Loss of FLOWERING LOCUS C Activity Eliminates the Late-Flowering Phenotype of FRIGIDA and Autonomous Pathway Mutations but Not Responsiveness to Vernalization (original) (raw)
Abstract
The MADS domain–containing transcription factor FLOWERING LOCUS C (FLC) acts as an inhibitor of flowering and is a convergence point for several pathways that regulate flowering time in Arabidopsis. In naturally occurring late-flowering ecotypes, the FRIGIDA (FRI) gene acts to increase FLC levels, whereas the autonomous floral promotion pathway and vernalization act to reduce FLC expression. Previous work has shown that the Landsberg erecta allele of FLC, which is not a null allele, is able to partially suppress the late-flowering phenotype of FRIGIDA and mutations in the autonomous pathway. In this study, using a null allele of FLC, we show that the late-flowering phenotype of FRIGIDA and autonomous pathway mutants are eliminated in the absence of FLC activity. In addition, we have found that the downregulation of SUPPRESSOR OF OVEREXPRESSION OF CONSTANS1 by FRI and autonomous pathway mutants also is mediated by FLC. Complete loss of FLC function, however, does not eliminate the effect of vernalization. Thus, FRI and the autonomous pathway may act solely to regulate FLC expression, whereas vernalization is able to promote flowering via _FLC_-dependent and _FLC_-independent mechanisms.
INTRODUCTION
The promotion of flowering in response to prolonged exposure to cold temperatures is an adaptation to prevent plants from flowering in the fall, prior to winter, and to enable them to flower in spring. This promotion, known as vernalization, does not initiate flowering directly but renders the meristem competent to respond to other environmental and developmental flowering signals (reviewed in Chouard, 1960; Michaels and Amasino, 2000). Although the physiology of vernalization has been studied extensively in many species, the molecular mechanism of vernalization remains largely unknown. In Arabidopsis, many naturally occurring ecotypes are relatively late flowering unless they are vernalized (Napp-Zinn, 1979; Karlsson et al., 1993), and thus they behave as winter annuals. Summer annual strains, in contrast, do not require vernalization for early flowering. Crosses between winter and summer annual strains have shown that the winter annual habit is caused by the interaction of two dominant genes: FLOWERING LOCUS C (FLC) (Koornneef et al., 1994; Lee et al., 1994) and FRIGIDA (FRI) (Burn et al., 1993; Lee et al., 1993; Clarke and Dean, 1994). FLC encodes a MADS domain–containing transcription factor that acts as an inhibitor of flowering, and FRI is required for FLC expression (Michaels and Amasino, 1999; Sheldon et al., 1999). Most summer annual ecotypes of Arabidopsis contain nonfunctional alleles of FRI (Johanson et al., 2000) and thus have low levels of FLC expression (Michaels and Amasino, 1999; Sheldon et al., 1999). Vernalization is able to eliminate the late-flowering phenotype caused by FLC and FRI (Lee and Amasino, 1995) and results in a downregulation of FLC levels (Michaels and Amasino, 1999; Sheldon et al., 1999). This suppression of FLC expression by vernalization is stable for the remainder of the plant life cycle, but FLC expression returns to high levels in the next generation. Transgenic plants containing FLC under the control of the constitutive 35S cauliflower mosaic virus promoter remain late flowering after cold treatment, indicating that FLC levels must be reduced for vernalization to be effective (Michaels and Amasino, 1999; Sheldon et al., 1999). Thus, the epigenetic downregulation of FLC appears to be a major target of the vernalization pathway, and this downregulation is a component of the acquisition of the competence to flower by the meristem.
In addition to studying naturally occurring variation, mutational analysis has been used to identify genes that control flowering in Arabidopsis. From mutagenized summer annual backgrounds, mutants have been identified in which the transition to flowering is delayed (Koornneef et al., 1991). These mutations identify genes that act as promoters of flowering, because a loss of function results in later flowering. On the basis of genetic and physiological characterization, these mutants are thought to operate in two pathways (reviewed in Koornneef et al., 1998; Simpson et al., 1999). Genes such as CONSTANS (CO) and GIGANTEA (GI) appear to be involved in promoting flowering in response to photoperiod. Arabidopsis is a facultative long-day (LD) plant, and the flowering of plants containing mutations in either of these genes is not promoted by inductive photoperiods. Thus, these mutants are day neutral or “blind” to photoperiod. Plants containing mutations in FCA, FPA, FVE, LUMINIDEPENDENS (LD), and FLOWERING LOCUS D are delayed in flowering but retain a photoperiod response and therefore are thought to promote flowering in a photoperiod-independent pathway often referred to as the autonomous pathway. Unlike photoperiod pathway mutants, the late-flowering phenotype of autonomous pathway mutants is strongly suppressed by vernalization. Thus, the late-flowering, vernalization-responsive phenotype of autonomous pathway mutants is similar to the winter annual habit of _FRI_-containing late-flowering ecotypes. Furthermore, like FRI, mutations in the autonomous pathway result in increased FLC expression that is eliminated by vernalization (Michaels and Amasino, 1999; Sheldon et al., 1999). These results indicate that the autonomous pathway promotes flowering, at least in part, by repressing FLC expression.
FLC was first identified genetically because the late-flowering phenotype of FRI and the autonomous pathway mutant ld is partially suppressed in a recessive manner by the Landsberg erecta (L_er_) FLC locus (Koornneef et al., 1994; Lee et al., 1994). Subsequent experiments have shown that other autonomous pathway mutants, but not photoperiod pathway mutants, also are partially suppressed by the L_er FLC_ locus (Sanda and Amasino, 1996b). Since the cloning of FLC, the L_er_ allele of FLC has been examined, but the recessive nature of this allele is unclear; there are no missense or nonsense mutations, and FLC mRNA levels and regulation are similar to those seen in other ecotypes (Sheldon et al., 2000; our unpublished results). Because the L_er_ allele of FLC is not a clear loss-of-function allele, we have reexamined the FLC dependence of the late-flowering phenotype of FRI and late-flowering autonomous and photoperiod pathway mutants by using a null allele of FLC. We also have used the null allele of FLC to determine if vernalization acts exclusively by downregulating FLC expression or if it can also promote flowering independent of FLC.
RESULTS
The Null Allele of FLC Is Able to Completely Suppress the Late-Flowering Phenotype of FRI
Previous studies have shown that the extreme late-flowering phenotype of lines containing dominant alleles of FRI and FLC is suppressed by the L_er FLC_ locus (Koornneef et al., 1994; Lee et al., 1994; Michaels and Amasino, 1999). However, the suppression mediated by the L_er FLC_ locus is incomplete; in the L_er_ background, FRI approximately doubles the number of vegetative nodes formed at the shoot apical meristem before flowering occurs (Koornneef et al., 1994; Lee et al., 1994). One model that accounts for this incomplete suppression is that the L_er_ allele of FLC has some low level of activity and the late-flowering phenotype of FRI in the L_er_ background is the result of increased FLC activity. Another model is that L_er_ contains an inactive allele of FLC and FRI acts to regulate flowering through both _FLC_-dependent and _FLC_-independent mechanisms.
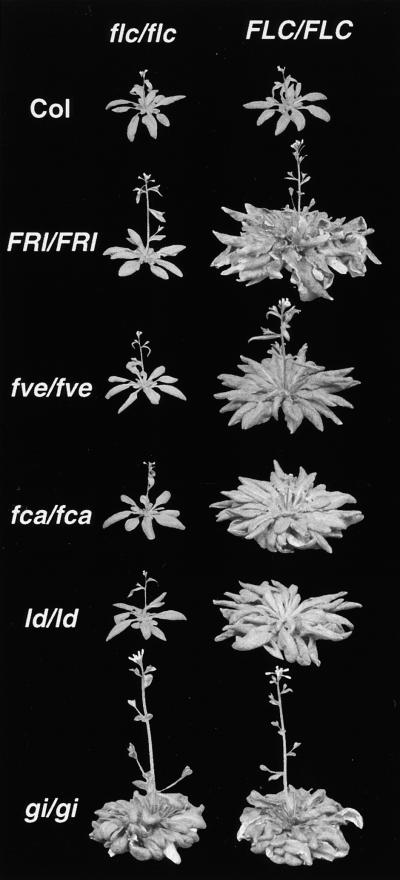
To distinguish between these models, we analyzed the flowering behavior of isogenic lines containing dominant or recessive FRI alleles in an flc mutant background. If FRI acts solely through FLC, then the FRI genotype should have no effect in the absence of FLC activity, whereas if FRI can delay flowering independent of FLC, FRI_-containing lines should remain late flowering in the absence of FLC activity. The results are shown in Figures 1 to 3. In the wild-type Columbia (Col) background, FRI strongly delays flowering, as has been shown previously (Lee et al., 1994). In an flc-3 mutant background, however, the effect of FRI is eliminated under both LD (Figures 1 and 2) and short-day (SD) conditions (Figure 3). Thus, the late-flowering phenotype of FRI is likely to result entirely from an upregulation of FLC activity, and the L_er allele of FLC is unlikely to be a null allele.
Figure 1.
Dependence of the Late-Flowering Phenotype of FRI and Certain Late-Flowering Mutants on FLC.
Plants at left are homozygous for an flc null allele. Plants at right contain wild-type FLC alleles and are shown as controls. Plants were grown under LD conditions.
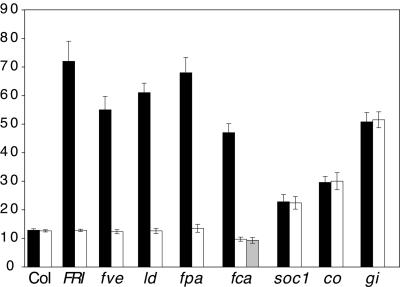
Figure 3.
Effect of FLC on Flowering Time under SD Conditions.
Black bars represent plants homozygous for wild-type FLC, and white bars represent plants homozygous for an flc null allele. All analyses were performed in the Col background except for those involving fca. For fca, F3 lines were grown from each of the F2 plants described in Figure 2, and their leaf numbers were averaged: white bar, fca/fca flc/flc; gray bar, FCA/FCA flc/flc. The white and gray bars show the effect of the FCA in the flc null background. Lines containing FRI, fve, ld, and fpa with wild-type FLC alleles did not flower after forming >80 leaves (data not shown). Error bars indicate ±sd. The y axis indicates the number of rosette leaves formed prior to flowering.
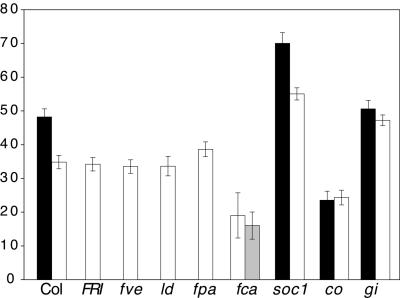
Figure 2.
Effect of FLC on Flowering Time under LD Conditions.
Black bars represent plants homozygous for wild-type FLC, and white bars represent plants homozygous for an flc null allele. All analyses were performed in the Col background except for those involving fca. For fca, an F2 population was generated from a cross between flc-3 in Col and an fca allele in Wassilewskija. Because of the different backgrounds, a minimum of 10 plants of each of the following genotypes was isolated in the F2 generation, and their leaf numbers were averaged: black bar, fca/fca FLC/FLC; white bar, fca/fca flc/flc; gray bar, FCA/FCA flc/flc. The white and gray bars show the effect of the FCA in the flc null background. Error bars indicate ±sd. The y axis indicates the number of rosette leaves formed prior to flowering.
FLC Affects Flowering in SD Conditions
In summer annual strains of Arabidopsis lacking FRI activity, FLC expression is not detected by RNA gel blot analysis from plants grown under LD (Michaels and Amasino, 1999; Sheldon et al., 1999) or SD (our unpublished results) conditions. This suggests that the expression, and subsequent effect on flowering time, of FLC may be dependent on FRI. To determine if a loss of FLC function affects flowering time in the absence of FRI, Col and flc-3 were grown under LD and SD. Under LD, the flowering time of flc-3 is indistinguishable from that of wild-type Col (Figures 1 and 2). Under SD, however, flc-3 flowers ∼12 leaves earlier than does the wild type (Figure 3). Thus, FLC affects flowering time in summer annual strains lacking FRI activity in SD conditions. Although the FLC transcript is not detected in the absence of FRI, the early-flowering phenotype of flc-3 in SD indicates that FLC must be expressed at some level and that this low level of expression can affect flowering in the absence of inductive photoperiods.
Autonomous Pathway Mutations Are Dependent on FLC for Their Late-Flowering Phenotype
The late-flowering phenotype of many autonomous pathway mutants is reduced by the L_er FLC_ locus, whereas photoperiod pathway mutants (co and gi) are relatively unaffected (Sanda and Amasino, 1996a, 1996b). Like FRI, mutations in autonomous pathway genes also result in increased FLC expression. Thus, as discussed above for the role of FRI, we determined the dependence of the late-flowering phenotype of these mutations on FLC. Accordingly, the flowering behavior of double mutants between the flc-3 null allele and fca, fpa, fve, ld, co, gi, and suppressor of overexpression of constans1 (soc1) was determined. The results are shown in Figures 1 to 3. Under LD conditions, the late-flowering phenotype of autonomous pathway mutations (fca, fpa, fve, and ld) was completely eliminated in the presence of a null allele of flc, whereas the flowering time of co, gi, and soc1 was unaffected by the absence of FLC. Under SD conditions, the late-flowering phenotype of ld, fca, and fve was eliminated in the flc-3 background, whereas fpa was slightly later flowering (Figure 3). The flowering time of non-autonomous pathway mutations was unaffected by FLC (co), or it showed an additive affect (gi and soc1) under SD (Figure 3). Thus, FCA, FPA, FVE, and LD are likely to act upstream of FLC and promote flowering by inhibiting FLC expression, whereas CO, GI, and SOC1 are likely to act in pathways that are downstream or independent of FLC.
The FLC Null Mutant Is Responsive to Vernalization
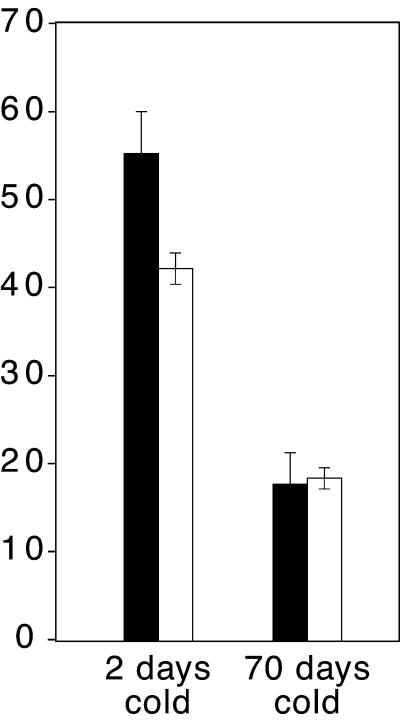
As described above, the late-flowering phenotype of FRI and autonomous pathway mutants can be eliminated by either vernalization, which permanently downregulates FLC expression, or loss-of-function mutations in FLC. These observations, coupled with the result that transgenic plants containing FLC under the control of the constitutive 35S promoter are late flowering and insensitive to vernalization (Michaels and Amasino, 1999; Sheldon et al., 1999), indicate that downregulation of FLC is a major function of the vernalization pathway. This raises the question of whether vernalization acts exclusively to downregulate FLC or whether it can also promote flowering via _FLC_-independent mechanisms. To address this question, an flc null allele and the wild type were cold treated and grown under SD, that is, under conditions in which the flowering of the flc mutant is delayed. The results are shown in Figure 4. After vernalization, both Col and flc-3 flowered after forming only half the number of leaves as non-cold-treated plants. Thus, the loss of FLC function does not eliminate the response to vernalization, and vernalization must promote flowering through _FLC_-independent pathways as well as through inactivation of FLC.
Figure 4.
Effect of Vernalization in an flc Null Mutant.
Black bars represent Col (homozygous for wild-type FLC), and white bars represent an flc null allele. Plants were cold treated for 2 or 70 days before growth under SD conditions. Error bars indicate ±sd. The y axis indicates the number of rosette leaves formed prior to flowering.
Effect of FRI and the Autonomous Pathway on SOC1 Expression Is Mediated by FLC
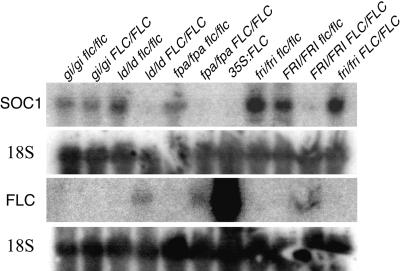
SOC1 is a MADS domain–containing transcription factor that acts as a promoter of flowering (Borner et al., 2000; Lee et al., 2000; Samach et al., 2000). SOC1 transcript levels are reduced in late-flowering backgrounds containing FRI or autonomous pathway mutations, but they are restored to wild-type levels after vernalization (Lee et al., 2000; Samach et al., 2000). The fact that SOC1 levels are regulated in a manner opposite that of FLC levels suggests that FLC may act as an inhibitor of SOC1 expression and that the effects of FRI, the autonomous pathway, and vernalization on SOC1 expression may be mediated by FLC (Lee et al., 2000; Samach et al., 2000). To test this model, SOC1 transcript levels were determined in lines containing FRI or autonomous pathway mutants with or without a functional allele of FLC. The results of RNA gel blot analyses are shown in Figure 5. In lines containing FRI, ld, or fpa with a wild-type FLC allele, SOC1 levels were suppressed. In the corresponding lines containing an flc null allele, however, SOC1 levels were similar to those in the wild type. Thus, the downregulation of SOC1 by FRI, ld, and fpa is blocked in the absence of FLC.
Figure 5.
RNA Gel Blot Analysis of SOC1 Expression in Various Genotypes.
RNA was isolated from 12-day-old plants grown under continuous light. FLC expression is shown for comparison. Blots were probed with 18S rDNA as a control for loading.
As a direct test of the ability of FLC to suppress SOC1 expression, SOC1 transcript levels were determined in a transgenic line containing FLC under the control of the constitutive 35S cauliflower mosaic virus promoter. As shown in Figure 5, SOC1 levels are suppressed in the FLC overexpression line, despite the absence of FRI or the presence of an autonomous pathway mutation. Thus, FLC expression is sufficient to suppress SOC1 levels.
DISCUSSION
Previous studies have shown that FLC acts as a repressor of flowering and is an integration point of several pathways that control flowering time (Koornneef et al., 1994; Lee et al., 1994; Sanda and Amasino, 1996b; Michaels and Amasino, 1999; Sheldon et al., 1999). The autonomous floral promotion pathway acts to suppress FLC expression; thus, mutations in autonomous pathway genes result in increased FLC levels and produce a late-flowering phenotype. In winter annual strains, the FRI gene acts to increase FLC expression and is epistatic to the suppression of FLC expression by the autonomous pathway. The late-flowering phenotype of both FRI and the autonomous pathway mutants is suppressed by vernalization (Napp-Zinn, 1979; Koornneef et al., 1991; Lee and Amasino, 1995), which causes a stable downregulation of FLC expression (Michaels and Amasino, 1999; Sheldon et al., 1999). Genetic analyses have shown that the L_er_ allele of FLC is able to partially suppress the late-flowering phenotype of FRI and autonomous pathway mutants (Koornneef et al., 1994; Lee et al., 1994; Sanda and Amasino, 1996a, 1996b). The L_er_ allele of FLC, however, is not a clear null allele (Sheldon et al., 2000; our unpublished results). Thus, the partial suppression of the late-flowering phenotype of FRI and autonomous pathway mutants in L_er_ could support two models: one in which the remaining late-flowering phenotype of FRI and autonomous pathway mutants in the L_er_ background is the result of residual FLC activity; or another in which the L_er_ allele of FLC is inactive, and FRI and autonomous pathway mutations delay flowering in part via an _FLC_-independent mechanism. Therefore, to determine the dependence of FRI and autonomous pathway mutations on FLC, we have reexamined the interactions between FLC and the autonomous pathway, FRI, and vernalization by using a null allele of FLC.
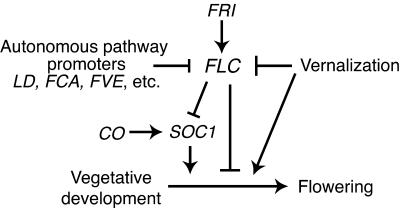
The data presented here demonstrate that in the presence of a null allele of FLC, the late-flowering phenotype of FRI and the autonomous pathway mutants ld, fca, and fve is eliminated. Thus, it appears that the late-flowering phenotype of these mutants is entirely the result of increased FLC expression (Figure 6). In contrast, we find that the late-flowering phenotype of soc1 and photoperiod pathway mutants such as co and gi is unaffected by a loss of FLC function. This is predicted from current models in which these genes act either independently (photoperiod pathway) (Koornneef et al., 1998; Simpson et al., 1999) or downstream (SOC1) (Lee et al., 2000; Samach et al., 2000) of FLC.
Figure 6.
Local Model for the Interaction of FLC, FRI, the Autonomous Pathway, and Vernalization in the Regulation of Flowering Time.
The late-flowering phenotype of fpa mutations, like that of fca, fve, and ld, is eliminated by a loss of FLC function in LD conditions. fpa, however, is distinguished from the other autonomous pathway mutants by a slight late-flowering phenotype under SD conditions in the absence of FLC; fpa/flc double mutants flower approximately four leaves later than does the flc single mutant. We have also noticed that the fpa mutant exhibits a smaller rosette diameter in SD conditions, reminiscent of a gibberellin (GA) deficiency. This phenotype was not observed in other autonomous pathway mutants. Meier et al. (2001) have also reported this SD phenotype as well as increased expression of genes involved in GA metabolism in fpa mutants. Thus, FPA appears to have functions beyond the regulation of FLC, and the slight late-flowering phenotype in SD in the absence of FLC may be a result of altered GA metabolism. (GA deficiency severely delays flowering in SD [Wilson et al., 1992].)
In the Col background, which lacks FRI activity, the flc-3 mutation has no effect on flowering time under inductive LD photoperiods, but it causes slightly earlier flowering than it does in the wild type under SD conditions. FLC transcript is undetectable by gel blot analysis of RNA from Col (Michaels and Amasino, 1999; Sheldon et al., 1999); thus, the lack of an effect of flc mutations on flowering time in LD conditions is not surprising. The early-flowering phenotype of the mutant under SD conditions, however, indicates that FLC must be present at some low level even in the absence of FRI or autonomous pathway mutations and that this low expression level has a measurable affect on flowering in noninductive photoperiods.
We also examined the effect of FLC on SOC1 expression. Previous studies have shown that SOC1 has an expression pattern opposite that of FLC (Lee et al., 2000; Samach et al., 2000): SOC1 is downregulated by FRI or mutations in the autonomous pathway and is upregulated by vernalization. The antagonistic expression patterns of FLC and SOC1 are consistent with a model in which FLC acts as a negative regulator of SOC1 and that FRI, the autonomous pathway, and vernalization affect SOC1 levels via changes in FLC expression (Lee et al., 2000; Samach et al., 2000).
In this study, we have directly tested the role of FLC in regulating SOC1 expression, and our results support this model. In the presence of FRI or autonomous pathway mutations, SOC1 levels are suppressed. However, if a null allele of FLC is combined with FRI or autonomous pathway mutations, the suppression is eliminated. Therefore, FLC is required for the suppression of SOC1 by FRI or autonomous pathway mutations. Furthermore, overexpression of FLC from a constitutive promoter can suppress SOC1 levels in the absence of FRI or autonomous pathway mutations. Thus, FLC expression is sufficient to suppress SOC1 levels. It should be noted, however, that SOC1 expression is not controlled exclusively via FLC, because overexpression of the photoperiod pathway gene CO, which does not affect FLC levels, is able to increase SOC1 expression (Samach et al., 2000). Also, the inhibition of flowering by FLC in lines containing FRI or autonomous pathway mutants is unlikely to be due solely to the downregulation of SOC1 because the late-flowering phenotypes of these lines are much more severe than are those of the soc1 null allele. Thus, the downregulation of SOC1 alone cannot account for the lateness of flowering in lines containing FRI or autonomous pathway mutants, and FLC likely acts to block flowering via SOC1 downregulation as well as via _SOC1_-independent mechanisms (Figure 6).
Recent experiments have shown that FLC expression plays a key role in creating a vernalization requirement in Arabidopsis (Michaels and Amasino, 1999, 2000; Sheldon et al., 1999, 2000). Lines containing FRI or autonomous pathway mutations have relatively high levels of FLC expression and are late flowering unless vernalized, which leads to a mitotically stable inactivation of FLC expression (Michaels and Amasino, 1999; Sheldon et al., 1999). The data presented here show that FLC inactivation by loss-of-function mutations also is effective at promoting flowering in these vernalization-requiring backgrounds. Conversely, transgenic plants with constitutive FLC expression are late flowering and insensitive to vernalization (Michaels and Amasino, 1999; Sheldon et al., 1999). Thus, the downregulation of FLC expression by cold appears to be key in the promotion of flowering by vernalization. The results presented in this study, however, demonstrate that the flc-3 null allele of FLC remains responsive to vernalization. Thus, although FLC clearly is a major target of the vernalization pathway, vernalization is capable of promoting flowering via _FLC_-independent mechanisms as well (Figure 6).
FLC plays a central role in the regulation of flowering time in Arabidopsis by integrating signals from FRI, the autonomous floral promotion pathway, and the vernalization pathway. The data presented here show that the effects of FRI and autonomous pathway mutants on flowering time and SOC1 expression are dependent on a functional allele of FLC. The vernalization pathway, however, is able to promote flowering in the absence of FLC and thus is capable of promoting flowering via _FLC_-independent as well as _FLC_-dependent mechanisms.
METHODS
Plant Materials
The Arabidopsis thaliana FLC null allele flc-3, FRI/FRI;FLC/FLC, FRI/FRI;flc/flc, ld-1, and co-1 are in the Columbia (Col) background and have been described previously (Redei, 1962; Lee et al., 1993; Michaels and Amasino, 1999). fca was isolated from T-DNA mutagenesis in the Wassilewskija background. fpa and gi were isolated from T-DNA mutagenesis in the Col background. The mutants fve and soc1 were kindly provided by Jose Martinez-Zapater (Universidad Autonoma, Madrid, Spain) and Marty Yanofsky (University of California–San Diego, La Jolla) and are in the Col background.
Plant Growth Conditions
All plants were grown under ∼100 μE m−2 sec−1 cool-white fluorescent light at 22°C. Long-day (LD) conditions consisted of 16 hr of light followed by 8 hr of darkness; short-day (SD) conditions consisted of 8 hr of light followed by 16 hr of darkness. For experiments involving vernalization, seeds were plated on agar-solidified medium containing 0.65 g/L Peter's Excel 15-5-15 fertilizer (Grace Sierra, Milpitas, CA) and were kept at room temperature overnight to allow them to become metabolically active before being transferred to 2°C for 70 days. During cold treatment, samples were kept under SD conditions. A minimum of 10 plants was averaged for each data point.
RNA Gel Blot Analysis
Total RNA was isolated using RNA Isolator (Genosys Biotechnologies, The Woodlands, TX), according to the manufacturer's instructions. For RNA gel blots, 15 μg of RNA was separated by denaturing formaldehyde agarose gel electrophoresis as described by Sambrook et al. (1989). RNA gel blots were probed with a 32P-ATP–labeled cDNA fragment that did not contain the conserved MADS box domains of FLC or SOC1. Blots also were probed with an 18S rRNA probe as a control for the quantity of RNA loaded.
Acknowledgments
This work was supported by the College of Agricultural and Life Sciences of the University of Wisconsin and by grants to R.M.A. from the United States Department of Agriculture National Research Initiative Competitive Grants Program and the National Science Foundation.
References
- Borner, R., Kampmann, G., Chandler, J., Gleissner, R., Wisman, E., Apel, K., and Melzer, S. (2000). A MADS domain gene involved in the transition to flowering in Arabidopsis. Plant J. 24**,** 591–599. [DOI] [PubMed] [Google Scholar]
- Burn, J.E., Smyth, D.R., Peacock, W.J., and Dennis, E.S. (1993). Genes conferring late flowering in Arabidopsis thaliana. Genetica 90**,** 147–155. [Google Scholar]
- Chouard, P. (1960). Vernalization and its relations to dormancy. Annu. Rev. Plant Physiol. 11**,** 191–238. [Google Scholar]
- Clarke, J.H., and Dean, C. (1994). Mapping FRI, a locus controlling flowering time and vernalization response in Arabidopsis thaliana. Mol. Gen. Genet. 242**,** 81–89. [DOI] [PubMed] [Google Scholar]
- Johanson, U., West, J., Lister, C., Michaels, S., Amasino, R., and Dean, C. (2000). Molecular analysis of FRIGIDA, a major determinant of natural variation in Arabidopsis flowering time. Science 290**,** 344–347. [DOI] [PubMed] [Google Scholar]
- Karlsson, B.H., Sills, G.R., and Nienhuis, J. (1993). Effects of photoperiod and vernalization on the number of leaves at flowering in 32 Arabidopsis thaliana (Brassicaceae) ecotypes. Am. J. Bot. 80**,** 646–648. [Google Scholar]
- Koornneef, M., Hanhart, C.J., and van der Veen, J.H. (1991). A genetic and physiological analysis of late-flowering mutants in Arabidopsis thaliana. Mol. Gen. Genet. 229**,** 57–66. [DOI] [PubMed] [Google Scholar]
- Koornneef, M., Blankestijn-de Vries, H., Hanhart, C., Soppe, W., and Peeters, T. (1994). The phenotype of some late-flowering mutants is enhanced by a locus on chromosome 5 that is not effective in the Landsberg erecta wild-type. Plant J. 6**,** 911–919. [Google Scholar]
- Koornneef, M., Alonso-Blanco, C., Peeters, A.J., and Soppe, W. (1998). Genetic control of flowering time in Arabidopsis. Annu. Rev. Plant Physiol. Plant Mol. Biol. 49**,** 345–370. [DOI] [PubMed] [Google Scholar]
- Lee, H., Suh, S.S., Park, E., Cho, E., Ahn, J.H., Kim, S.G., Lee, J.S., Kwon, Y.M., and Lee, I. (2000). The AGAMOUS-LIKE 20 MADS domain protein integrates floral inductive pathways in Arabidopsis. Genes Dev. 14**,** 2366–2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, I., and Amasino, R.M. (1995). Effect of vernalization, photoperiod, and light quality on the flowering phenotype of Arabidopsis plants containing the FRIGIDA gene. Plant Physiol. 108**,** 157–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, I., Bleecker, A., and Amasino, R. (1993). Analysis of naturally-occurring late flowering in Arabidopsis thaliana. Mol. Gen. Genet. 237**,** 171–176. [DOI] [PubMed] [Google Scholar]
- Lee, I., Michaels, S.D., Masshardt, A.S., and Amasino, R.M. (1994). The late-flowering phenotype of FRIGIDA and LUMINIDEPENDENS is suppressed in the Landsberg erecta strain of Arabidopsis. Plant J. 6**,** 903–909. [Google Scholar]
- Meier, C., Bouquin, T., Nielsen, M.E., Raventos, D., Mattsson, O., Rocher, A., Schomburg, F., Amasino, R.M., and Mundy, J. (2001). Gibberellin response mutants identified by luciferase imaging. Plant J., in press. [DOI] [PubMed]
- Michaels, S., and Amasino, R. (1999). FLOWERING LOCUS C encodes a novel MADS domain protein that acts as a repressor of flowering. Plant Cell 11**,** 949–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaels, S., and Amasino, R. (2000). Memories of winter: Vernalization and the competence to flower. Plant Cell Environ. 23**,** 1145–1154. [Google Scholar]
- Napp-Zinn, K. (1979). On the genetical basis of vernalization requirement in Arabidopsis thaliana (L.) Heynh. In La physiologie de la floraison, P. Champagnat and R. Jaques, eds (Paris: Centre National de la Recherche Scientifique), pp. 217–220.
- Redei, G.P. (1962). Supervital mutants in Arabidopsis. Genetics 47**,** 443–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samach, A., Onouchi, H., Gold, S.E., Ditta, G.S., Schwarz-Sommer, Z., Yanofsky, M.F., and Coupland, G. (2000). Distinct roles of CONSTANS target genes in reproductive development of Arabidopsis. Science 288**,** 1613–1616. [DOI] [PubMed] [Google Scholar]
- Sambrook, J., Fritsch, E.F., and Maniatis, T. (1989). Molecular Cloning: A Laboratory Manual. (Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press).
- Sanda, S.L., and Amasino, R.M. (1996. a). Ecotype-specific expression of a flowering mutant phenotype in Arabidopsis thaliana. Plant Physiol. 111**,** 641–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanda, S.L., and Amasino, R.M. (1996. b). Interaction of FLC and late-flowering mutations in Arabidopsis thaliana. Mol. Gen. Genet. 251**,** 69–74. [DOI] [PubMed] [Google Scholar]
- Sheldon, C.C., Burn, J.E., Perez, P.P., Metzger, J., Edwards, J.A., Peacock, W.J., and Dennis, E.S. (1999). The FLF MADS box gene: A repressor of flowering in Arabidopsis regulated by vernalization and methylation. Plant Cell 11**,** 445–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldon, C.C., Rouse, D.T., Finnegan, E.J., Peacock, W.J., and Dennis, E.S. (2000). The molecular basis of vernalization: The central role of FLOWERING LOCUS C (FLC). Proc. Natl. Acad. Sci. USA 97**,** 3753–3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson, G.G., Gendall, T., and Dean, C. (1999). When to switch to flowering. Annu. Rev. Cell Dev. Biol. 15**,** 519–550. [DOI] [PubMed] [Google Scholar]
- Wilson, R.N., Heckman, J.W., and Somerville, C.R. (1992). Gibberellin is required for flowering in Arabidopsis thaliana under short days. Plant Physiol. 100**,** 403–408. [DOI] [PMC free article] [PubMed] [Google Scholar]