Conditional telomerase induction causes proliferation of hair follicle stem cells (original) (raw)
. Author manuscript; available in PMC: 2006 Feb 6.
Published in final edited form as: Nature. 2005 Aug 18;436(7053):1048–1052. doi: 10.1038/nature03836
Abstract
TERT, the protein component of telomerase1,2, serves to maintain telomere function through the de novo addition of telomere repeats to chromosome ends and is reactivated in 90% of human cancers. In normal tissues, TERT is expressed in stem cells and in progenitor cells3, but its role in these compartments is not fully understood. Here, we show that conditional transgenic induction of TERT in mouse skin epithelium causes a rapid transition from telogen, the resting phase of the hair follicle cycle, to anagen, the active phase, thereby facilitating robust hair growth. TERT overexpression promotes this developmental transition by causing proliferation of quiescent, multipotent stem cells in the hair follicle bulge region. This new function for TERT does not require the telomerase RNA component (TERC), which encodes the template for telomere addition, and therefore operates through a novel mechanism independent of its activity in synthesizing telomere repeats. These data indicate that, in addition to its established role in extending telomeres, TERT can promote proliferation of resting stem cells through a non-canonical pathway.
Keywords: telomerase, telomere, stem cell, hair follicle, epidermis
In stem cell and progenitor cell compartments3–5, TERT serves an important role in keeping telomeres sufficiently long and stable to prevent the adverse consequences of dysfunctional telomeres on cell viability and chromosomal stability6–8. However, the need for expression of TERT in tissue stem cells and progenitor cells with long telomeres is less clear, especially in laboratory mice, whose telomeres are significantly longer than those of humans (40–60kb vs. 5–15kb). Moreover, recent findings indicate that TERT promotes tumor development even in settings of ample telomere reserve, although the mechanisms underlying these telomere length-independent activities of TERT remain unclear9–13. We therefore hypothesized that TERT may exert effects in stem cell and progenitor cell compartments that could explain both its regulation during lineage development and its poorly understood telomere length-independent activities.
To test this hypothesis, we turned to the mammalian hair follicle, an organ that harbors tightly regulated multipotent stem cells and that cycles between telogen and anagen14. Initiation of a new anagen cycle depends upon activation of a small number of quiescent stem cells that reside in the bulge, a niche at the follicle base15–19. These activated stem cells proliferate and differentiate into progenitor cells (matrix cells) that give rise to the differentiated lineages that comprise the hair shaft and root sheaths. This period of hair synthesis ceases when the new section of the anagen follicle is remodeled through apoptotic regression (catagen), resulting in another telogen phase (Fig 1a). To understand how telomerase is regulated during mouse hair follicle cycling, we analyzed telomerase activity in mouse skin, exploiting the fact that hair follicle cycles are synchronized for the first two postnatal periods of hair follicle growth, approximately 8 weeks20. Protein extracts from wild type mouse skin were obtained between postnatal days 4 and 52, to allow analysis of both the first and second postnatal hair cycles using the Telomere Repeat Amplification Protocol (TRAP assay). Telomerase activity strongly correlated with the first and second anagen phases and was not detected during telogen phases (Fig 1b). These data indicate that, in mouse hair follicles, as in human21, telomerase is associated with the anagen phase, a period of intense progenitor cell activity.
Figure 1.
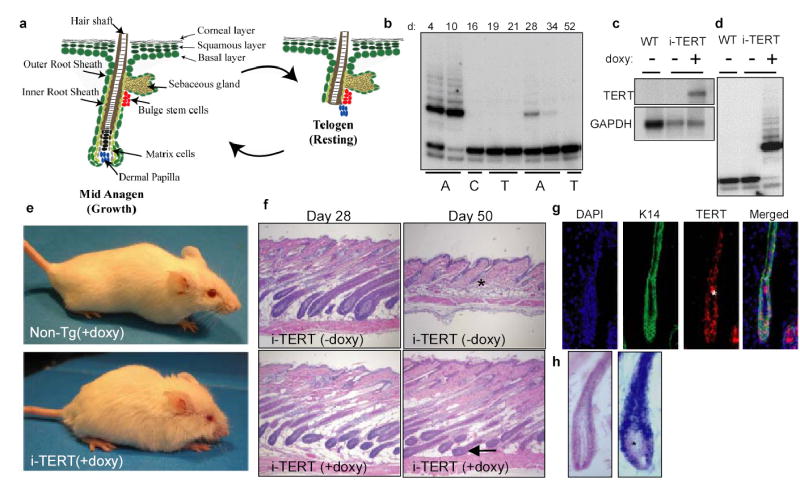
Conditional activation of TERT promotes the anagen phase of the hair follicle cycle. a, Diagram of hair follicle cycling b, TRAP on Non-Tg skin samples at days 4, 10 (anagen-A), 16 (catagen-C), 19, 21 (telogen-T), 28, 34 (A), and 52 (T). c–d, Northern blot analysis and TRAP on skin extracts from i-TERT and WT mice at day 50. e, Non-Tg (+doxy) and i-TERT (+doxy) mice at day 70. f, H&E skin sections (*=telogen hair follicle, arrow= anagen hair follicle), 20x. g, RNA in situ hybridization for TERT mRNA and immunofluorescence for K14 in i-TERT (+doxy) skin (*=autofluorescence). h, RNA in situ hybridization for TERT mRNA (blue) in i-TERT(+doxy) skin section (right) and WT anagen skin (left) (*=dermal papilla).
To determine if TERT can modulate adult tissue stem and progenitor cell function, we engineered a conditional TERT transgenic system in mice using a tetracycline-regulated approach22. The mouse TERT cDNA was cloned under control of a tetracycline responsive promoter (tetop-TERT+). To drive expression of TERT, we chose a CMV enhancer/β-actin promoter (actin-rtTA+) because this element was previously shown to be active in stem cells23 and in many epithelial tissues, including skin24,25. Tetop-TERT+ mice were intercrossed with actin-rtTA+ mice to generate actin-rtTA+; tetop-TERT+ (termed inducible TERT or i-TERT) mice. To induce expression of TERT, i-TERT mice were administered drinking water containing the tetracycline analogue, doxycycline. Northern blot and TRAP assay confirmed doxycycline-dependent induction of the TERT transgene and increased telomerase activity in the skin of i-TERT mice (Fig 1c, d) as well as in other tissues (data not shown). Remarkably, within several weeks of doxycycline treatment, the coats of i-TERT mice were significantly altered, reminiscent of mice with known mutations that affect hair follicle cycling26,27 (Fig 1e).
To determine if abnormalities in hair follicle cycling might underlie the altered hair phenotype, we analyzed skin biopsies from i-TERT mice after doxycycline administration beginning at day 21. Hair follicles were appropriately in anagen at day 28 in i-TERT mice on and off doxycycline, and in littermate controls. By day 50, follicles from i-TERT mice off doxycycline and from non-transgenic mice had exited anagen and were in the second post-natal telogen phase. In marked contrast, hair follicles from i-TERT mice on doxycycline were consistently in anagen at day 50 (Fig 1f). This effect was doxycycline-dependent and occurred with 100% penetrance in i-TERT mice (18/18 in anagen) (chi square analysis, p<0.0001 for i-TERT on vs. off doxycycline, Table S1). RNA in situ hybridization revealed a pan-epithelial expression pattern of transgenic TERT in skin that included the Keratin-14+ compartment, but spared the dermal papilla, indicating that transgenic TERT mRNA is expressed principally in hair follicle and skin epithelium (Fig 1g,h & 3g). Together, these data show that conditional induction of TERT in adult hair follicle epithelium promotes the anagen phase.
Figure 3.
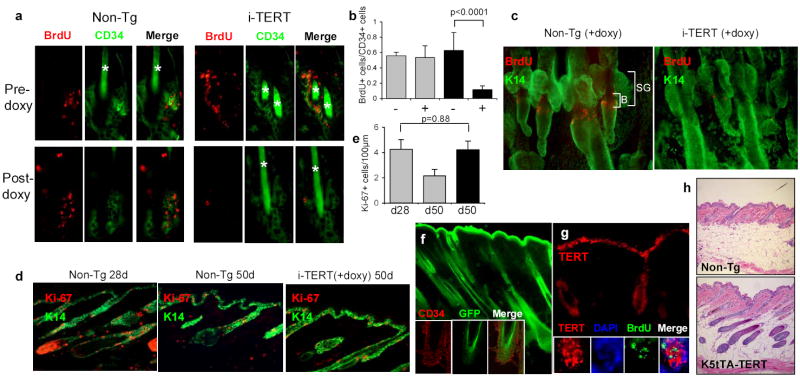
TERT activates stem cells, depleting BrdU label from LRCs. a, Immunofluorescence for BrdU (red) and CD34 (green) shows maintenance of LRCs in Non-Tg group, but dramatic loss of label in i-TERT mice after doxy treatment (pre-doxy = day 55, post-doxy= day 90). b, Quantification of LRC data from (a), showing number of BrdU+ cells/CD34+ cells. i-TERT (black bars, n=4 mice), Non-Tg (gray bars, n=3 mice), - indicates pre-doxy, + indicates post-doxy. c, LRC analysis from whole mounts of epidermis from tail of mice labeled with BrdU at day 10, switched to doxy at day 40 and analyzed at day 65. (BrdU=red, K14=green). d, Immunofluorescence using Ki-67 (red) to mark proliferating cells and K14 (green) to identify basal layer of skin. e, Quantitation of proliferation index in (d) as Ki-67+ cells/100μm length of basal layer. n=2 mice for each comparison. f, GFP epifluorescence costained with CD34 (inset, confocal microscopy) in skin section from an actin-GFP mouse. g, RNA in situ analysis for TERT mRNA in i-TERT(+doxy) mouse skin. (inset) TERT mRNA expression (cytoplasmic) overlaps in bulge with LRCs, marked by BrdU (nuclear). h, H&E sections from K5tTA+; tetop-TERT+ (−doxy) (bottom) and Non-Tg (top) mice, 20X. Error bars indicate standard deviation. p values based on t-test. *=autofluorescence of hair.
To determine if expression of TERT is sufficient to induce a transition from telogen to anagen, i-TERT mice were treated with doxycycline after hair follicles had entered the prolonged second telogen (day 40 in FVB mice (Fig. 2; data not shown)) and were biopsied at regular intervals for 12 days. TERT mRNA and telomerase activity progressively increased from day 3 through day 9 (Fig 2a). Whereas hair follicles from non-transgenic mice remained in telogen during the duration of the time course, follicles from i-TERT mice treated with doxycycline entered anagen by day 9 and were in mid-anagen20 by day 12 (Fig 2b) (chi-square analysis, p=0.005, Table S2). The kinetics of anagen entry closely followed the timing of TERT induction. To determine if induction of anagen by TERT enabled hair growth, i-TERT mice were treated with doxycycline-drinking water beginning at day 45 and shaved at day 55. Fourteen days after shaving, i-TERT mice administered doxycycline showed significant hair growth as compared with both i-TERT mice off doxycycline and non-transgenic controls, in which hair did not grow during this interval (Fig 2c). Strikingly, these results show that induction of TERT in telogen follicles is sufficient to initiate a transition to the anagen phase and promote new hair synthesis.
Figure 2.
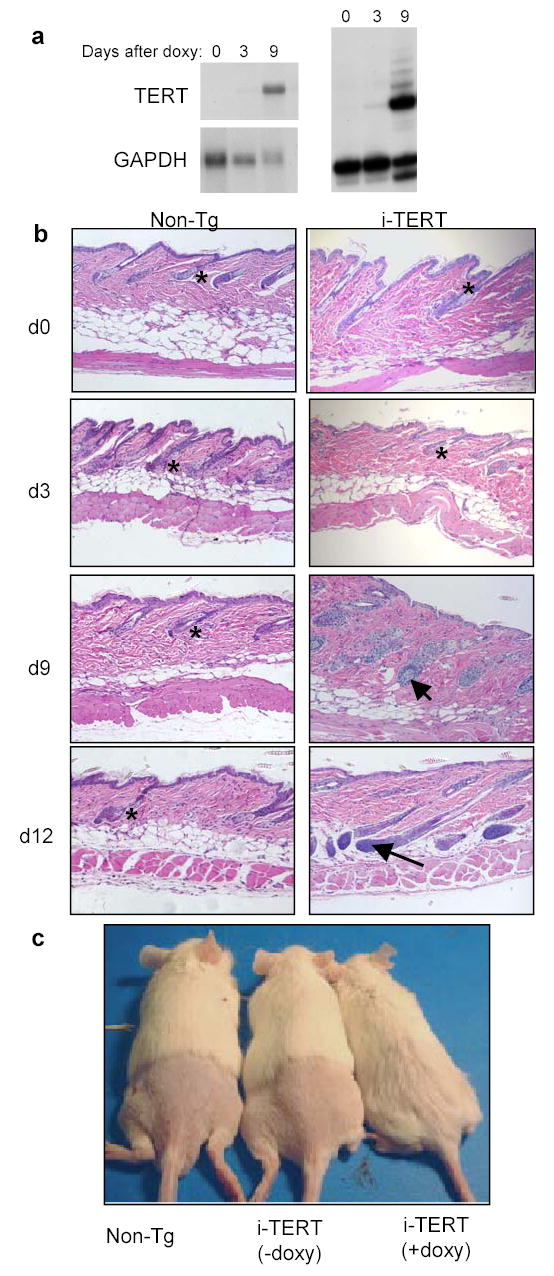
TERT induction triggers a rapid transition from telogen to anagen. a, Northern blot (left) and TRAP assay (right) show increased TERT expression and telomerase activity by nine days of doxycycline treatment. b, Follicles in i-TERT mice entered anagen (arrow) by day 9, whereas Non-Tg controls remained in telogen (*), H&E, 20x. c, Hair growth was observed only in i-TERT mice (+doxy) (right), but not in i-TERT mice (−doxy) (center) or Non-Tg littermates (left).
Because activation of bulge stem cells is integral to the initiation of a new anagen cycle15,16,18, we hypothesized that TERT’s effects on the hair follicle cycle might be mediated through the stem cell compartment. To address this hypothesis, we employed a label retaining technique that has been used successfully to mark hair follicle bulge stem cells by repeated injections of BrdU followed by a long chase period15. Cohorts of i-TERT mice and non-transgenic controls were injected with BrdU at 10 days of age. During the second telogen, mice in each group were biopsied, switched to doxycycline drinking water, and biopsied again between days 80 and 100. Label retaining cells (LRCs) were visualized by double immunostaining with antibodies against BrdU and CD34, a cell membrane marker for hair follicle stem cells28,29. LRCs were present in similar numbers in both i-TERT and non-transgenic mice at age 55 days, before the switch to doxycycline water (approximately 0.6 BrdU+ cells/CD34+ cell). After five weeks of doxycycline treatment, BrdU label in CD34+ stem cells was retained in non-transgenic mice at comparable levels, consistent with previous observations that BrdU label persists in quiescent bulge cells for more than six months17. In marked contrast, BrdU label was profoundly depleted in the CD34+ cell population in the bulge by induction of TERT in i-TERT mice (76% reduction in BrdU+ cells/CD34+ cell, p<0.0001) (Fig. 3a,b). Despite the loss of BrdU label, CD34+ cells in the bulge remained in similar numbers, indicating that, under the influence of TERT, stem cells divide but likely self-renew to maintain the CD34+ population. A similar reduction in LRCs in i-TERT mice was seen in epidermal wholemounts, corroborating the effects seen in dorsal skin sections (Fig. 3c). These data show that TERT causes hair follicle bulge cells to proliferate, diluting BrdU label from this quiescent stem cell population.
To determine if TERT more broadly enhances keratinocyte proliferation, we measured the proliferation index in the basal layer of the interfollicular epidermis (Fig. 3d). Despite expression of transgenic TERT mRNA in this compartment, proliferation was not substantially altered in the basal layer in i-TERT mice compared to non-transgenic littermates in anagen (4.2 Ki-67+ cells/100μm for i-TERT day 50 compared to 4.3 Ki-67+ cells/100μm for non-transgenic day 28) (Fig. 3e). Furthermore, we found no changes in structure, differentiation, or signaling in either hair follicle or interfollicular epidermis in i-TERT mice (Fig. S2, S3). We therefore conclude that the principle effects of TERT in this system occur through activation of quiescent hair follicle stem cells.
To understand if these results are consistent with a direct effect for TERT on stem cells, we asked if transgenic TERT is expressed in the stem cell compartment. We found that the promoter element used to direct rtTA expression is strongly active in CD34+ bulge cells in actin-GFP mice24 (Fig. 3f). Furthermore, TERT mRNA was co-expressed with BrdU in LRCs in the bulge region in i-TERT mice (Fig. 3g). While induction of anagen can occur through signals from the dermal papilla30, the lack of detectable levels of TERT mRNA in the dermal papilla (Fig. 1h) makes it unlikely in this case. To confirm that TERT exerts its effect through the epithelium, we intercrossed tetop-TERT mice with a transgenic mouse in which the Keratin-5 promoter drives expression of the tetracycline transactivator (tTA) in the basal layer and outer root sheath (K5-tTA, tet off configuration)18. Compound K5-tTA+; tetop-TERT+ mice were bred on doxycycline and weaned off doxycycline-drinking water at day 21 to induce the TERT transgene. Expression of TERT mRNA in skin epithelium (data not shown) induced anagen in 5/5 K5-tTA+; tetop-TERT+ mice, whereas all littermate control biopsies were in telogen (6/6, p=0.0009 by Chi square analysis)(Fig. 3h). These data show that TERT’s effects in promoting anagen are intrinsic to the K5 compartment of the skin epithelium, the layer where the hair follicle stem cells reside.
These effects for TERT in facilitating a switch from telogen to anagen could occur through TERT’s established role in telomere synthesis or through a novel mechanism, independent of its known enzymatic function. Telomere synthesis requires both TERT and TERC; therefore, if the effects of TERT are retained in a TERC−/− background, telomere extension cannot be required for stem cell activation events mediated by TERT. To answer this question, TERC+/− mice were intercrossed with inducible TERT alleles to derive cohorts of i-TERT mice that were TERC+/+, TERC+/− and TERC−/−. Mice in each group were treated with doxycycline beginning in telogen at day 40. Histological analysis at day 55 revealed that conditional activation of TERT induced anagen in 5/5 i-TERT; TERC−/− mice (Fig. 4a) (p=0.0003 for i-TERT; TERC−/− mice on doxycycline vs. i-TERT mice off doxycycline, Table S3). These results were identical to those obtained from i-TERT mice in either TERC+/+ or TERC+/− backgrounds (6/6 in anagen), indicating that TERC is not required for TERT to induce anagen. TRAP assays and RT-PCR analysis confirmed the absence of telomerase activity and TERC RNA in the skin of i-TERT; TERC−/− mice (Fig. 4b). These data prove that the mechanism by which TERT triggers hair follicles to transition from telogen to anagen does not require telomere synthesis.
Figure 4.
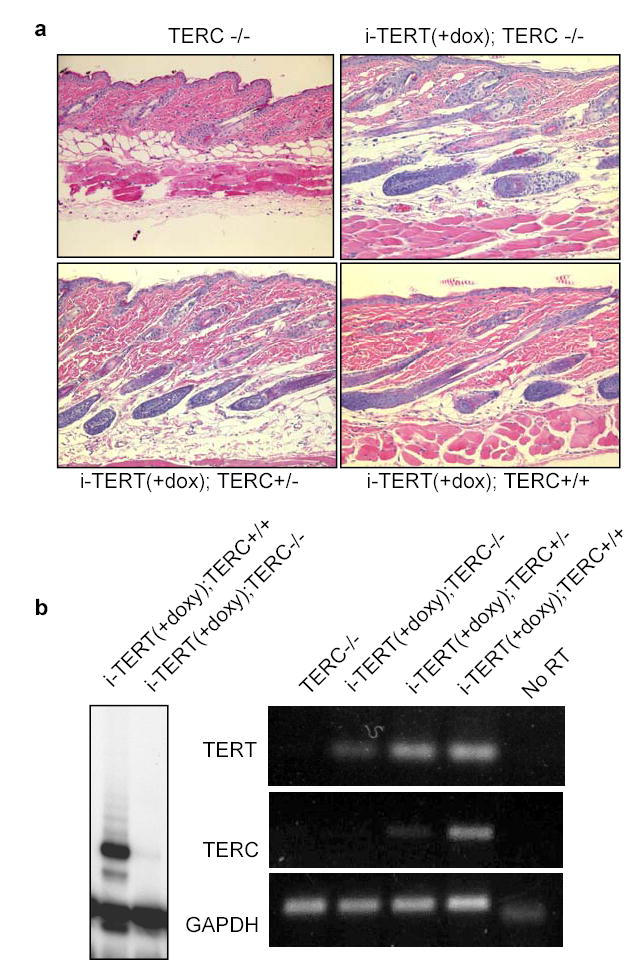
TERT's activity in facilitating a transition from telogen to anagen is independent of its function in telomere synthesis. a, TERT induced anagen in mice with TERC+/+, TERC+/−, and TERC−/− backgrounds, H&E 20x. b, Skin samples from i-TERT; TERC−/− mice lacked telomerase activity by TRAP (left) and lacked TERC expression by RT-PCR (right).
Here we show that conditional activation of TERT protein in mouse skin is sufficient to induce proliferation of hair follicle stem cells and that this function is independent of its role in telomere synthesis. In this transgenic context, either altered timing of TERT expression or higher protein levels may be important in mediating these new activities for TERT. Our data encourage further studies to understand the role of the endogenous TERT protein in stem cells, including loss-of-function studies and experiments designed to determine if TERT's function in stem cell proliferation is cell autonomous. We propose that as TERT is dynamically regulated during the course of stem and progenitor cell maturation, or during advancing stages of cancer development, TERT may directly support these processes. These data provide a novel framework for understanding the recently identified telomere length-independent functions of TERT9–12 and suggest new strategies for manipulating TERT for therapeutic purposes in treating disorders associated with tissue injury and aging.
Methods
Generation of transgenic mice
TERT was placed under control of a tetracycline-inducible promoter by subcloning a 3.5kb EcoRI fragment of the mouse TERT cDNA into the EcoRI site of pUHD10-322. To create actin-rtTA, an EcoRI-BamHI fragment of the rtTA cDNA22 was subcloned into the EcoR1 site of pCAGS24 by blunt-ended ligation. Prokaryotic sequences were excised from each plasmid and the gel-isolated DNA fragments were separately injected into pronuclei of FVB/N fertilized zygotes. Founder mice were screened by PCR and Southern blot. Actin-rtTA transgene positive mice were intercrossed with tetop-TERT transgene positive mice to generate i-TERT double transgenic mice for characterization. Doxycycline (2 mg/ml in 5% sucrose for rtTA, 10μg/ml in water for tTA) was administered in drinking water in light-protected bottles and changed biweekly. TERC+/− mice were backcrossed to the FVB/N strain for six generations, then intercrossed with the i-TERT alleles to yield i-TERT mice on TERC+/+, TERC+/− and TERC−/− backgrounds. All mice were treated in accordance with AAALAC approved guidelines at Stanford University.
Histology
Skin biopsies were obtained from dorsal skin of mice under anesthesia and wounds were closed with 9mm staples (Roboz). Biopsy specimens were either fixed overnight in 10% formalin then embedded in paraffin or fixed overnight in embedded in OCT and frozen on an isopropanoldry ice slurry. Formalin fixed, paraffin embedded tissue sections were stained with hematoxylin and eosin (H&E) for microscopic analysis.
RNA in situ analysis
Digoxygenin-labeled anti-sense RNA probes were synthesized in vitro using digoxygenin-UTP (Roche). In situ analysis was performed on 10μm frozen sections or 5μm paraffin sections. Probes were detected using a horseradish peroxidase conjugated anti-digoxygenin antibody and RNA in situs were developed using biotinyl tyramide (Dako) followed by Cy3-Strepavidin (Vector Laboratories). Slides were mounted with Vectashield plus DAPI (Vector Laboratories). For chromogenic RNA in situ analysis, probes were detected using an alkaline phosphatase conjugated anti-digoxygenin antibody and were developed using NBT/BCIP (Roche) and counterstained with nuclear fast red (Vector Laboratories).
Immunofluorescence
All assays were performed on 10μm frozen sections. Samples were fixed in 10% formalin then blocked with either 10% normal goat serum in TBS-T or MOM IgG Blocking solution (Vector laboratories). Sections were incubated in primary antibody overnight at 4°C and detected with FITC conjugated anti-rat (Jackson ImmunoResearch), or Cy3 conjugated anti-rat (Jackson ImmunoResearch). Primary antibodies included: mouse anti-Ki-67 (Pharmingen), rabbit anti-K14 (Covance), rat anti-BrdU (BD), rat-anti-CD34 (Pharmingen). For BrdU detection, slides were pre-treated in 1N HCl for 1hr at 37°C.
Analysis of Label Retaining Cells
To label follicle stem cells, 10-day-old mice were injected with 250μg of BrdU every 12 hours for four injections to mark proliferating epidermal keratinocytes. Skin samples were obtained from the mice after an extended chase period of 45–90 days. BrdU immunofluorescence was performed on frozen sections to visualize label retaining cells, followed by costaining for CD34.
Tail Wholemount Immunolabelling
Wholemounts of tail epidermis were prepared and stained for BrdU and K14 as described17.
Northern blots, RT-PCR, and telomerase activity assays
Tissues were snap frozen in liquid nitrogen and then ground with mortar and pestle. RNA was isolated from organs or cells by means of homogenization in Trizol. 5 μg of total RNA was fractionated on a 0.8% formaldehyde gel, transferred to Hybond-N membrane (Amersham), and hybridized with TERT or GAPDH 32P-labeled DNA probes. For RT-PCR, the reverse transcriptase reaction was performed on 1μg of total RNA using Superscript II, and PCR was performed with primer pairs specific for TERT or GAPDH. For telomerase repeat amplification protocol (TRAP) assays, protein was extracted from 50–100 mg of tissue in CHAPS lysis buffer, and a standard TRAP reaction was performed (TRAPEZE, Chemicon).
Supplementary Material
Sfig1
Sfig2
Sfig3
Sfig4
Sfig5
Sfig6
Supp data
Supp table 4
Supp tables 1-
Acknowledgments
We wish to acknowledge expert technical assistance from the Stanford Transgenic Core Facility and from Pauline Chu in the Stanford Comparative Medicine Histology Research Core Lab. We wish to thank R. DePinho for the use of TERC−/− mice, A. Glick for the use of K5-tTA mice, T. Sun for the gift of AE13 and AE15 antibodies, and K. Braun and V. Horsley for important technical insights. We appreciate helpful comments and insights from T. de Lange, R. Nusse, I. Weissman, L. Attardi, J. Sage, A. Brunet, M. Cleary, P. Khavari and members of the Artandi laboratory. K.Y.S. is supported by Medical Scientist Training Program Grant GM07365. A.E.O is supported by NIH ARO 46786. This work was supported by the Rita Allen Foundation, V Foundation, the NIH, and the Stanford Cancer Council.
References
- 1.Blackburn EH. Switching and signaling at the telomere. Cell. 2001;106:661–73. doi: 10.1016/s0092-8674(01)00492-5. [DOI] [PubMed] [Google Scholar]
- 2.Smogorzewska A, De Lange T. Regulation of telomerase by telomeric proteins. Annu Rev Biochem. 2004;73:177–208. doi: 10.1146/annurev.biochem.73.071403.160049. [DOI] [PubMed] [Google Scholar]
- 3.Morrison SJ, Prowse KR, Ho P, Weissman IL. Telomerase activity in hematopoietic cells is associated with self-renewal potential. Immunity. 1996;5:207–216. doi: 10.1016/s1074-7613(00)80316-7. [DOI] [PubMed] [Google Scholar]
- 4.Yui J, Chiu CP, Lansdorp PM. Telomerase activity in candidate stem cells from fetal liver and adult bone marrow. Blood. 1998;91:3255–62. [PubMed] [Google Scholar]
- 5.Forsyth NR, Wright WE, Shay JW. Telomerase and differentiation in multicellular organisms: turn it off, turn it on, and turn it off again. Differentiation. 2002;69:188–97. doi: 10.1046/j.1432-0436.2002.690412.x. [DOI] [PubMed] [Google Scholar]
- 6.Blasco MA, et al. Telomere shortening and tumor formation by mouse cells lacking telomerase RNA. Cell. 1997;91:25–34. doi: 10.1016/s0092-8674(01)80006-4. [DOI] [PubMed] [Google Scholar]
- 7.Lee HW, et al. Essential role of mouse telomerase in highly proliferative organs. Nature. 1998;392:569–74. doi: 10.1038/33345. [DOI] [PubMed] [Google Scholar]
- 8.Rudolph KL, et al. Longevity, stress response, and cancer in aging telomerase-deficient mice. Cell. 1999;96:701–12. doi: 10.1016/s0092-8674(00)80580-2. [DOI] [PubMed] [Google Scholar]
- 9.Gonzalez-Suarez E, et al. Increased epidermal tumors and increased skin wound healing in transgenic mice overexpressing the catalytic subunit of telomerase, mTERT, in basal keratinocytes. Embo J. 2001;20:2619–2630. doi: 10.1093/emboj/20.11.2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Artandi SE, et al. Constitutive telomerase expression promotes mammary carcinomas in aging mice. Proc Natl Acad Sci U S A. 2002;99:8191–8196. doi: 10.1073/pnas.112515399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stewart SA, et al. Telomerase contributes to tumorigenesis by a telomere length-independent mechanism. Proc Natl Acad Sci U S A. 2002;99:12606–11. doi: 10.1073/pnas.182407599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang S, Khoo CM, Naylor ML, Maser RS, DePinho RA. Telomere-based crisis: functional differences between telomerase activation and ALT in tumor progression. Genes Dev. 2003;17:88–100. doi: 10.1101/gad.1029903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blasco MA, Hahn WC. Evolving views of telomerase and cancer. Trends Cell Biol. 2003;13:289–94. doi: 10.1016/s0962-8924(03)00085-0. [DOI] [PubMed] [Google Scholar]
- 14.Alonso L, Fuchs E. Stem cells in the skin: waste not, Wnt not. Genes Dev. 2003;17:1189–200. doi: 10.1101/gad.1086903. [DOI] [PubMed] [Google Scholar]
- 15.Cotsarelis G, Sun TT, Lavker RM. Label-retaining cells reside in the bulge area of pilosebaceous unit: implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell. 1990;61:1329–37. doi: 10.1016/0092-8674(90)90696-c. [DOI] [PubMed] [Google Scholar]
- 16.Taylor G, Lehrer MS, Jensen PJ, Sun TT, Lavker RM. Involvement of follicular stem cells in forming not only the follicle but also the epidermis. Cell. 2000;102:451–61. doi: 10.1016/s0092-8674(00)00050-7. [DOI] [PubMed] [Google Scholar]
- 17.Braun KM, et al. Manipulation of stem cell proliferation and lineage commitment: visualisation of label-retaining cells in wholemounts of mouse epidermis. Development. 2003;130:5241–55. doi: 10.1242/dev.00703. [DOI] [PubMed] [Google Scholar]
- 18.Tumbar T, et al. Defining the epithelial stem cell niche in skin. Science. 2004;303:359–63. doi: 10.1126/science.1092436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morris RJ, et al. Capturing and profiling adult hair follicle stem cells. Nat Biotechnol. 2004;22:411–7. doi: 10.1038/nbt950. [DOI] [PubMed] [Google Scholar]
- 20.Muller-Rover S, et al. A comprehensive guide for the accurate classification of murine hair follicles in distinct hair cycle stages. J Invest Dermatol. 2001;117:3–15. doi: 10.1046/j.0022-202x.2001.01377.x. [DOI] [PubMed] [Google Scholar]
- 21.Ramirez RD, Wright WE, Shay JW, Taylor RS. Telomerase activity concentrates in the mitotically active segments of human hair follicles. J Invest Dermatol. 1997;108:113–7. doi: 10.1111/1523-1747.ep12285654. [DOI] [PubMed] [Google Scholar]
- 22.Gossen M, Bujard H. Tight control of gene expression in mammalian cells by tetracycline- responsive promoters. Proc Natl Acad Sci U S A. 1992;89:5547–51. doi: 10.1073/pnas.89.12.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wright DE, et al. Cyclophosphamide/granulocyte colony-stimulating factor causes selective mobilization of bone marrow hematopoietic stem cells into the blood after M phase of the cell cycle. Blood. 2001;97:2278–85. doi: 10.1182/blood.v97.8.2278. [DOI] [PubMed] [Google Scholar]
- 24.Okabe M, Ikawa M, Kominami K, Nakanishi T, Nishimune Y. 'Green mice' as a source of ubiquitous green cells. FEBS Letters. 1997;407:313–319. doi: 10.1016/s0014-5793(97)00313-x. [DOI] [PubMed] [Google Scholar]
- 25.Sawamura D, et al. Promoter/enhancer cassettes for keratinocyte gene therapy. J Invest Dermatol. 1999;112:828–30. doi: 10.1046/j.1523-1747.1999.00583.x. [DOI] [PubMed] [Google Scholar]
- 26.Hebert JM, Rosenquist T, Gotz J, Martin GR. FGF5 as a regulator of the hair growth cycle: evidence from targeted and spontaneous mutations. Cell. 1994;78:1017–25. doi: 10.1016/0092-8674(94)90276-3. [DOI] [PubMed] [Google Scholar]
- 27.Gat U, DasGupta R, Degenstein L, Fuchs E. De Novo hair follicle morphogenesis and hair tumors in mice expressing a truncated beta-catenin in skin. Cell. 1998;95:605–14. doi: 10.1016/s0092-8674(00)81631-1. [DOI] [PubMed] [Google Scholar]
- 28.Trempus CS, et al. Enrichment for living murine keratinocytes from the hair follicle bulge with the cell surface marker CD34. J Invest Dermatol. 2003;120:501–11. doi: 10.1046/j.1523-1747.2003.12088.x. [DOI] [PubMed] [Google Scholar]
- 29.Blanpain C, Lowry WE, Geoghegan A, Polak L, Fuchs E. Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell. 2004;118:635–48. doi: 10.1016/j.cell.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 30.Sato N, Leopold PL, Crystal RG. Induction of the hair growth phase in postnatal mice by localized transient expression of Sonic hedgehog. J Clin Invest. 1999;104:855–64. doi: 10.1172/JCI7691. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sfig1
Sfig2
Sfig3
Sfig4
Sfig5
Sfig6
Supp data
Supp table 4
Supp tables 1-