to delete 2.9 kb (ΔDMDΔG), 1.3 kb (ΔG), and 3.8 kb (Δ3.8kb-5′H19) of sequence 5′ to the _Xba_I site at −0.8 kb relative to the H19 transcription start site (Fig. 1). For each vector, a _loxP_-flanked PGK-neo cassette and an H19 sequence isolated from a 129Sv/J mouse genomic library (Stratagene) were cloned into pBKSII (Stratagene) (Fig. 2A). The same 2.6-kb _Xba_I-_Bam_HI H19 fragment was used for the right arm of each vector. A 6.6-kb _Bam_HI-_Kpn_I (ΔDMDΔG) fragment, an 8.2-kb _Bam_HI-_Hin_dIII (ΔG) fragment, and a 5.6-kb _Bam_HI-_Sac_I (Δ3.8kb-5′H19) H19 fragment were used for the left arms. The ΔDMDΔG and Δ3.8kb-5′H19 vectors also include a 2.3-kb _Sal_I fragment containing a diphtheria toxin A cassette for negative selection (38). The vectors were linearized by _Not_I digestion.
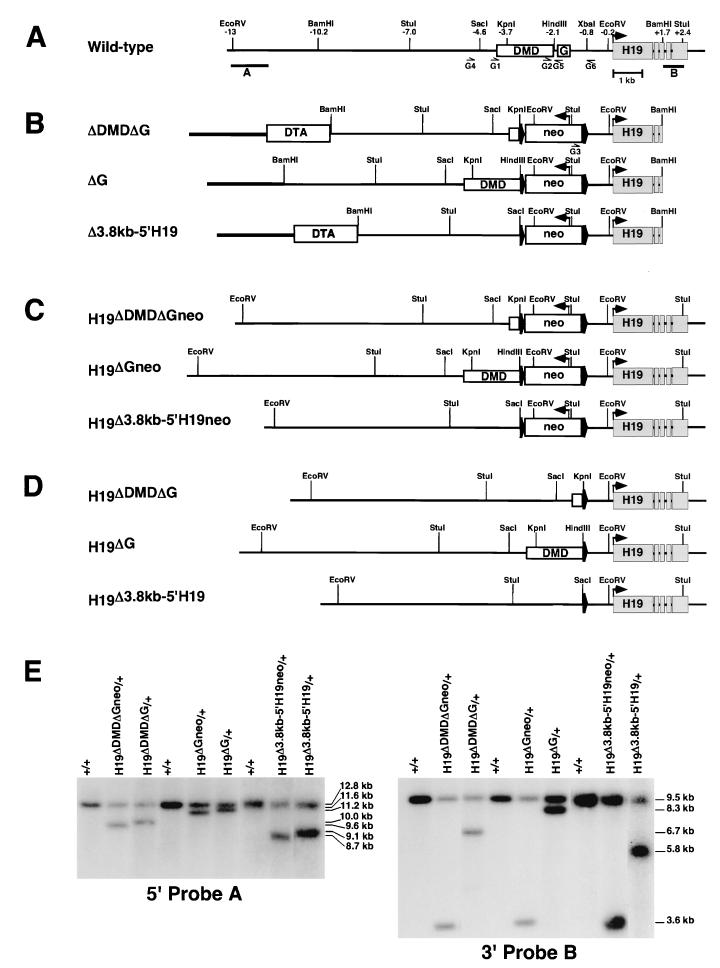
FIG. 2.
Generation of the H19ΔDMDΔG, H19ΔG, and H19Δ3.8kb-5′H19 alleles. The H19 locus is illustrated before and after homologous recombination of the target vectors and Cre-mediated excision of the PGK-neo cassette. The DMD, G-rich repeat sequence (G), and H19 exons are depicted in boxes. (A) Endogenous H19 locus. Positions of restriction sites (in kilobases) are relative to the H19 transcription start site. Correctly targeted clones were identified with the use of external probes A (1.7-kb _Eco_RV-_Eco_RI fragment) and B (0.75-kb _Bam_HI-_Stu_I fragment). (B) Targeting vectors. The PGK-neo cassette (neo) (boxed) flanked by loxP sites (wide arrowheads), H19 DNA (thin line), and pBKSII (thick line) is illustrated. The directions of the neo and H19 transcripts are indicated (narrow arrowheads). Targeting vectors ΔDMDΔG and Δ3.8kb-5′H19 also included the diphtheria toxin A (DTA) negative selection cassette (boxed). The sequence that is deleted from each vector is described in the legend to Fig. 1. (C) Targeted alleles generated from homologous recombination of each of the targeting vectors. A loxP_-flanked PGK-neo cassette replaced the deleted 5′_H19 sequences. (D) PGK-_neo_-excised targeted alleles following Cre-mediated recombination. A loxP site remains at each targeted locus. (E) Southern analysis of the targeted alleles before and after Cre-mediated removal of the PGK-neo cassette. Probes A and B were hybridized to _Eco_RV- and _Stu_I-digested DNA, respectively. The sizes of the DNA fragments are shown to the right. The primers (G1 to G6) (Table 1) used to assay the targeted alleles are shown below the schematics in panels A and B.
Targeted disruption in ES cells and detection of mutant alleles.
Embryonic stem (ES) cells, embryonic day 14.1 (35), were used for the H19 gene targeting experiments. The cell culture and electroporation methods, Southern blot analysis, generation of chimeric and germ line mice, and genotyping of tail biopsies were performed as previously described (51) with the following exceptions. Targeted H19ΔDMDΔGneoR and H19ΔGneoR clones were transiently transfected with a Cre recombinase-expressing plasmid (45) for removal of the PGK-neo cassette. Mice harboring the H19Δ3.8kb-5′H19neoR allele were mated to transgenic mice expressing Cre recombinase for germ line excision of the PGK-neo cassette (14). Mutant alleles were identified by Southern analysis (Fig. 2) and PCR amplification with primers that flank the deleted sequences (Table 1; Fig. 2A and B). Primer pairs G1 and G5 were used to assay H19ΔDMD (400 bp), G1 and G6 were used to assay H19ΔDMDΔG (390 bp), G2 and G6 were used to assay H19ΔG (320 bp), G3 and G6 were used to assay H19Δ3.8kb-5′H19neoR (350 bp), and G4 and G6 were used to assay H19Δ3.8kb-5′H19 (360 bp). PCR amplification was performed on 50 to 200 ng of tail and liver DNA with Ready-To-Go PCR beads (Amersham) and primers at a final concentration of 0.4 μM as follows: 2 min of denaturation at 95°C; 32 cycles of 1 min at 94°C, 1 min at 58°C, and 1 min at 72°C; and 10 min of extension at 72°C.
TABLE 1.
Primers used for genotyping, bisulfite analysis, and reverse transcription-PCR
| Primer no. (name)a | H19 position no.b | Sequence (5′-3′) |
|---|---|---|
| G1 (H19-3.9F) | −3953 (1303) | TGCAAGGAGACCATGCCTATTCTTG |
| G2 (H19-2.3F) | −2286 (2971) | CAATGTTCATAAGGGTCATGGGGTG |
| G3 (PGK-_neo_F) | NAc | CCACTTGTGTAGCGCCAAGTGCC |
| G4 (H19-4.8F) | −4811 (445) | CCAACTGAGAGGGCCATAGTGTGAG |
| G5 (H19-2.0R) | −2031 (3227) | CGTAAAGTGTCACAAATGCCTGATCCC |
| G6 (HSEQ1R) | −750 (4551) | CCACAGAGTCAGCATCCAC |
| B1 (BMsp2t1) | −3978 (1278) | GAGTATTTAGGAGGTATAAGAATT |
| B2 (BMsp2t2) | −3952 (1304) | GTAAGGAGATTATGTTTATTTTTGG |
| B3 (BHhalt4) | −3532 (1726) | CCTCATTAATCCCATAACTAT |
| B4 (BHhalt3) | −3507 (1751) | ATCAAAAACTAACATAAACCCCT |
| B5 (BHha7t) | −2031 (3225) | TAAAATATCACAAATACCTAATCCCT |
| B6 (BHSEQm1) | −709 (4551) | CCATTACTCTTAACTTCTATTAAA |
| B7 (BHSEQm2) | −733 (4527) | AATTATACAACTAAAATCCACAAAATCAAC |
| B8 (BTV3-1) | −2310 (2938) | GGTAAATTTATGGGTTATTTAAGG |
| B9 (BTV3-2) | −2286 (2972) | AATGTTTATAAGGGTTATGGGGTGG |
| B10 (BTV3-3) | −1861 (3397) | CCTAAATTCAATAAAACATTACAA |
| B11 (BTV3-4) | −1884 (3374) | CCCAACCTCTACTTTTATAAC |
| RT1 (HI3) | +1722 (7242)d | CCTCAAGATGAAAGAAATGGT |
| RT2 (HE5) | +2528 (8048)d | AACACTTTATGATGGAACTGC |
Mutant mice were further bred to C57BL/6J mice. Allelic expression and methylation patterns were analyzed in the progeny of heterozygous mutant mice that were bred to the B6(CAST-H19) strain of mice (53). These mice have Mus musculus castaneus (CAST) H19 and Igf2 alleles on a C57BL/6J background. The maternal parent is designated first in hybrid mice. Heterozygous mutant mice were intercrossed to generate homozygous mutant mice.
RNA isolation and analysis.
Tissues (liver, tongue, kidney, gut, heart, lung, and brain) were collected from neonatal (0 to 5 day) mice, and total RNA was prepared by the lithium chloride method (4). RNase protection assays (RPAs) were performed to assess H19 (8, 13), Igf2 (36), and rpL32 (20) RNA levels. Products were resolved on 7% polyacrylamide-7 M urea gels.
H19 allele-specific expression assay.
The H19 allelic expression assay was conducted on cDNA (cDNA Cycle kit; Invitrogen) prepared from 1 μg of total RNA by using the LightCycler Real Time PCR system (Roche Molecular Biochemicals). Primers were designed to amplify a coding region of the H19 gene (642 bp, GenBank accession number AF049091). The forward primer lay over an exon-exon boundary at intron 3 of the H19 gene (RT1) while the reverse primer was localized within exon 5 (RT2) (Table 1). Fluorescence resonance energy transfer hybridization probes were designed to the C57BL/6J amplicon: H19 sensor probe, 5′-CCACCTGTCGTCCATCTCC-3′; and H19 anchor probe, 5′-TCTGAGGGCAACTGGGTGTGG-3′. The H19 sensor probe spans a single nucleotide polymorphism at nucleotide 7954 between C57BL/6J (G) and CAST (A) and was labeled with fluorescein at the 3′ end. The H19 anchor probe was labeled with LC-Red640 at the 5′ end and was phosphorylated at the 3′ end. To a Ready-To-Go PCR bead, 6.22 μl of sterile water and 0.38 μl of TaqStart Antibody (Clontech) were added, and the reaction mixture was incubated at room temperature for 5 min. After incubation, a final concentration of 3.0 mM MgCl2, a 0.3 μM concentration of each primer, and a 0.15 μM concentration of each probe were added to the mix and the volume was brought to 12.5 μl. From this reaction mix, 10 μl was removed and added to a LightCycler glass capillary (Roche Molecular Biochemicals) and 10 μl of cDNA (1/100 to 1/250 diluted in H2O) and H2O were added for a final reaction volume of 20 μl. Samples were denatured at 95°C for 2 min and then amplified for 38 to 45 cycles, depending on tissue type, at 95°C for 0 s, 55°C for 15 s, and 72°C for 25 s. A single fluorescence acquisition occurred at the end of each annealing step. After amplification, a final denaturation step was conducted at 95°C for 0 s, followed by a single annealing step at 50°C for 15 s and a melting curve analysis with fluorescence acquisition occurring continuously as the temperature was increased from 50 to 85°C in increments of 0.2°C. A cooling step at 40°C for 30 s was performed at the end of the melting curve analysis. The data were analyzed by using the LightCycler Software Data Analysis function. After background subtraction, the contribution of each allele was calculated as the peak height or peak area of the melting curve generated at the allele-specific temperature, approximately 67.5°C for C57BL/6J and 61.5°C for CAST. The H19 allelic expression pattern was calculated as the fraction of expression from the paternal mutant allele relative to the expression from the maternal CAST allele. A _Cac_8I restriction site unique to CAST DNA was used to confirm the efficacy of the LightCycler Data Analysis by digesting amplified sample and resolving the products on a 7% polyacrylamide gel.
DNA isolation and methylation: Southern analysis.
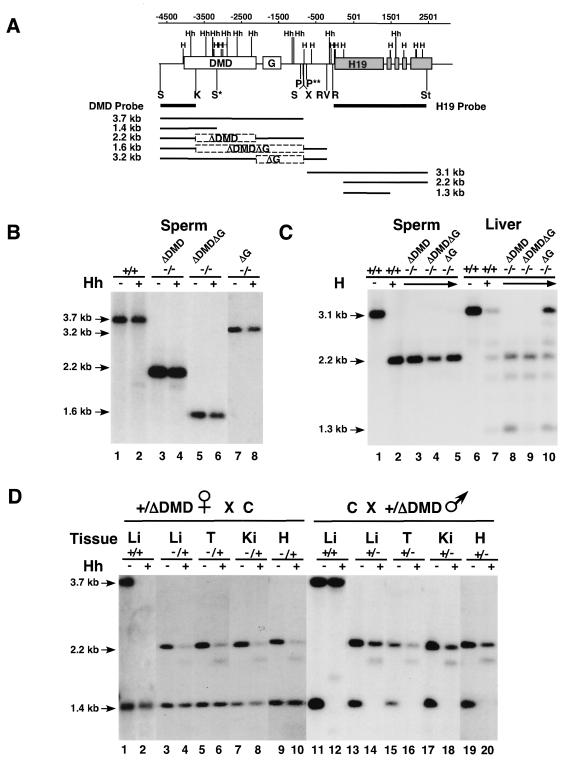
DNA was isolated from tissues as previously described (7). Genomic DNA (10 μg) was digested with _Pvu_II and _Stu_I in combination with _Hpa_II to analyze the methylation of the H19 transcription unit. To analyze methylation of the DMD, DNA was digested with _Sac_I (H19ΔDMD) or _Sac_I and _Eco_RV (H19ΔDMDΔG and H19ΔG) in combination with _Hha_I. The probes used for the analyses were a 2.5-kb _Eco_RI-_Stu_I fragment (H19 transcription unit) and a 0.9-kb _Sac_I-_Kpn_I fragment (DMD) (see Fig. 5).
FIG. 5.
Methylation status of the H19ΔDMD, H19ΔDMDΔG, and H19ΔG alleles. (A) Schematic of genomic Southern analysis. The first line indicates the position (in base pairs) relative to the start of transcription. The second line shows the H19 endogenous locus with the DMD, G-rich repeat (G), and H19 transcription unit designated by boxes and the _Hha_I (Hh) and _Hpa_II (H) restriction sites located above the line. Sites between −4000 and +500 relative to the H19 transcription start site are methylated on the paternal allele in wild-type mice. Located beneath the second line are the two probes used in the Southern analysis and the major fragments detected for each allele. The ΔDMD, ΔDMDΔG, and ΔG deletions are designated by dashed boxes. To assay the methylation status of the DMD sequence, genomic DNA was digested with _Sac_I or _Sac_I plus _Hha_I for wild-type (+/+) and ΔDMD DNA and with _Sac_I plus _Eco_RV or _Sac_I, _Eco_RV, and _Hha_I for ΔDMDΔG and ΔG DNA. To assay the H19 transcription unit, wild-type and mutant DNA was digested with _Pvu_II plus _Stu_I or _Pvu_II, _Stu_I, and _Hpa_II. Homozygous and heterozygous mutant mice were analyzed. The genotype of the samples and the absence (−) or presence (+) of _Hha_I or _Hpa_II in the digestion are noted above each Southern blot in panels B, C, and D. Restriction sites relevant to the methylation analysis are shown (_Hha_I [Hh], _Hpa_II [H], _Eco_RI [R], _Eco_RV [RV], _Pvu_II [P], _Sac_I [S], _Stu_I [St], and _Xba_I [X]). S* is a _Sac_I site unique to the C allele, and P** is a _Pvu_II site unique to the B and mutant alleles. (B) The methylation status of the DMD in sperm. Sperm from wild-type (lanes 1 and 2) and homozygous mutant (lanes 3 to 8) adult males was hypermethylated. (C) The methylation status of the H19 transcription unit in sperm and neonatal liver tissue. Wild-type and homozygous mutant sperm DNA (lanes 1 to 5) was hypomethylated as was neonatal liver DNA from ΔDMD and ΔDMDΔG deletion mice (lanes 8 and 9). Mice inheriting the ΔG deletion displayed a wild-type pattern of paternally specific methylation (lane 10 and data not shown). (D) The methylation status of the DMD in neonatal tissues. The paternal ΔDMD allele (lanes 13 to 20) is more methylated than the maternal ΔDMD allele (lanes 3 to 10) in liver (Li), tongue (T), kidney (Ki), and heart (H) DNA.
DNA bisulfite modification and sequencing analysis.
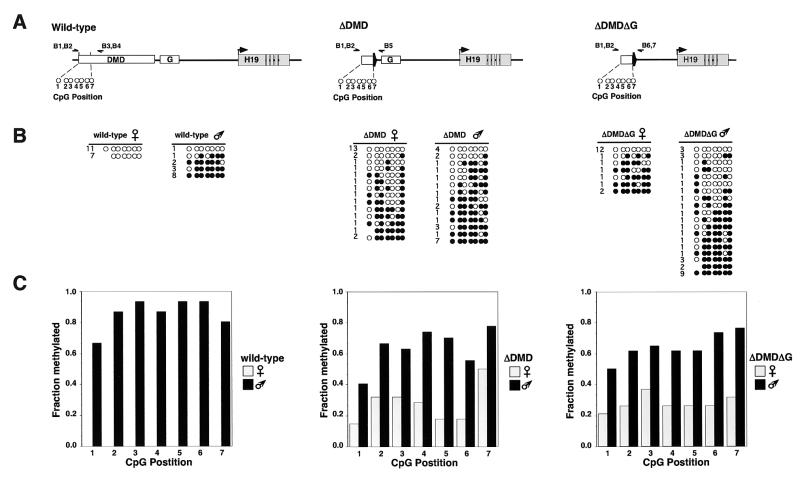
Neonatal liver and kidney DNA isolated from heterozygous neonates that had inherited the mutant alleles maternally (H19ΔDMD/+, H19ΔDMDΔG/+) and paternally (+/H19ΔDMD, +/H19ΔDMDΔG) were subjected to bisulfite modification. Briefly, 5 μg of DNA was digested with _Xba_I (H19ΔDMD) or _Xba_I and _Eco_RV (H19ΔDMDΔG, H19ΔG) for 1 h, extracted with phenol-chloroform, ethanol precipitated with 1 M NH4 acetate, and resuspended in 100 μl of TE (10 mM Tris-HCl, 1 mM EDTA, pH 8.0). DNA was then subjected to bisulfite modification, subcloning, and sequencing as described previously (17, 52) with modification. PCR amplification was performed with Ready-To-Go PCR beads with primers (0.5 μM) specific for the top strand of bisulfite-mutagenized DNA (Table 1) (primers B1 to B11). Primers for the mutant alleles flanked the 5′ DMD and the loxP sequence. Primer pairs used for the first and second rounds of PCR amplification of the 5′ DMD sequence were B1-B4 and B2-B3 for the wild-type allele, B1-B5 and B2-B5 for the H19ΔDMD allele, and B1-B6 and B2-B7 for the H19ΔDMDΔG allele, and primer pairs used for the 3′ DMD sequence were B8-B10 and B9-B11 for the wild-type allele and B8-B6 and B9-B7 for the H19ΔG allele (see Fig. 6). The data obtained for each set of primers were compiled from two or more independent PCRs.
FIG. 6.
Methylation profile of individual DNA strands in the 5′ DMD region in neonatal liver. (A) Schematic of the wild-type, H19ΔDMD, and H19ΔDMDΔG alleles analyzed by the bisulfite mutagenesis and sequencing assay. The seven CpG dinucleotides at the 5′ DMD region that are common to the wild-type and deletion alleles are depicted as open circles. CpG dinucleotides 1 (1330), 2 (1360), 3 (1362), 4 (1382), 5 (1384), 6 (1391), and 7 (1397) are located between −3926 and −3729 relative to the H19 transcription start site of the wild-type allele (the GenBank accession no. U19619 position is given in parentheses). CpG dinucleotide 6 is within the _Hha_I site assayed by the genomic Southern blot in Fig. 5. The primers used to amplify the 5′ DMD region are shown above each allele (B1 to B7) (Table 1). (B) The methylation status of individual DNA strands of the wild-type, H19ΔDMD, and H19ΔDMDΔG alleles. Filled circles correspond to methylated cytosines, and open circles correspond to unmethylated cytosines. The absence of a circle indicates that the residue was not assayed. The number of times a given profile was observed is indicated to the left of each strand. (C) Summary of the methylation profiles of the seven CpG dinucleotides on the wild-type, H19ΔDMD, and H19ΔDMDΔG alleles. Gray and black bars represent the fraction of methylated cytosines on the maternal and paternal alleles, respectively. In contrast to wild-type alleles that are methylated exclusively on the paternal alleles, mutant ΔDMD and ΔDMDΔG alleles show an intermediate level of methylation with more methylation on the mutant paternal allele than on the mutant maternal allele.
RESULTS
Generation of the ΔDMDΔG, ΔG, and Δ3.8kb-5′H19 mice.
It was previously demonstrated that the DMD is required for the imprinted expression of H19 and Igf2 by deleting 1.6 kb of this 2-kb sequence from the endogenous locus (51). The ΔDMD (Fig. 1) deletion removes the majority of the nuclease hypersensitive regions (28, 34) and three of the four 21-bp conserved repeats that confer CTCF-dependent in vitro insulator activity on the maternal allele (9, 27, 33). To determine if an additional sequence 5′ to H19 is required for H19 and Igf2 imprinting, three other deletion alleles were generated (Fig. 1). A similar targeting strategy was used to generate each of the ΔDMDΔG, ΔG, and Δ3.8kb-5′H19 alleles in ES cells and in mice (Fig. 2). Progeny from reciprocal mating of B6(CAST-H19) mice and mutant mice lacking the PGK-neo cassette were assayed for parent-specific expression of H19 and Igf2 as well as parent-specific methylation at the H19 locus.
The G-rich repeat element can be deleted without loss of imprinted expression.
One of the hallmark characteristics of imprinted genes is a repetitive element. Given that repeats may assume alternative chromatin structure, one can envision a role for repetitive elements in imprinted gene regulation. Alternatively, this presence of repetitive elements in imprinted loci may be purely coincidental, and therefore, the role of these elements must be tested in vivo. To this end, we generated the ΔG deletion that removes a 1.3-kb region containing the 461-bp G-rich repetitive element that resides between the DMD and the H19 promoter (H19ΔG) (Fig. 1). In contrast to results from the ΔDMD deletion, analysis of the H19ΔG allele revealed that the ΔG deletion had no effect on the repression of the paternal H19 and the maternal Igf2 alleles (Fig. 3A and 4B and data not shown). Strict imprinted expression of these genes was maintained. In addition, maternal transmission of the H19ΔG allele had no effect on relative H19 expression levels (Fig. 4A and data not shown) and paternal transmission of the H19ΔG allele had no effect on relative Igf2 expression levels (Fig. 3B and data not shown), demonstrating that the deleted sequence is not required for wild-type levels of H19 and Igf2 expression. This study confirms the H19 transgenic study that indicated that the G-rich repetitive element is not required for the imprinting of H19 in neonatal livers (48). These experiments also showed that the DMD position relative to the H19 start of transcription is flexible, since the movement of the DMD 1.3 kb closer to the H19 promoter on the H19ΔG allele had no effect on DMD function.
FIG. 3.
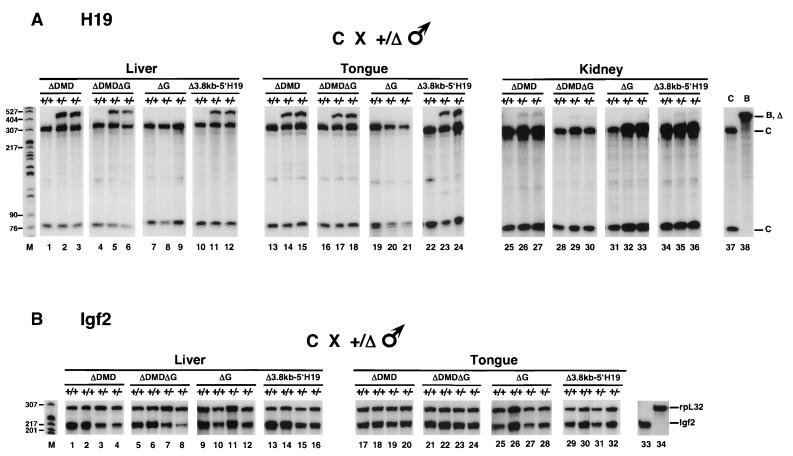
H19 and Igf2 expression in mice inheriting the paternal H19ΔDMD, H19ΔDMDΔG, H19ΔG, and H19Δ3.8kb-5′H19 alleles. Tissues were isolated from neonatal mice generated from the mating of B6(CAST-H19) (C) females and heterozygous mutant males (+/δ) on a C57BL/6J (B) background. The wild-type C and B alleles are designated with a plus sign and the deletion (Δ) alleles are designated with a minus sign above each lane. Total liver (3 μg), tongue (2 μg), and kidney (10 μg) RNA from wild-type (+/+) and mutant (+/−) littermates were analyzed by RPAs for the indicated deletions. (A) Allele-specific expression of H19. The distinct C and common B and deletion allele-protected fragments are designated. Biallelic expression of H19 was detected in liver, tongue, and kidney RNA (lanes 2, 3, 5, 6, 11, 12, 14, 15, 17, 18, 23, 24, 26, 27, 29, 30, 35, and 36) and in heart, lung, brain, and gut RNA (data not shown) upon paternal inheritance of the H19ΔDMD, H19ΔDMDΔG, and H19Δ3.8kb-5′H19 alleles. Relative levels of H19 expression from the mutant paternal allele to the wild-type maternal allele varied among tissues (liver > brain, tongue > heart, lung, gut > kidney). While these results were confirmed by LightCycler Real-Time PCR, less variation in H19 expression levels among samples for a given tissue was measured by the LightCycler PCR than by RPA. H19ΔDMD and H19Δ3.8kb-5′H19 results are summarized in Table 2. H19 expression was not detected from the paternal H19ΔG allele in liver, tongue, and kidney RNA (lanes 8, 9, 20, 21, 32, and 33) nor in heart, lung, and brain RNA (data not shown). Lanes 37 and 38 are control C and B neonatal liver RNAs, respectively. (B) Relative levels of expression of Igf2 compared to rpL32. RNA was assayed simultaneously with the Igf2 and rpL32 probes. In neonatal liver tissue, Igf2 expression from the H19ΔDMD, H19ΔDMDΔG, and H19Δ3.8kb-5′H19 paternal alleles is reduced by 60 to 75% relative to levels detected from the paternal allele of wild-type littermates (lanes 1 to 8 and 13 to 16). In contrast, Igf2 was less affected on the paternal H19ΔDMD, H19ΔDMDΔG, and H19Δ3.8kb-5′H19 alleles in tongue tissue (lanes 17 to 24 and 29 to 32) and in kidney, heart, and lung tissue (data not shown). H19ΔDMD and H19Δ3.8kb-5′H19 results are summarized in Table 3. There was no effect of the paternal ΔG deletion on Igf2 expression in the neonatal liver (lanes 9 to 12), tongue (lanes 25 to 28), and other (data not shown) tissues assayed. Control neonatal liver RNA was assayed with Igf2 (lane 33) and rpL32 (lane 34) probes. Size markers (in base pairs) are indicated to the left in lane M.
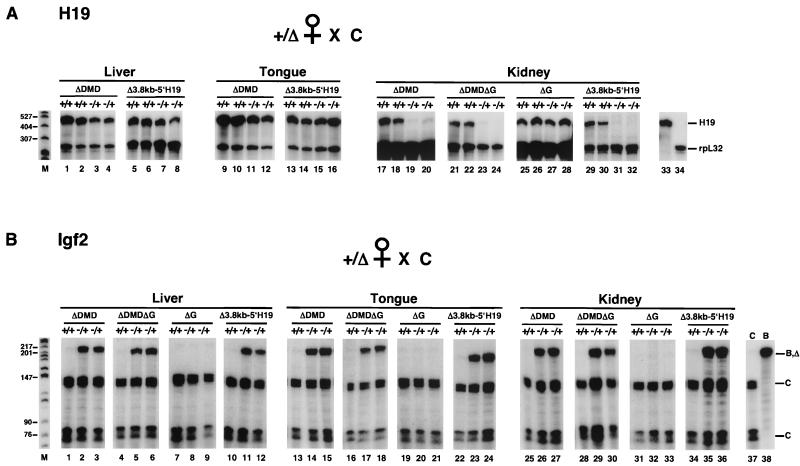
FIG. 4.
H19 and Igf2 expression in mice inheriting the maternal H19ΔDMD, H19ΔDMDΔG, H19ΔG, and H19Δ3.8kb-5′H19 alleles. Tissues were isolated from neonatal mice generated from the mating of female heterozygous mutants (+/Δ) on a C57BL/6J (B) background and B6(CAST-H19) (C) males. The wild-type C and B alleles are designated with a plus sign and the deletion (Δ) alleles are designated with a minus sign above each lane. Total liver (3 μg), tongue (2 μg), and kidney (10 μg) RNA from wild-type (+/+) and mutant (−/+) littermates was analyzed by RPAs for the indicated deletions. (A) Relative levels of expression of H19 compared to rpL32. RNA was assayed simultaneously with the H19 and rpL32 probes. In neonatal liver and tongue RNA, H19 expression from the H19ΔDMD, H19ΔDMDΔG, and H19Δ3.8kb-5′H19 alleles was reduced by 40 to 65% relative to that of wild-type littermates (lanes 1 to 16 and data not shown). In contrast, H19 was more severely affected by the maternal H19ΔDMD, H19ΔDMDΔG, and H19Δ3.8kb-5′H19 alleles in kidney (>90% reduction) (lanes 17 to 24 and 29 to 32) and in heart and lung (75 to 90% reduction) (data not shown) RNA. H19ΔDMD and H19Δ3.8kb-5′H19 results are summarized in Table 3. The ΔG deletion had no effect on H19 expression in the neonatal kidney (lanes 25 to 28) and other (data not shown) tissues assayed. Northern analysis confirmed the RPA results (data not shown). Control neonatal liver RNA was assayed with H19 (lanes 33) and rpL32 (lane 34) probes. (B) Allele-specific expression of Igf2. The distinct C and common B and deletion allele-protected fragments are designated. Biallelic expression of Igf2 was detected in liver, tongue, and kidney RNA (lanes 2, 3, 5, 6, 11, 12, 14, 15, 17, 18, 23, 24, 26, 27, 29, 30, 35, and 36), and heart, lung, brain, and gut RNA (data not shown) upon maternal inheritance of the H19ΔDMD, H19ΔDMDΔG, and H19Δ3.8kb-5′H19 alleles. Relative levels of Igf2 expression from the mutant maternal allele to the wild-type paternal allele were 25 to 40% in liver tissue and >50% in all other tissues. H19ΔDMD and H19Δ3.8kb-5′H19 results are summarized in Table 2. Igf2 expression was not detected from the maternal H19ΔG allele in neonatal liver, tongue, and kidney RNAs (lanes 8, 9, 20, 21, 32, and 33) nor in heart and lung RNA (data not shown). Control C (lane 37) and B (lane 38) neonatal liver RNA was assayed. Size markers (in base pairs) are indicated to the left in lane M.
Although the ΔG deletion alone had no effect on H19 and Igf2 imprinting, there was still the possibility that removing the G-rich repetitive element in the context of a DMD deletion would exacerbate the loss of imprinting of these genes. To test this hypothesis, the H19ΔDMDΔG allele was generated by removing 2.9 kb of sequence, including the majority of the DMD and all of the G-rich repeat sequence. Similar to the H19ΔDMD allele, H19 was activated on the paternal H19ΔDMDΔG allele and Igf2 was activated on the maternal H19ΔDMDΔG allele (Fig. 3A and 4B). The finding that the ΔDMD and ΔDMDΔG deletions exhibited similar levels of H19 and Igf2 expression indicated that ΔG in the context of the ΔDMD deletion had no additional detrimental effect on the imprinting of this locus and supported the notion that the 461-bp G-rich repeat element was not required for imprinted expression.
The DMD deletions activate H19 and Igf2 in a tissue-specific manner.
In our initial study of the H19ΔDMD allele, we analyzed imprinting in neonatal liver tissue since this is a relatively homogeneous tissue with high levels of H19 and Igf2 expression. We found that paternal H19 RNA derived from the mutant alleles was approximately 50% of that which was normally observed in wild-type liver tissues. The activation of Igf2 on the mutant maternal allele was approximately 30% of wild-type levels. In the present study of the ΔDMD and ΔDMDΔG deletions, we compared H19 and Igf2 expression in neonatal liver, tongue, kidney, heart, lung, gut, and brain tissues. While imprinting was disrupted in all tissues, the extent of derepression of the normally silent H19 and Igf2 alleles varied among tissues (Fig. 3A and 4B and data not shown). H19 was most highly activated on the mutant paternal allele in liver tissue, slightly less activated in tongue and brain tissue, and significantly less activated in kidney, heart, lung, and gut tissue (Fig. 3A, values for ΔDMD in Table 2, and data not shown). It should be noted, however, that H19 is typically expressed at low levels in kidney, heart, lung, and brain tissue. In contrast, Igf2 was least activated on the mutant maternal allele in liver tissue (Fig. 4B, ΔDMD values in Table 2, and data not shown). The biallelic Igf2 expression pattern that is normally observed in brain tissue was unaffected by the deletions (Table 2). These data indicate that H19 and Igf2 expression are regulated either directly by tissue-specific elements within the DMD or via other regulatory elements that may interact with the DMD in a tissue-specific manner.
TABLE 2.
Activation of paternal H19 and maternal Igf2 expression from mutant alleles
| Tissue type | Deletion | Mutant paternal/wild-type maternal H19 ratioa | Mutant maternal/wild-type paternal Igf2 ratioa |
|---|---|---|---|
| Liver | ΔDMD | 0.51 ± 0.03 (6)b | 0.29 ± 0.06 (27) |
| Δ3.8kb-5′H19 | 0.53 ± 0.02 (6)b | 0.30 ± 0.05 (6) | |
| Tongue | ΔDMD | 0.37 ± 0.02 (6)b | 0.52 ± 0.07 (8) |
| Δ3.8kb-5′H19 | 0.32 ± 0.04 (6)b | 0.56 ± 0.09 (6) | |
| Kidney | ΔDMD | <0.06 ± NA (4)b | 0.51 ± 0.06 (6) |
| Δ3.8kb-5′H19 | <0.06 ± NA (6)b | 0.68 ± 0.09 (6) | |
| Heart | ΔDMD | 0.10 ± 0.022 (3)b | 0.62 ± 0.12 (6) |
| Δ3.8kb-5′H19 | 0.14 ± 0.014 (3)b | 0.77 ± 0.22 (5) | |
| Lung | ΔDMD | 0.14 ± 0.07 (3)b | 0.54 ± 0.07 (6) |
| Δ3.8kb-5′H19 | 0.23 ± 0.16 (3) | 0.96 ± 0.06 (3) | |
| Brain | ΔDMD | 0.38 ± 0.05 (3)b | 0.94 ± 0.28 (3)c |
| Δ3.8kb-5′H19 | 0.48 ± 0.05 (3) | NDd | |
| Gut | ΔDMD | 0.19 ± 0.09 (3)b | 0.35 ± 0.04 (9) |
| Δ3.8kb-5′H19 | 0.19 ± 0.06 (3) | NDd |
Tissue-specific reduction of maternal H19 and paternal Igf2 expression from the ΔDMD and ΔDMDΔG mutant alleles.
We assessed the tissue-specific effect of the ΔDMD and ΔDMDΔG deletions on the normally active maternal H19 and paternal Igf2 alleles. Maternal inheritance of the DMD deletions caused tissue-specific reductions in H19 expression (Fig. 4A, ΔDMD values in Table 3, and data not shown). H19 RNA was 40 to 65% lower in liver, tongue, and brain tissue and dramatically reduced (>90%) in kidney tissue. Heart and lung tissue exhibited intermediate effects from the DMD deletions. Hence, in most tissues, the level of H19 RNA from the mutant maternal alleles was similar to the level observed from the mutant paternal alleles. In tongue tissue, however, we did detect a slightly higher level of H19 RNA from the mutant maternal allele than from the mutant paternal allele (ΔDMD values in Tables 2 and 3 and data not shown).
TABLE 3.
Reduction of maternal H19 and paternal Igf2 expression from mutant alleles
| Tissue type | Deletion | Mutant maternal/wild-type maternal H19 ratioa | Mutant paternal/wild-type paternal Igf2 ratioa |
|---|---|---|---|
| Liver | ΔDMD | 0.44 ± 0.05, 0.41 ± 0.11 | 0.28 ± 0.04, 0.35 ± 0.03 |
| Δ3.8kb-5′H19 | 0.26 ± 0.05, 0.37 ± 0.03 | 0.26 ± 0.05, 0.38 ± 0.10 | |
| Tongue | ΔDMD | 0.59 ± 0.12 | 0.75 ± 0.12 |
| Δ3.8kb-5′H19 | 0.53 ± 0.06 | 0.71 ± 0.06 | |
| Kidney | ΔDMD | ≤0.10 | ∼1.0b |
| Δ3.8kb-5′H19 | ≤0.10 | ∼1.0b | |
| Heart | ΔDMD | 0.26 ± 0.04 | ∼1.0b |
| Δ3.8kb-5′H19 | 0.15 ± 0.05 | ∼1.0b | |
| Lung | ΔDMD | 0.11 ± 0.03 | ∼1.0b |
| Δ3.8kb-5′H19 | ≤0.10 | ∼1.0b | |
| Brain | ΔDMD | 0.56 ± 0.13 | ∼1.0b |
| Δ3.8kb-5′H19 | 0.53 ± 0.11 | NDc |
Paternal inheritance of the deletions also disrupted Igf2 expression in a tissue-specific manner (Fig. 3B). The Igf2 RNA was on average 70% lower in liver tissue, <30% lower in tongue tissue, and unchanged in all other tissues (Fig. 3B, ΔDMD values in Table 3, and data not shown). We detected the most significant reduction of Igf2 expression in liver tissue, the tissue in which Igf2 was least activated from the mutant maternal allele (compare Fig. 3B and 4B). Consequently, the paternal and maternal H19ΔDMD and H19ΔDMDΔG alleles express similar levels of Igf2.
The parent-specific methylation pattern of the H19ΔDMD, H19ΔDMDΔG, and H19ΔG alleles.
The paternally specific methylation that is acquired on the DMD in sperm is regarded as a mark that is critical for the regulation of H19 and Igf2 imprinting (18, 40, 52). In contrast, paternally specific methylation of the H19 promoter and 5′ end of the H19 transcription unit is acquired during embryogenesis so that in all somatic tissues paternally specific methylation extends from the DMD to the 5′ end of the H19 transcription unit (52, 53). It was previously determined that the H19ΔDMD allele acquires sperm-specific methylation and that in neonatal liver tissue both mutant parental alleles are partially methylated at the remaining DMD sequence and unmethylated at the H19 transcription unit (51).
To determine if removing an additional sequence 5′ to H19 further perturbed the methylation imprint, we compared the methylation status of the H19ΔDMD, H19ΔDMDΔG, and H19ΔG alleles in sperm and neonatal tissues. Analysis of the methylation status of the remaining DMD sequence and the 5′ end of the H19 transcription unit in sperm revealed that the wild-type methylation pattern was acquired on the mutant alleles: the DMD was hypermethylated (Fig. 5B) and the 5′ end of the H19 transcription unit was unmethylated (Fig. 5C, lanes 1 to 5) in ΔDMD, ΔDMDΔG, and ΔG deletion sperm DNA. Similarly, examination of neonatal liver DNA isolated from ΔG heterozygous and homozygous mutant mice revealed that the DMD and H19 transcription unit possessed the wild-type pattern of methylation (Fig. 5C and data not shown). In contrast, neonatal liver tissue derived from heterozygous and homozygous mutant H19ΔDMD and H19ΔDMDΔG mice exhibited hypomethylation of the H19 transcription unit and partial methylation of the remaining DMD sequence on the mutant alleles (Fig. 5C and D and data not shown). Thus, although the wild-type pattern of methylation is acquired on the mutant alleles in sperm DNA, the H19ΔDMD and H19ΔDMDΔG alleles fail to maintain the methylation pattern in somatic tissues. Additionally, no striking differences in methylation were revealed between ΔDMD and ΔDMDΔG DNA.
To determine if tissue-specific methylation differences were associated with the tissue-specific differences in H19 expression observed for the ΔDMD and ΔDMDΔG mice (Fig. 3 and 4 and Tables 2 and 3), we assayed genomic DNA methylation in neonatal liver, tongue, kidney, heart, and brain tissue. The mutant parental alleles were equally hypomethylated at the 5′ end of the H19 transcription unit (data not shown) and equivalently methylated at the remaining 5′ DMD sequence in all _H19ΔDMD_- and _H19ΔDMDΔG_-derived tissues (Fig. 5D, lanes 3 to 10 and 13 to 20, and data not shown). We conclude that differential DNA methylation patterns neither cause nor reflect the tissue-specific H19 expression patterns observed for the mutant alleles. These methylation patterns were maintained after six generations of crossing with C57BL/6J mice (data not shown).
Parent-specific methylation is detected at each of the remaining seven DMD CpG dinucleotides on the H19ΔDMD and H19ΔDMDΔG alleles.
While the DMD deletions remove most of the DMD sequence, seven DMD CpG dinucleotides remain on the ΔDMD and ΔDMDΔG alleles. A closer examination of the CpG dinucleotide within the remaining _Hha_I site 5′ to the ΔDMD and ΔDMDΔG deletions revealed slightly more methylation on the mutant paternal alleles than on the mutant maternal alleles (Fig. 5D, lanes 3 to 10 and 13 to 20). To quantify this methylation difference at all seven CpGs, we used the bisulfite mutagenesis and sequencing assay. The wild-type and mutant alleles were independently amplified from bisulfite-modified DNA (Fig. 6A and Table 1). As suggested by Southern analysis, the H19ΔDMD and H19ΔDMDΔG alleles were methylated at each of the seven CpG dinucleotides on both the maternal and paternal mutant alleles in liver tissue (Fig. 6). While the level of methylation for the mutant alleles was intermediate between the maternal and paternal wild-type alleles, the seven CpG dinucleotides were more likely to be methylated on the mutant paternal alleles than on the mutant maternal alleles (Fig. 6). Similar results were obtained when kidney DNA was assayed (data not shown). Thus, the H19ΔDMD and H19ΔDMDΔG alleles retained some parentally specific methylation in the remaining DMD sequence. In addition, no tissue-specific methylation at the 5′ DMD sequence was observed.
The analysis of the H19ΔDMD and H19ΔDMDΔG alleles revealed that the CpG dinucleotides of the loxP vector sequence were also methylated on both the paternal and maternal mutant alleles (data not shown). Because the introduced sequence could contribute to the observed methylation on the mutant alleles, we assayed the methylation status of the 3′ DMD and loxP vector sequence of the H19ΔG alleles. As with the wild-type 3′ DMD CpG dinucleotides, the H19ΔG DMD and loxP vector CpG dinucleotides were also methylated exclusively on the paternal allele in neonatal liver DNA (H19ΔG/+, +/H19ΔG) (data not shown). These results show that rather than causing the disruption in allelic methylation, the introduced loxP vector sequence acquired the methylation imprint of the adjacent sequence. It should be noted that the analysis of the wild-type allele also revealed that six CpG dinucleotides external to the previously defined 3′ end of the DMD (−2083 _Hin_dIII site in Fig. 1) (52) were also methylated exclusively on the paternal allele (data not shown). Hence, the deleted DMD sequence as opposed to the loxP vector sequence likely accounts for the loss of differential methylation at the H19 locus.
The 3.8-kb deletion shows no additional effect on imprinting when compared to the 1.6-kb DMD deletion.
Both the ΔDMD and ΔDMDΔG deletions retained some differential methylation at the 5′ portion of the DMD. Therefore, we tested whether a larger deletion that eliminates the entire 2-kb DMD and all four 21-bp repeats (CTCF sites), would lead to a greater impact on H19 and Igf2 imprinting (Fig. 1). We did not detect significant differences in H19 and Igf2 expression from the paternal allele by deleting the entire 2 kb of DMD (H19Δ3.8kb-5′H19) compared to deleting 1.6 kb of the DMD sequence (H19ΔDMD) (Fig. 3 and Tables 2 and 3). In addition, the single remaining CTCF site did not appear to confer residual insulator activity since its deletion on the maternal H19Δ3.8kb-5′H19 allele did not result in a significant increase in Igf2 expression relative to the H19ΔDMD and H19ΔDMDΔG alleles in most tissues, with the exception of lung tissue (Fig. 4 and Tables 2 and 3). Likewise, H19 expression from the H19ΔDMD and H19Δ3.8kb-5′H19 maternal alleles was similar in most tissues. Moreover, the 5′ end of the H19 transcription unit was equally hypomethylated on both of the parental H19Δ3.8kb-5′H19 alleles in somatic tissues (data not shown). Consequently, the deleted 1.6-kb DMD sequence likely accounts for the loss of H19 and Igf2 imprinting that is detected in this and other targeting deletion studies that remove the entire DMD sequence (32, 47, 51).
DISCUSSION
A previous study of the 1.6-kb DMD deletion (ΔDMD) demonstrated that the DMD was required for both H19 and Igf2 imprinting in neonatal liver tissue and that the DMD functions to silence H19 on the paternal allele and Igf2 on the maternal allele (51). In addition, the DMD was shown to be required for complete activation of the maternal H19 and paternal Igf2 alleles and for paternally specific methylation at the H19 locus. Here, we further analyze the ΔDMD deletion and three additional deletions of the DMD region in multiple neonatal tissues. We show that the ΔDMD perturbs imprinting in all tissues and that relative expression levels from both parental alleles in heterozygous mutant mice vary in a tissue-specific manner. In addition, this expression is not further affected by removing more sequence 3′ to the DMD or an additional upstream sequence, including the remaining 5′ DMD sequence. We conclude that the original 1.6-kb ΔDMD deletion is sufficient to cause deregulation of H19 and Igf2 imprinted expression in all tissues.
Similar to what we report above, a 9.2-kb targeted deletion at the H19 locus spanning the sequence 5′ to the DMD, the DMD, and the G-rich repetitive element also resulted in perturbation of H19 and Igf2 imprinting in all tissues assayed (32). Interestingly, maternal transmission of a conditional allele that removed a 6.2-kb segment from −7.0 to −0.8 kb 5′ of H19 in terminally differentiated muscle activated the silent Igf2 allele while H19 remained unaffected after paternal transmission of this deletion (47). Deregulated expression resulting from these larger deletions was suggested to be the actual consequence of removing the 2-kb DMD (32, 47). The question that arises from these and similar studies is whether the roles attributed to the DMD in H19 and Igf2 imprinting can be associated with the same or different regions of the DMD.
The first indication that the DMD functions as an insulator element in regulating Igf2 imprinting was demonstrated by movement of the endodermal enhancers between the endogenous Igf2 gene and the DMD (55), resulting in Igf2 expression from the normally silent maternal allele. Through the use of in vitro enhancer-blocking assays, the DMD has since been shown to function as an insulator that is dependent upon the conserved CTCF-binding repeat sequences (9, 27, 32, 33). As CTCF binding and in vitro DMD enhancer-blocking activity are methylation sensitive (9, 27, 29) and the DMD repeat sequences footprint specifically on the maternal allele in vivo (50), CTCF is an excellent candidate for regulating H19 and Igf2 imprinting. Although DMD repeat element R1 (Fig. 1) confers insulator activity in an in vitro enhancer-blocking assay (9) and the 5′ R1-spanning DMD sequence acquires more methylation on the paternal than on the maternal ΔDMD allele, the R1 element alone does not confer significant insulator activity at the endogenous locus, as is indicated by the nonimprinted expression of Igf2 in ΔDMD mice. Thus in vitro assessment of insulator activity of individual DMD repeat elements may not reflect the sequence requirements for insulator function at the endogenous H19 and Igf2 locus, which is governed by complex long-range enhancer-mediated interactions. In a mouse transgenic assay, the sequence spanning three of the four DMD repeats (R2 to R4) (Fig. 1) mediated enhancer-blocking activity; however, the sequence which spans R2 alone did not exhibit insulator activity (27). Furthermore, a 1.2-kb targeted deletion of the DMD sequence spanning repeats R3 and R4 did not appear to perturb Igf2 imprinting (19), indicating that repeats R1 and R2 together may confer insulator activity at the endogenous locus. Thus, it appears that at least two CTCF-binding repeats are required for insulator activity at the H19 and Igf2 imprinting domain. It remains to be determined if any two CTCF-binding repeats will mediate insulator activity at the endogenous H19 and Igf2 locus and whether a sequence other than the repeats is required for this activity. In support of sequence requirements other than the CTCF-binding repeats, in vitro assays indicated that an additional 5′ flanking sequence enhanced the insulator activity of the DMD (9, 32).
At other loci, the number of CTCF sites varies. Only one CTCF site is present at the chicken β-globin insulator element (11). In contrast, two methylation-sensitive CTCF-binding sites flank the CTG repeats of the human DM1 locus that is associated with myotonic dystrophy (23). These sites, in combination with CTG repeats, are proposed to function as an insulator. In the mouse, a single CTCF-binding site has been reported within an intron of the Gtl2 locus, a maternally expressed nontranslated gene that is linked to the oppositely imprinted Dlk1 gene (22, 46). In the human, two CTCF-binding sites have been found within the GTL2 promoter (56). Thus, it will be important to determine if these CTCF sites can function as an insulator or if these sites have one of the other regulatory functions (such as transcriptional activation or repression) that have been ascribed to CTCF (39).
The DMD sequence requirements for H19 silencing versus Igf2 insulation may differ. Paternal transmission of a 1.2-kb deletion that removed DMD repeats R3 and R4 caused activation of the paternal H19 allele, even though the remaining DMD sequence acquired paternally specific methylation (19). In contrast, Igf2 was not activated after maternal transmission of the deletion. From these studies, the authors concluded that the DMD sequence requirements for H19 silencing differ from those for Igf2 insulator activity. Our data indicate that the DMD sequence spanning repeats R2, R3, and R4 is required for both H19 silencing and Igf2 insulation. The amount of DMD sequence deleted, rather than the specific sequence itself, could be responsible for the distinct imprinting defects observed for the different deletions. Additional gene targeting experiments are required to resolve this issue.
While the DMD provides allele-specific silencing and insulator activities, it also appears to play a role in modulating tissue-specific expression of H19 and Igf2. Paternal transmission of the 1.2-kb 5′H19 deletion was reported to perturb H19 silencing in a subset of tissues (19). The 9.2-kb 5′ H19 deletion exhibited tissue-specific perturbations in H19 and Igf2 imprinting (32). Our data show that the DMD deletions cause tissue-specific changes in H19 and Igf2 activation from the normally imprinted allele. We also detect reduced levels of H19 and Igf2 expression from the normally expressed alleles relative to their wild-type counterparts, and the levels of expression from the mutant maternal and paternal alleles are nearly equivalent in all tissues assayed. Although the DMD is required for imprinting in all tissues, it is unclear how the tissue-specific effects are conferred. One possibility is that the DMD interacts differently with tissue-specific enhancers within this locus (1, 30, 32, 37). Additionally, the DMD may exhibit unique interactions with the mesoderm-specific silencer and repressor elements that regulate Igf2 (2, 15), one of which coincides with the differentially methylated region (DMR) 5′ of Igf2 (DMR1). DMR1 and another DMR within the Igf2 gene (DMR2) are exclusively methylated on the paternal allele; however, upon maternal inheritance of a 13-kb deletion that removes the DMD, maternal Igf2 is expressed and the DMRs become methylated (24). A complete understanding of tissue-specific imprinted regulation will require the elucidation of the mechanism by which insulators and enhancers operate in vertebrates as well as the identification of additional tissue-specific regulatory elements in the H19 and Igf2 imprinting domain.
Our ΔDMD targeted deletion clearly demonstrates the necessity, but not the sufficiency, of the DMD sequence for regulating H19 and Igf2 imprinting. Targeted deletions have also characterized tissue-specific transcriptional regulatory elements required for H19 and Igf2 imprinting and expression (15, 37). The single-copy yeast and bacterial artificial chromosome transgenic models together suggest that the sequence from immediately upstream of Igf2 to as far as 130 kb downstream of H19 is required to recapitulate H19 and Igf2 imprinted expression in all tissues (1-3, 32). H19 minitransgenes that consist of 5′ H19 sequence, the H19 transcription unit or a reporter gene, and 3′ H19 sequence that spans the endodermal enhancers have been used to elucidate the sequence required for H19 imprinting in liver tissue (12, 16, 21, 41, 48). To date, the shortest low-copy-number transgene that confers H19 imprinted methylation and expression in liver tissue includes 5.5 kb of 5′ H19 sequence, the H19 transcription unit, and 8 kb of 3′ H19 (16). Interestingly, the methylation of a low-copy-number enhancerless transgene containing 5.5 kb of 5′ H19 sequence and the H19 transcription unit is imprinted, demonstrating that DMD can acquire imprinted methylation in the absence of enhancer-mediated transcription (16). It remains to be proven whether the entire 2-kb DMD alone is sufficient to imprint exogenous targets.
Repetitive elements are often associated with imprinted genes. Recently, a repetitive element within the _U2afbp_-rs1 locus has been reported to be dispensable for imprinted methylation of this gene (49). In contrast, a repetitive element at the Rasgrf1 locus is essential for its imprinting (P. D. Soloway, personal communication). Here we show that deleting a 1.3-kb segment between the DMD and H19 promoter that contains the G-rich repeat does not affect H19 and Igf2 imprinting in any neonatal tissue tested. Hence, we corroborate the findings that the G-rich repeat element was not required for imprinting of an H19 transgene in neonatal liver tissue (48) and that removal of the endogenous G-rich repeat element in combination with the H19 transcription unit did not perturb Igf2 imprinting (42). Curiously, our ΔG deletion also removed a sequence that acquires parent-specific DNA methylation (52, 53) and histone H4 acetylation (26), other hallmarks that are commonly associated with imprinted genes. Thus, since the ΔG deletion does not affect imprinting, these parent-specific characteristics of the sequence between the DMD and H19 promoter are most likely a consequence of the spreading of epigenetic silencing signals from the DMD to the H19 gene during postimplantion development (44, 52, 53).
In conclusion, further mutational analysis of the endogenous DMD region is required to unravel the role of specific sequences in activation versus the silencing of parental alleles and for tissue-specific enhancer-mediated interactions that result in imprinted gene expression. For example, it will be interesting to determine whether eliminating or altering CTCF-binding repeats will disrupt H19 as well as Igf2 imprinting in vivo. Furthermore, it will also be crucial to determine whether the DMD harbors other parent-specific differences, such as histone modifications associated strictly with active or inactive chromatin (31), as they may play an integral part in establishing epigenetic signals at the DMD locus. Together, these studies at the H19 and Igf2 imprinting domain will elucidate the complex mechanisms of long-range tissue-specific enhancer-mediated interactions, insulation, and silencing, all of which regulate imprinted gene expression.
Acknowledgments
We thank J. Richa and the University of Pennsylvania Transgenic Core Facility for the production of chimeric mice. We thank Nora Engel and Raluca Verona for comments on the manuscript.
This work was supported by U.S. Public Health Service grant GM51279 and the Howard Hughes Medical Institute. J.L.T. was supported by National Research Service Award postdoctoral fellowship GM18458. M.R.W.M. was supported by the Lalor Foundation.
REFERENCES
- 1.Ainscough, J. F., L. Dandolo, and M. A. Surani. 2000. Appropriate expression of the mouse H19 gene utilises three or more distinct enhancer regions spread over more than 130 kb. Mech. Dev. 91**:**365-368. [DOI] [PubMed] [Google Scholar]
- 2.Ainscough, J. F., R. M. John, S. C. Barton, and M. A. Surani. 2000. A skeletal muscle-specific mouse Igf2 repressor lies 40 kb downstream of the gene. Development 127**:**3923-3930. [DOI] [PubMed] [Google Scholar]
- 3.Ainscough, J. F.-X., T. Koide, M. Tada, S. Barton, and M. A. Surani. 1997. Imprinting of Igf2 and H19 from a 130 kb YAC transgene. Development 124**:**3621-3632. [DOI] [PubMed] [Google Scholar]
- 4.Auffray, C., and F. Rougeon. 1980. Purification of mouse immunoglobulin heavy-chain messenger RNAs from total myeloma tumor RNA. Eur. J. Biochem. 107**:**303-314. [DOI] [PubMed] [Google Scholar]
- 5.Barlow, D. P. 1997. Competition—a common motif for the imprinting mechanism? EMBO J. 16**:**6899-6905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bartolomei, M. S., and S. M. Tilghman. 1997. Genomic imprinting in mammals. Annu. Rev. Genet. 31**:**493-525. [DOI] [PubMed] [Google Scholar]
- 7.Bartolomei, M. S., A. L. Webber, M. E. Brunkow, and S. M. Tilghman. 1993. Epigenetic mechanisms underlying the imprinting of the mouse H19 gene. Genes Dev. 7**:**1663-1673. [DOI] [PubMed] [Google Scholar]
- 8.Bartolomei, M. S., S. Zemel, and S. M. Tilghman. 1991. Parental imprinting of the mouse H19 gene. Nature 351**:**153-155. [DOI] [PubMed] [Google Scholar]
- 9.Bell, A. C., and G. Felsenfeld. 2000. Methylation of a CTCF-dependent boundary controls imprinted expression of the Igf2 gene. Nature 405**:**482-485. [DOI] [PubMed] [Google Scholar]
- 10.Bell, A. C., A. G. West, and G. Felsenfeld. 2001. Insulators and boundaries: versatile regulatory elements in the eukaryotic genome. Science 291**:**447-450. [DOI] [PubMed] [Google Scholar]
- 11.Bell, A. C., A. G. West, and G. Felsenfeld. 1999. The protein CTCF is required for the enhancer blocking activity of vertebrate insulators. Cell 98**:**387-396. [DOI] [PubMed] [Google Scholar]
- 12.Brenton, J. D., R. A. Drewell, S. Viville, K. J. Hilton, S. C. Barton, J. F.-X. Ainscough, and M. A. Surani. 1999. A silencer element identified in Drosophila is required for imprinting of H19 reporter transgenes in mice. Proc. Natl. Acad. Sci. USA 96**:**9242-9247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brunkow, M. E., and S. M. Tilghman. 1991. Ectopic expression of the H19 gene in mice causes prenatal lethality. Genes Dev. 5**:**1092-1101. [DOI] [PubMed] [Google Scholar]
- 14.Cho, H., J. Mu, J. K. Kim, J. L. Thorvaldsen, Q. Chu, E. B. Crenshaw III, K. H. Kaestner, M. S. Bartolomei, G. I. Shulman, and M. J. Birnbaum. 2001. Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKB beta). Science 292**:**1728-1731. [DOI] [PubMed] [Google Scholar]
- 15.Constancia, M., W. Dean, S. Lopes, T. Moore, G. Kelsey, and W. Reik. 2000. Deletion of a silencer element in Igf2 results in loss of imprinting independent of H19. Nat. Genet. 26**:**203-206. [DOI] [PubMed] [Google Scholar]
- 16.Cranston, M. J., T. L. Spinka, D. A. Elson, and M. S. Bartolomei. 2001. Elucidation of the minimal sequence required to imprint H19 transgenes. Genomics 73**:**98-107. [DOI] [PubMed] [Google Scholar]
- 17.Davis, T. L., K. T. Tremblay, and M. S. Bartolomei. 1998. Imprinted expression and methylation of the mouse H19 gene are conserved in extraembryonic lineages. Dev. Genet. 23**:**111-118. [DOI] [PubMed] [Google Scholar]
- 18.Davis, T. L., G. J. Yang, J. R. McCarrey, and M. S. Bartolomei. 2000. The H19 methylation imprint is erased and reestablished differentially on the parental alleles during male germ cell development. Hum. Mol. Genet. 9**:**2885-2894. [DOI] [PubMed] [Google Scholar]
- 19.Drewell, R. A., J. D. Brenton, J. F. Ainscough, S. C. Barton, K. J. Hilton, K. L. Arney, L. Dandolo, and M. A. Surani. 2000. Deletion of a silencer element disrupts H19 imprinting independently of a DNA methylation epigenetic switch. Development 127**:**3419-3428. [DOI] [PubMed] [Google Scholar]
- 20.Dudov, K. P., and R. P. Perry. 1984. The gene encoding the mouse ribosomal protein L32 contains a uniquely expressed intron-containing gene and an unmutated processed gene. Cell 37**:**457-468. [DOI] [PubMed] [Google Scholar]
- 21.Elson, D. A., and M. S. Bartolomei. 1997. A 5′ differentially methylated sequence and the 3′ flanking region are necessary for H19 transgene imprinting. Mol. Cell. Biol. 17**:**309-317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferguson-Smith, A. C., and M. A. Surani. 2001. Imprinting and the epigenetic asymmetry between parental genomes. Science 293**:**1086-1089. [DOI] [PubMed] [Google Scholar]
- 23.Filippova, G. N., C. P. Thienes, B. H. Penn, D. H. Cho, Y. J. Hu, J. M. Moore, T. R. Klesert, V. V. Lobanenkov, and S. J. Tapscott. 2001. CTCF-binding sites flank CTG/CAG repeats and form a methylation-sensitive insulator at the DM1 locus. Nat. Genet. 28**:**335-343. [DOI] [PubMed] [Google Scholar]
- 24.Forne, T., J. Oswald, W. Dean, J. R. Saam, B. Bailleul, L. Dandolo, S. M. Tilghman, J. Walter, and W. Reik. 1997. Loss of the maternal H19 gene induces changes in Igf2 methylation in both cis and trans. Proc. Natl. Acad. Sci. USA 94**:**10243-10248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frevel, M. A. E., J. J. Hornberg, and A. E. Reeve. 1999. A potential imprint control element: identification of a conserved 42 bp sequence upstream of H19. Trends Genet. 15**:**216-218. [DOI] [PubMed] [Google Scholar]
- 26.Grandjean, V., L. O'Neill, T. Sado, B. Turner, and A. Ferguson-Smith. 2001. Relationship between DNA methylation, histone H4 acetylation and gene expression in the mouse imprinted _Igf2_-H19 domain. FEBS Lett. 488**:**165-169. [DOI] [PubMed] [Google Scholar]
- 27.Hark, A. T., C. J. Schoenherr, D. J. Katz, R. S. Ingram, J. M. Levorse, and S. M. Tilghman. 2000. CTCF mediates methylation-sensitive enhancer-blocking activity at the H19/Igf2 locus. Nature 405**:**486-489. [DOI] [PubMed] [Google Scholar]
- 28.Hark, A. T., and S. M. Tilghman. 1998. Chromatin conformation of the H19 epigenetic mark. Hum. Mol. Genet. 7**:**1979-1985. [DOI] [PubMed] [Google Scholar]
- 29.Holmgren, C., C. Kanduri, G. Dell, A. Ward, R. Mukhopadhya, M. Kanduri, V. Lobanenkov, and R. Ohlsson. 2001. CpG methylation regulates the Igf2/H19 insulator. Curr. Biol. 11**:**1128-1130. [DOI] [PubMed] [Google Scholar]
- 30.Ishihara, K., N. Hatano, H. Furuumi, R. Kato, T. Iwaki, K. Miura, Y. Jinno, and H. Sasaki. 2000. Comparative genomic sequencing identifies novel tissue-specific enhancers and sequence elements for methylation-sensitive factors implicated in Igf2/H19 imprinting. Genome Res. 10**:**664-671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jenuwein, T., and C. D. Allis. 2001. Translating the histone code. Science 293**:**1074-1080. [DOI] [PubMed] [Google Scholar]
- 32.Kaffer, C. R., M. Srivastava, K.-Y. Park, E. Ives, S. Hsieh, J. Batlle, A. Grinberg, S.-P. Huang, and K. Pfeifer. 2000. A transcriptional insulator at the imprinted H19/Igf2 locus. Genes Dev. 14**:**1908-1919. [PMC free article] [PubMed] [Google Scholar]
- 33.Kanduri, C., V. Pant, D. Loukinov, E. Pugacheva, C. F. Qi, A. Wolffe, R. Ohlsson, and V. V. Lobanenkov. 2000. Functional association of CTCF with the insulator upstream of the H19 gene is parent of origin-specific and methylation-sensitive. Curr. Biol. 10**:**853-856. [DOI] [PubMed] [Google Scholar]
- 34.Khosla, S., A. Aitchison, R. Gregory, N. D. Allen, and R. Feil. 1999. Parental allele-specific chromatin configuration in a boundary/imprinting-control element upstream of the mouse H19 gene. Mol. Cell. Biol. 19**:**2556-2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuhn, R., K. Rajewsky, and W. Muller. 1991. Generation and analysis of interleukin-4 deficient mice. Science 254**:**707-710. [DOI] [PubMed] [Google Scholar]
- 36.Leighton, P. A., R. S. Ingram, J. Eggenschwiler, A. Efstratiadis, and S. M. Tilghman. 1995. Disruption of imprinting caused by deletion of the H19 gene region in mice. Nature 375**:**34-39. [DOI] [PubMed] [Google Scholar]
- 37.Leighton, P. A., J. R. Saam, R. S. Ingram, C. L. Stewart, and S. M. Tilghman. 1995. An enhancer deletion affects both H19 and Igf2 expression. Genes Dev. 9**:**2079-2089. [DOI] [PubMed] [Google Scholar]
- 38.McCarrick, J. W. I., J. R. Parnes, R. H. Seong, D. Solter, and B. B. Knowles. 1993. Positive-negative selection gene targeting with the diphtheria toxin A-chain gene in mouse embryonic stem cells. Transgenic Res. 2**:**183-190. [DOI] [PubMed] [Google Scholar]
- 39.Ohlsson, R., R. Renkawitz, and V. Lobanenkov. 2001. CTCF is a uniquely versatile transcription regulator linked to epigenetics and disease. Trends Genet. 17**:**520-527. [DOI] [PubMed] [Google Scholar]
- 40.Olek, A., and J. Walter. 1997. The pre-implantation ontogeny of the H19 methylation imprint. Nat. Genet. 17**:**275-276. [DOI] [PubMed] [Google Scholar]
- 41.Pfeifer, K., P. Leighton, and S. M. Tilghman. 1996. The structural gene of H19 is required for transgene imprinting. Proc. Natl. Acad. Sci. USA 93**:**13876-13883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reed, M. R., C. F. Huang, A. D. Riggs, and J. R. Mann. 2001. A complex duplication created by gene targeting at the imprinted H19 locus results in two classes of methylation and correlated Igf2 expression phenotypes. Genomics 74**:**186-196. [DOI] [PubMed] [Google Scholar]
- 43.Reik, W., and J. Walter. 2001. Genomic imprinting: parental influence on the genome. Nat. Rev. Genet. 2**:**21-32. [DOI] [PubMed] [Google Scholar]
- 44.Sasaki, H., A. C. Ferguson-Smith, A. S. W. Shum, S. C. Barton, and M. A. Surani. 1995. Temporal and spatial regulation of H19 imprinting in normal and uniparental mouse embryos. Development 121**:**4195-4202. [DOI] [PubMed] [Google Scholar]
- 45.Sauer, B., and N. Henderson. 1990. Targeted insertion of exogenous DNA into the eukaryotic genome by the Cre recombinase. New Biol. 2**:**441-449. [PubMed] [Google Scholar]
- 46.Schmidt, J. V., P. G. Matteson, B. K. Jones, X. J. Guan, and S. M. Tilghman. 2000. The Dlk1 and Gtl2 genes are linked and reciprocally imprinted. Genes Dev. 14**:**1997-2002. [PMC free article] [PubMed] [Google Scholar]
- 47.Srivastava, M., S. Hsieh, A. Grinberg, L. Williams-Simons, S.-P. Huang, and K. Pfeifer. 2000. H19 and Igf2 monoallelic expression is regulated in two distinct ways by a shared cis acting regulatory region upstream of H19. Genes Dev. 14**:**1186-1195. [PMC free article] [PubMed] [Google Scholar]
- 48.Stadnick, M. P., F. M. Pieracci, M. J. Cranston, E. Taksel, J. L. Thorvaldsen, and M. S. Bartolomei. 1999. Role of a 461 bp G-rich repetitive element in H19 transgene imprinting. Dev. Genes Evol. 209**:**239-248. [DOI] [PubMed] [Google Scholar]
- 49.Sunahara, S., K. Nakamura, K. Nakao, Y. Gondo, Y. Nagata, and M. Katsuki. 2000. The oocyte-specific methylated region of the U2afbp-rs/U2af1-rs1 gene is dispensable for its imprinted methylation. Biochem. Biophys. Res. Commun. 268**:**590-595. [DOI] [PubMed] [Google Scholar]
- 50.Szabo, P. E., S.-H. Tang, A. Rentsendorj, G. P. Pfeifer, and J. R. Mann. 2000. Maternal-specific footprints at putative CTCF sites in the H19 imprinting control region give evidence for insulator function. Curr. Biol. 10**:**607-610. [DOI] [PubMed] [Google Scholar]
- 51.Thorvaldsen, J. L., K. L. Duran, and M. S. Bartolomei. 1998. Deletion of the H19 differentially methylated domain results in loss of imprinted expression of H19 and Igf2. Genes Dev. 12**:**3693-3702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tremblay, K. D., K. L. Duran, and M. S. Bartolomei. 1997. A 5′ 2-kilobase-pair region of the imprinted mouse H19 gene exhibits exclusive paternal methylation throughout development. Mol. Cell. Biol. 17**:**4322-4329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tremblay, K. D., J. R. Saam, R. S. Ingram, S. M. Tilghman, and M. S. Bartolomei. 1995. A paternal-specific methylation imprint marks the alleles of the mouse H19 gene. Nat. Genet. 9**:**407-413. [DOI] [PubMed] [Google Scholar]
- 54.Warnecke, P. M., J. M. Mann, M. Frommer, and S. J. Clark. 1998. Bisulfite sequencing in preimplantation embryos: DNA methylation profile of the upstream region of the mouse imprinted H19 gene. Genomics 51**:**182-190. [DOI] [PubMed] [Google Scholar]
- 55.Webber, A. L., R. S. Ingram, J. M. Levorse, and S. M. Tilghman. 1998. Location of enhancers is essential for the imprinting of H19 and Igf2 genes. Nature 391**:**711-715. [DOI] [PubMed] [Google Scholar]
- 56.Wylie, A. A., S. K. Murphy, T. C. Orton, and R. L. Jirtle. 2000. Novel imprinted DLK1/GTL2 domain on human chromosome 14 contains motifs that mimic those implicated in IGF2/H19 regulation. Genome Res. 10**:**1711-1718. [DOI] [PMC free article] [PubMed] [Google Scholar]