Differential Transmission of Human Immunodeficiency Virus Type 1 by Distinct Subsets of Effector Dendritic Cells (original) (raw)
Abstract
Dendritic cells (DC) support human immunodeficiency virus type 1 (HIV-1) transmission by capture of the virus particle in the mucosa and subsequent transport to the draining lymph node, where HIV-1 is presented to CD4+ Th cells. Virus transmission involves a high-affinity interaction between the DC-specific surface molecule DC-SIGN and the viral envelope glycoprotein gp120 and subsequent internalization of the virus, which remains infectious. The mechanism of viral transmission from DC to T cells is currently unknown. Sentinel immature DC (iDC) develop into Th1-promoting effector DC1 or Th2-promoting DC2, depending on the activation signals. We studied the ability of these effector DC subsets to support HIV-1 transmission in vitro. Compared with iDC, virus transmission is greatly upregulated for the DC1 subset, whereas DC2 cells are inactive. Increased transmission by DC1 correlates with increased expression of ICAM-1, and blocking studies confirm that ICAM-1 expression on DC is important for HIV transmission. The ICAM-1-LFA-1 interaction is known to be important for immunological cross talk between DC and T cells, and our results indicate that this cell-cell contact is exploited by HIV-1 for efficient transmission.
Human immunodeficiency virus type 1 (HIV-1) infects human CD4+ T cells via interactions between the viral envelope glycoprotein gp120 and the CD4 receptor and a chemokine coreceptor on the T cell (9). Sexual transmission of HIV-1 requires the help of dendritic cells (DC) to cross the mucosal barrier before infection of T cells can occur (19, 23, 33-35, 41, 43). DC residing in peripheral tissues are able to capture HIV-1 and to facilitate transport to a draining lymph node, which becomes the center of viral replication. Although HIV-1 can infect certain DC, such as Langerhans cells (4, 5, 16, 30, 47), other DC specifically bind HIV-1 and present the virus particle to T cells without becoming infected themselves (2, 3, 14, 16). The recently identified DC-specific receptor DC-SIGN (CD209) facilitates specific binding of HIV-1, HIV-2, and simian immunodeficiency virus (SIV) through interaction with the viral envelope glycoprotein gp120 and mediates internalization of virions, which remain in an infectious form in an intracellular compartment (11, 14, 24, 31). The mechanism of subsequent virus transmission to T cells remains unknown.
DC are professional antigen-presenting cells that take up antigen at sites of pathogen entry (1). Upon encounter with antigen, sentinel immature DC (iDC) develop into mature effector DC (mDC) that are specialized to stimulate naïve T cells. In vitro studies with monocyte-derived DC indicate that these effector DC express distinct molecules (20, 21). Depending on the type of pathogen and the microenvironment of the iDC, different subsets of effector DC develop, which promote the development of Th1 cells or Th2 cells from naïve precursors. In this way, the type of T-cell response is adapted to the type of invading pathogen and the source of infected tissue (21). These distinct subsets of effector DC bias the polarization of Th cells into Th1 cells (DC1), Th2 cells (DC2), or both (DC0) (8). The differential DC maturation is illustrated in Fig. 1A. Unbiased DC0 cells are obtained with maturation factors (MF), i.e., interleukin-1β (IL-1β) and tumor necrosis factor alpha (TNF-α), or lipopolysaccharide (LPS) and induce both IL-4-producing Th1 cells and gamma interferon (IFN-γ)-producing Th2 cells (37), of which the balance varies depending on the cell donor and the antigen dose (36). The presence of IFN-γ, double-stranded RNA (dsRNA) [poly(I-C)], or viral RNA induces the development of DC into effector DC1 cells, characterized by their capacity to promote Th1 responses in naïve T cells (6, 40, 44, 46). DC2 cells can be induced by cholera toxin, helminths, and prostaglandins, and these cells express high levels of OX40L that bias Th2 responses (10, 13, 22, 48).
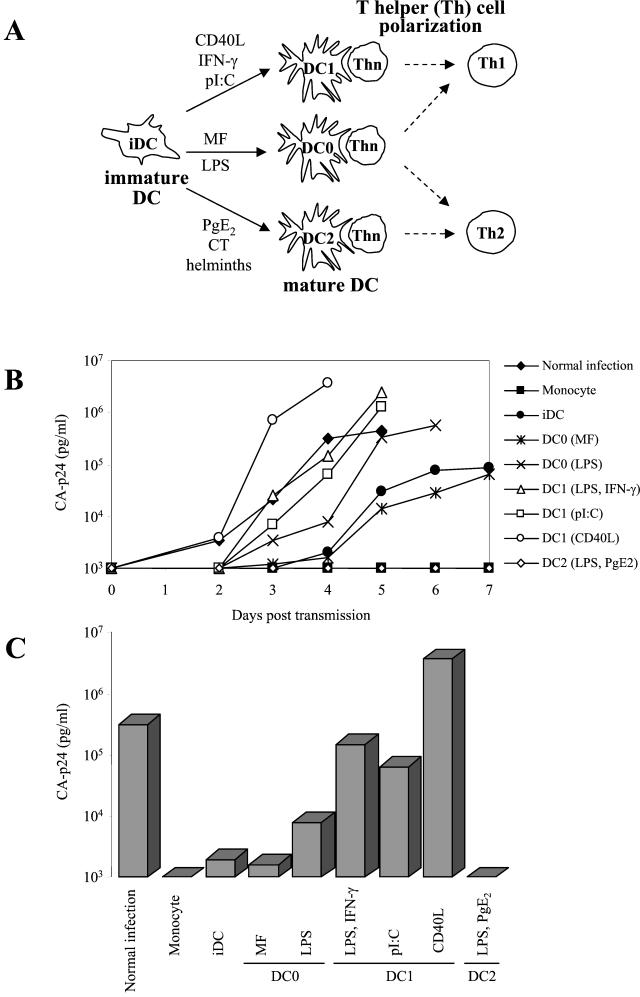
FIG. 1.
Differential HIV-1 transmission by DC subsets. (A) Maturation of iDC to obtain distinct subsets of mDC. Purified monocytes were cultured for 6 days in the presence of GM-CSF and IL-4 to obtain CD1a+ CD14− CD83− iDC. These iDC were then cultured with diverse stimuli for 2 additional days to obtain CD1a+ CD83+ mDC of the DC1, DC2, and DC0 types. The Th cell-polarizing capacities of the DC subsets are indicated. (B) Replication of HIV-1 in T cells after transmission by different subsets of DC. In brief, 50 × 103 DC were pulsed with HIV-1 LAI (150 pg of CA-p24 per well) for 2 h, and unbound virus was washed out, except in the control experiment without DC (normal infection). DC were subsequently cocultured with 50 × 103 SupT1 T cells, and virus spread in SupT1 cells after transmission was monitored for 7 days by CA-p24 production. (C) The same data from day 4 in panel B are represented as bars. Similar results were obtained in more than 10 independent experiments.
To study the ability of differentially matured DC to support HIV-1 transmission, we used an in vitro assay for DC-mediated HIV-1 infection of T cells. We found that the efficiency of virus transmission to T cells is largely influenced by the type of DC subset. The DC1 subset shows a markedly increased ability to mediate HIV-1 transmission compared to iDC, which correlates with increased surface expression of ICAM-1. Antibody blocking studies indicate that ICAM-1 plays an important role in transmission. The DC2 subset is very inefficient in HIV-1 transmission, and the DC0 cells display an intermediate phenotype, similar to iDC. Our observations suggest that the DC1 subset with high ICAM-1 expression is a key player in HIV-1 transmission and that cell-cell contact between the DC and the T cell, mediated by ICAM-1 and LFA-1, is instrumental in efficient virus transmission.
MATERIALS AND METHODS
Cytokines, antigens, and reagents.
Human recombinant granulocyte-macrophage colony-stimulating factor (rGM-CSF) (500 U/ml) was a gift from Schering-Plough, Uden, The Netherlands. Human recombinant IFN-γ (rIFN-γ) (1,000 U/ml) was a gift from P. H. van der Meide (Biomedical Primate Research Center, Rijswijk, The Netherlands). Human recombinant IL-2 (rIL-2) was obtained from Chiron, Amsterdam, The Netherlands. Human rIL-4 (250 U/ml) and human recombinant TNF-α (rTNF-α) (50 ng/ml) were obtained from PBH (Hannover, Germany). Human rIL-1β (10 ng/ml) was obtained from Boehringer Mannheim (Mannheim, Germany). Prostaglandin E2 (PgE2), poly(I-C) (Sigma, St. Louis, Minn.), and cholera toxin (CT; Sigma) were used at 10−6 M, 20 μg/ml, and 1 μg/ml, respectively. LPS (Difco, Detroit, Mich.) was used at a final concentration of 100 ng/ml. Superantigen Staphylococcus aureus enterotoxin B (SEB; Sigma Chemical Co., St. Louis, Mo.) was used at a final concentration of 1 ng/ml. Stromal cell-derived factor 1 (SDF-1) (R&D, Minneapolis, Minn.) was used at a final concentration of 2.0 μg/ml, and azidothymidine (AZT) was used at 10 μM. Antibodies to ICAM-1,2,3 and LFA-1,2,3 were acquired from Immunotech, Marseille, France, and used at final concentrations of 10 μg/ml for blocking experiments and 1 μg/ml for fluorescence-activated cell sorter (FACS) staining. The anti-DC-SIGN antibody was a gift from Yvette van Kooyk, Nijmegen, The Netherlands.
In vitro generation of iDC from PBMC and subsequent maturation.
Venous blood from healthy donors was collected by venipuncture in sodium-heparin-containing tubes (VT100H; Venoject, Terumo Europe, Leuven, Belgium). Peripheral blood mononuclear cells (PBMC) were isolated by density centrifugation on Lymphoprep (Nycomed, Torshov, Norway). Subsequently, PBMC were layered on a Percoll (Pharmacia, Uppsala, Sweden) gradient with three density layers (1.076, 1.059, and 1.045 g/ml). The light fraction with predominantly monocytes was collected, washed, and seeded in 24-well culture plates (Costar, Cambridge, Mass.) at a density of 5 × 105 cells per well. After 60 min at 37°C, nonadherent cells were removed, and adherent cells were cultured in Iscove's modified Dulbecco's medium (IMDM; Life Technologies Ltd., Paisley, United Kingdom) with gentamicin (86 μg/ml; Duchefa, Haarlem, The Netherlands) and 1% fetal clone serum (HyClone, Logan, Utah) and supplemented with GM-CSF and IL-4 to obtain iDC as described elsewhere (13). At day 3, the culture medium with supplements was refreshed. At day 6, CD1a+ CD14− iDC were treated with different reagents to initiate distinct maturation pathways.
Immature DC were treated either with MF (rIL-1β/rTNF-α), CT, LPS, or poly(I-C) or a combination of LPS and PgE2 or IFN-γ. Maturation in response to CD40 ligand (CD40L) was obtained by stimulation of iDC with irradiated mouse fibroblast cells (J558 cells) stably expressing CD40L. After 48 h, fully mature CD1a+ (>95%) CD83+ (>90%) mDC were obtained. The cytokine secretion profiles and Th-polarizing properties of different mDC will be described elsewhere. All subsequent tests were performed after harvesting and extensive washing of the cells to remove all induction factors.
Flow cytometry.
Mouse anti-human monoclonal antibodies (MAbs) against the following molecules were used: CD1a (OKT6; Ortho Diagnostic System, Beerse, Belgium); CD83 (Hb15a, immunoglobulin G2b [IgG2b]; Immunotech, Marseille, France); CD86 (IgG2a; Innogenetics, Ghent, Belgium); ICAM-1, ICAM-2, and ICAM-3 (all three obtained from R&D Systems, Abingdon, United Kingdom); CD4 (Becton Dickinson, San Jose, Calif.); CCR5 and CXCR4 (both obtained from PharMingen, San Diego, Calif.); and DC-SIGN. Bound MAbs were detected by fluorescein isothiocyanate (FITC)-conjugated goat F(ab′)2 anti-mouse IgG and IgM (Jackson Immunoresearch Laboratories Inc., West Grove, Pa.). Samples were analyzed with a FACScan (Becton Dickinson).
T cells.
CD4+ CD45RA+ CD45RO naïve Th cells (>98% pure as assessed by flow cytometry) were purified from peripheral blood lymphocytes (PBL; heterologous to DC) by using a human CD4+ CD45RO− column kit (R&D). Naïve T cells, PBL, and the SupT1 T-cell line were cultured in RPMI medium (Life Technologies Ltd., Paisley, United Kingdom) supplemented with 10% fetal calf serum, penicillin (100 U/ml), and streptomycin (100 μg/ml). For naïve T cells and PBL, we also added rIL-2 (100 U/ml) and SEB.
Virus stocks.
SupT1 T cells and C33A cervix carcinoma cells were transfected by electroporation and CaPO4 precipitation, respectively, with 10 μg of the molecular clone of the T-tropic HIV-1 LAI strain as described previously (7). The virus-containing supernatant was harvested 3 days posttransfection, filtered, and stored at −80°C. The concentration of virus was measured by CA-p24 enzyme-linked immunosorbent assay (ELISA).
Mixed lymphocyte reaction.
Mature DC were tested for their ability to stimulate allogeneic naïve T cells in a mixed lymphocyte reaction (MLR). CD4+ CD45RA+ CD45RO− Th cells (2.5 × 105/200 μl) were cocultured in 96-well flat-bottom culture plates with increasing numbers of different mDC. T-cell proliferation was measured after 5 days by the thymidine incorporation assay. [3H]thymidine (0.3 μQ/well; Radiochemical Centre, Amersham, Little Chalfont, United Kingdom) was added to the culture for 16 h, and the sample was analyzed by liquid scintillation counting.
HIV capture by DC.
DC (150 × 103/100 μl per well) were incubated with a high virus dose (20 ng of CA-p24) for 2 h at 37°C. Cells were washed extensively with phosphate-buffered saline (PBS) to remove unbound virus and were subsequently lysed to release the captured CA-p24, which was measured by ELISA.
HIV-1 transmission assay.
For the HIV-1 transmission assay, a previously described assay (14, 32) was used with some modifications. In short, iDC or fully mature CD83+ mDC were incubated on a 96-well plate (40 × 103 cells/100 μl per well) with virus (0.15 ng of CA-p24 per well, unless indicated otherwise) for 2 h in RPMI medium. We used the CXCR4-using primary virus isolate LAI. The DC were washed twice with PBS to remove unbound virus and cocultured with T cells (40 × 103/100 μl per well) for 7 days in RPMI medium. Virus spread in T cells was measured with the CA-p24 ELISA, and the cultures were inspected for the appearance of HIV syncytia. The CA-p24 values at day 4 postinfection are shown for most experiments. In some experiments, DC were incubated with AZT, SDF-1, or anti-ICAM-1 antibodies during the virus pulse. These inhibitors were washed out together with unbound virus prior to coculture with T cells. Maturation of DC by CD40L was performed with irradiated mouse cells stably expressing CD40L (J558 cells). Because these cells are present in the transmission assay, we tested their ability to promote HIV-1 transmission. The irradiated mouse cells do not support HIV-1 transmission. To control for the presence of contaminating T cells and the presence of residual free virus after washing, we performed transmission experiments with the precursor monocytes, which were consistently negative in transmission. In some experiments, T cells were added to virus-preincubated DC in a Transwell culture dish, such that both types of cells were separated by a membrane. To test the effect of soluble DC-derived factors on HIV-1 transmission, we collected the supernatants of immature DC and differentially matured DC, which were activated with J558-CD40L for 24 h. Supernatants were harvested, filtered, and frozen at −20°C before use in the infection experiments.
RESULTS
DC subsets differ significantly in their ability to transmit HIV-1.
Monocyte-derived iDC were treated with different stimuli to generate distinct subsets of CD83+ mDC. The different maturation pathways are illustrated in Fig. 1A, and the expression of the maturation marker CD83 is included in the FACS analysis of Fig. 3. The final state of maturation was confirmed by upregulation of CD80, CD86, and HLA-DR, downregulation of the mannose receptor, and the loss of phagocytotic capacity (results not shown). Based on their ability to induce the development of IFN-γ-producing Th1 cells or IL-4-producing Th2 cells from naïve precursors, the mDC were designated DC1 or DC2, and unbiased mDC were termed “DC0.” These effector phenotypes are stable over time. A detailed description of the DC subsets, their cytokine production profile, and their T-cell effector function is presented elsewhere (8). DC1 cells were generated either by IFN-γ treatment or by poly(I-C) stimulation. We also obtained cells with DC1-like properties by treatment of iDC with CD40L. Stimulation of iDC with CT or with LPS plus PgE2 resulted in two types of DC2 cells. The DC0 cells were induced with maturation factor IL-1β plus TNF-α or LPS. In this study, we analyzed the DC subsets for their ability to transmit HIV-1 to CD4+ T cells.
FIG. 3.
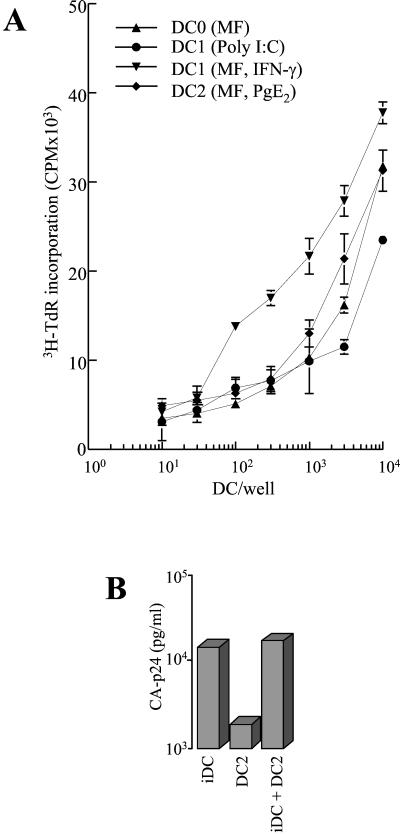
(A) mDC subsets and stimulation of T cells. iDC were matured as described in the legend to Fig. 1. mDC (10 × 104 cells per well) were cultured in different numbers with 2.5 × 105 allogeneic naïve (CD45RA+) CD4+ T cells. After 5 days, T-cell proliferation was measured by [3H]thymidine incorporation (shown in cpm). Similar results were obtained with DC from two other donors. (B) HIV-1 transmission in mixed DC cultures. Transmission was performed with iDC, DC2, and a mixture of iDC and DC2. The CA-p24 values measured at day 4 posttransmission are shown.
We used a previously described transmission assay with some modifications (14, 32). DC were incubated for 2 h with the CXCR4-using primary HIV-1 isolate LAI, washed extensively to remove unbound virus, and cocultured with SupT1 T cells for 7 days. Virus spread was measured in the culture supernatant by CA-p24 ELISA. The results of a representative transmission experiment with the different DC subsets are shown in Fig. 1, which represents the replication kinetics after transmission (Fig. 1B) and the CA-p24 values at day 4 (Fig. 1C). Consistent with previous results, iDC were able to transfer HIV-1 to T cells. Interestingly, we observed profound differences for the three classes of mDC. All DC1 samples displayed a dramatically increased HIV-1 transmission capacity compared to iDC. The DC1 transmission efficiency is comparable to that of direct infection of T cells without the wash to remove unbound virus. The DC2 cells were largely inactive, and the DC0 cells showed an intermediate transmission activity similar to that of iDC. Similar results were obtained with DC derived from different donors (Table 1). To control for the presence of contaminating T cells and the presence of residual free virus after washing, we performed transmission experiments with the precursor monocytes, which were consistently negative in transmission, presumably because these cells lack DC-SIGN (15). DC in the absence of T cells did not support virus replication (results not shown).
TABLE 1.
Virus transmission by DC derived from different donors
| DC type | Virus spread (pg of CA-p24/ml)a | ||||
|---|---|---|---|---|---|
| Donor 1 | Donor 2 | Donor 3 | Donor 4 | Donor 5 | |
| iDC | 240,000 | 7,800 | 2,000 | 22,000 | 14,600 |
| DC0b | 44,000 | <1,000 | 1,600 | 6,500 | NDe |
| DC1c | 250,000 | 720,000 | 65,000 | 36,000 | 145,000 |
| DC2d | 6,400 | <1,000 | <1,000 | 3,500 | 1,800 |
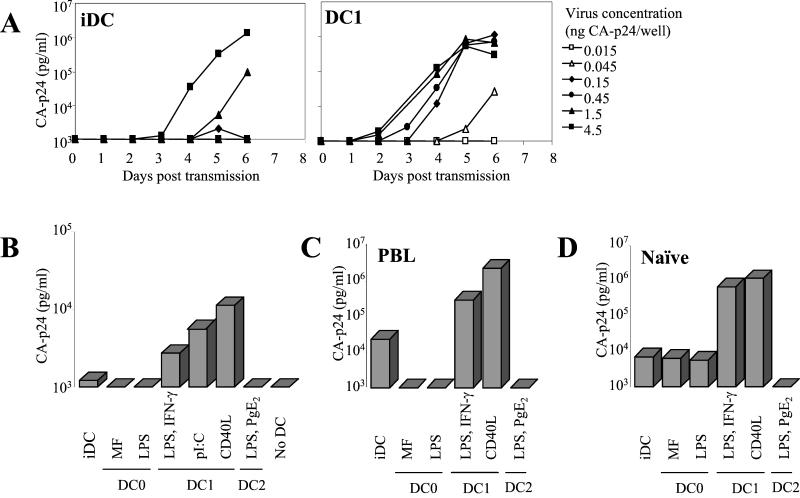
We titrated the amount of HIV-1 in the transmission assay with the relatively inefficient transmitter iDC and the efficient transmitter DC1 (Fig. 2A). A virus dose of only 45 pg of CA-p24 is sufficient for DC1 cells to initiate a spreading infection in T cells, but at least 30-fold more virus is needed with iDC as a transmitter. In another experiment, we omitted the wash step that removes unbound virus (Fig. 2B). This experiment was performed with an extremely low virus dose (15 pg of CA-p24) that is not sufficient to initiate a spreading infection of T cells in the absence of DC. T-cell infection can be rescued with iDC, but again the DC1 subset is the most efficient virus transmitter. We further analyzed DC-mediated transmission to primary T cells. The different effector DC display the same relative transmission efficiencies in a coculture with heterologous PBL (Fig. 2C). Experiments with purified naïve T cells produced similar results (Fig. 2D), indicating that DC1 cells are particularly suited for HIV-1 transmission.
FIG. 2.
DC1 cells are superior in HIV-1 transmission. (A) Transmission assay as described in the legend of Fig. 1B. The amount of virus incubated with iDC or DC1 was titrated, and virus spread in SupT1 cells was measured. The DC1 cells were obtained by CD40L stimulation. (B) DC-mediated enhancement of HIV-1 infection. A minimal dose of 15 pg of CA-p24 per well of C33A-produced virus was used to infect T cells in the absence or presence of distinct DC subsets. No wash step was performed to remove unbound virus. (C and D) Transmission to primary T cells. DC were preincubated with virus (150 pg of CA-p24 per well), subsequently washed, and cocultured with PBL (C) or naïve T cells (Thn) (D). The CA-p24 values of day 4 posttransmission are shown.
To investigate whether differences in the induction of T-cell proliferation by the DC subsets could account for the observed differences in virus spread in T cells, we performed MLR. DC0, DC1, and DC2 subsets were incubated with naïve T cells, and T-cell proliferation was measured by [3H]thymidine incorporation (Fig. 3A). Among the mDC subsets, we measured no significant difference in their capacity to induce T-cell proliferation, supporting the idea that the DC subsets differ in their ability to transmit HIV-1. We also performed transmission experiments with a mixture of the efficiently transmitting iDC cells and the inactive DC2 cells (Fig. 3B). The presence of DC2 cells did not inhibit the efficient transmission obtained with iDC cells. This result confirms that the inability of DC2 cells to transmit virus is not due to inhibition of T-cell proliferation. Instead, we reason that DC2 cells lack a factor that is critical for HIV-1 transmission.
Phenotypic analysis of DC subsets.
We analyzed several DC surface markers to elucidate the mechanism underlying the profound differences in transmission efficiency. We thereby focused on molecules that are important in DC-HIV and DC-T-cell interactions. The DC marker CD1a and DC maturation markers CD83 and CD86 were included as controls. Binding of the virus particle is mediated by DC-SIGN, which is expressed upon generation of iDC from monocytes (results not shown [15]). FACS analyses showed that DC-SIGN expression is slightly decreased upon DC maturation, but to a similar extent for the different mDC subsets (Fig. 4). Next, we investigated the expression of the HIV receptors (and coreceptors) as factors for potential entry of HIV-1 in DC (results not shown). CD4 was not differentially expressed, and CCR5 was in fact downregulated on all mDC subsets. Elevated CXCR4 expression was only apparent for the DC2 subset, but this subset has a poor capacity to transmit HIV-1. Downregulation of CCR5 and upregulation of CXCR4 during maturation of DC have been described previously (38). We then focused on adhesion molecules on DC that are involved in DC-T-cell contacts. Most of these molecules, such as LFA-1, ICAM-3, CD40, and CD86, are equally expressed on the DC subsets, and ICAM-2 is not expressed on mDC (Fig. 4) (data not shown [40]). Interestingly, ICAM-1 varies significantly among the DC subsets, and its expression correlates with the transmission capacity (Fig. 4). In particular, DC1 cells have higher levels of ICAM-1 expression than iDC, DC2, and DC0 cells. Similar results were obtained with DC derived from different donors (Table 2). This raises the interesting possibility that DC1 cells transmit HIV-1 efficiently because these cells interact more efficiently with the recipient T cell through the well-established ICAM-1-LFA-1 interaction.
FIG. 4.
DC1 cells express enhanced levels of ICAM-1 but not DC-SIGN. iDC were matured as described in the legend to Fig. 1. The expression of CD1a, CD86, CD83, ICAM-1, ICAM-3, and DC-SIGN was determined by FACS. (The broken line represents the isotype-matched control, and the solid line represents the specific staining.) The mean fluorescence intensity is indicated. Similar results were obtained with DC of four different donors (see also Table 2).
TABLE 2.
CD83 and ICAM-1 expression by DC derived from different donors
| MAb target | Mean fluorescence intensitya | ||||
|---|---|---|---|---|---|
| Donor 1 | Donor 2 | Donor 3 | Donor 4 | Donor 5 | |
| CD83 | |||||
| iDC | 0 | 0 | 0 | 0 | 0 |
| DC0b | 27 | 86 | 79 | 65 | 15 |
| DC1c | 32 | 100 | 140 | 58 | 14 |
| DC2d | 40 | 128 | NDe | 67 | 21 |
| ICAM-1 | |||||
| iDC | 109 | 329 | 347 | 268 | 78 |
| DC0b | 356 | 827 | 1,068 | 640 | 107 |
| DC1c | 639 | 1,899 | 2,279 | 992 | 306 |
| DC2d | 185 | 311 | ND | 207 | 122 |
Cell-cell contact by means of the ICAM-1-LFA-1 interaction facilitates HIV-1 transmission.
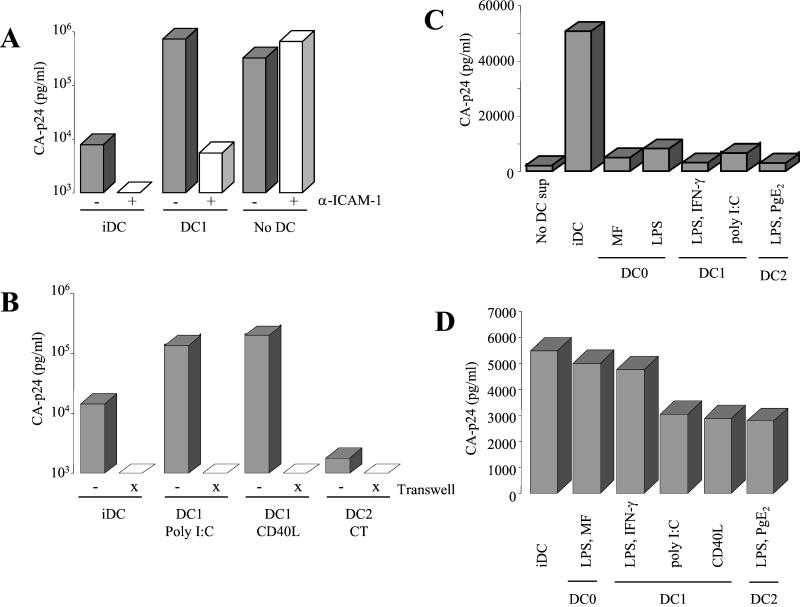
Antibody blocking studies were performed to establish the importance of ICAM-1 expression for DC-mediated virus transmission (Fig. 5A). Virus transmission was performed in the presence or absence of an antibody to ICAM-1 that blocks LFA-1 binding. DC1-mediated transmission was compared with that of iDC, and a normal infection (without a wash step) served as a control experiment. Blocking of ICAM-1 greatly reduced the transmission efficiency of both iDC and DC1 cells, but the spreading infection in T cells was not affected. Preincubation of DC with antibodies to LFA-1 or ICAM-3 did not block virus transmission to T cells (results not shown). To test whether direct cell-cell contact is required for the efficient virus transmission observed for DC1 cells, we performed an experiment with the DC (preincubated with virus) and T cells in two compartments separated by a membrane that is permeable only for virus particles (transwell culture dish; Fig. 5B). Virus transmission from DC1 cells to T cells was completely abolished in the transwell experiment, indicating the requirement for cell-cell contact. The same result was obtained in transmission studies with iDC. Thus, iDC are also likely to use the ICAM-1-LFA-1 interaction, which is confirmed by the blocking experiment with anti-ICAM-1 antibodies (Fig. 5A). Alternatively, it is possible that DC-secreted factors play a role in the transmission of HIV-1 to T cells. To test this, we performed a regular T-cell infection in the presence of conditioned DC medium (Fig. 5C). The supernatants of the mDC subsets did not affect the T-cell infection rate significantly, but the supernatant from iDC displayed an enhancement effect. Similar results were obtained when the supernatant was added 1 day postinfection (results not shown), suggesting that the stimulatory effect of the iDC supernatant is not at the level of virus entry. Combined with the slightly elevated DC-SIGN expression on iDC, these results provide an explanation for the fair transmission capacity of iDC, despite a moderate ICAM-1 level. Most importantly, these results exclude the possibility that the high transmission capacity of DC1 is due to a soluble factor. These observations suggest that the interaction between ICAM-1 on DC1 and LFA-1 on T cells is critical for efficient HIV-1 transmission.
FIG. 5.
Cell-cell contact via ICAM-1-LFA-1 is essential for DC1-mediated transmission. (A) Blocking of ICAM-1. Transmission of HIV-1 by DC was performed in the absence or presence of a neutralizing anti-ICAM-1 antibody. The antibody was present in the 2-h DC-HIV-1 incubation and washed out together with unbound virus prior to coculture with T cells. The DC1 cells used in this experiment were obtained by poly(I-C) stimulation. The right two bars represent a normal infection control. No wash was performed with these samples. (B) DC-mediated transmission was performed with or without a permeable membrane (transwell) between the virus-preincubated DC and T cells. (C) Effect of DC-conditioned medium on regular T-cell infection. SupT1 cells were infected with HIV-1 (150 pg of CA-p24) in the presence or absence of the respective DC supernatants. The CA-p24 values measured at day 3 postinfection are shown. (D) Virus capture by DC. DC (150 × 103) were incubated with virus (20 ng of CA-p24) for 2 h. After extensive washing, DC were lysed, and bound virus was quantitated by ELISA.
DC1 appear superior in the second phase of virus transmission, which is the presentation of HIV-1 to T cells. Based on the similar level of DC-SIGN expression on iDC and all mDC subsets, one would predict that DC1 is not special in the first phase of transmission, which is virus binding. To test this, iDC and the mDC subsets were incubated with HIV-1 for 2 h, washed extensively, and subsequently lysed to quantitate the amount of bound virus by CA-p24 ELISA. We measured a similar virus-binding activity with DC1, iDC, and DC0 (Fig. 5D). This result is consistent with a previous study indicating that the levels of efficiency of virus uptake are similar for iDC and mDC, although a different uptake mechanism was proposed (11).
DISCUSSION
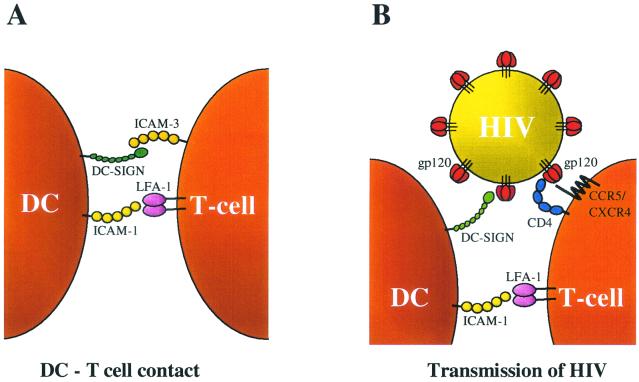
We studied the transmission of HIV by different subsets of effector DC (mDC) that were obtained from monocyte-derived iDC through distinct maturation pathways. These mDC display profound differences in their ability to support HIV-1 transmission to T cells. Compared with iDC, the DC1 subset shows greatly improved virus transmission efficiency to both a T-cell line and PBL target cells. In contrast, DC2 cells are poor transmitters, which is not caused by a negative impact of DC2 on the T cells. Unbiased mDC show an intermediate ability to mediate HIV transmission. We set out to analyze the mechanism of increased transmission by DC1. It could be excluded that secreted factors determine the enhanced transmission capacity of DC1. A major factor in the DC-HIV-1 interactions is the recently identified DC-SIGN molecule that binds the viral envelope glycoprotein gp120 (14, 15). We show that all mDC subsets express approximately equal levels of DC-SIGN (Fig. 4), and virus capture was similar for the DC subsets tested (Fig. 5D). Thus, there must be another reason for the increased transmission capacity of the DC1 subset. Our results indicate that increased ICAM-1 expression, which is observed exclusively for the DC1 subset, plays a major role in virus transmission to T cells by facilitating cell-cell contact. First, we demonstrated that DC-T-cell contact is critical for transmission in the transwell experiment (Fig. 5B). Second, HIV-1 transmission by DC1 could be blocked, albeit not completely, by anti-ICAM-1 antibodies (Fig. 5A). The partial inhibition may indicate that other cell surface molecules participate in the DC-T-cell contact. These combined results are translated in the transmission model shown in Fig. 6, which depicts three essential interactions: (i) the DC-HIV interaction through DC-SIGN-gp120; (ii) the DC-T-cell interaction through ICAM-1-LFA-1, and (iii) the HIV-T-cell interactions through gp120-CD4 and CCR5/CXCR4. In this model, the DC-SIGN arm of the DC binds HIV-1 and the ICAM-1 arm binds the T cell, thus juxtaposing the virus particle and the T-cell surface. The ICAM-1-LFA-1 interaction also plays important roles in immune reactions, e.g., in DC1-induced Th1 polarization (27, 39). Thus, HIV exploits the human immune system in its mode of transmission.
FIG. 6.
Transmission model. (A) In regular DC-T-cell contacts, the initial interaction involves ICAM-1 and LFA-1, as well as DC-SIGN and ICAM-3 (15). (B) In HIV-1 transmission, DC-SIGN captures the virus particle through interaction with gp120 (14). When the DC subsequently contact T cells through the ICAM-1-LFA-1 interaction, HIV-1 is juxtaposed to the T-cell surface with the CD4 and CXCR4 receptors.
An extensive microarray-proteomics analysis of the gene expression profile of maturing DC was recently reported (26). Interestingly, this survey showed decreased expression of integrins and other cell adhesion molecules and increased expression of cell motility genes, which is consistent with the enhanced migration properties of mature DC. Increased ICAM-1 expression may therefore be a relatively unique property of the DC1 subset that allows the functional interaction with T cells in the lymph node.
ICAM-1 has been implicated in DC-T-cell contacts through interaction with LFA-1 (45), and we now propose that this interaction facilitates HIV transmission. As an alternative explanation of our results, increased ICAM-1 expression on DC1 cells could establish a stronger DC-HIV interaction through binding of virion-associated LFA-1 (12). In fact, HIV and SIV specifically incorporate cellular adhesion molecules such as LFA-1, and these molecules facilitate adhesion of virions to T cells and thereby enhance viral infectivity (18, 29). The incorporation of LFA-1 on HIV-1 particles varies with the cell type that is used for virus production. To critically test this hypothesis, we produced virus in cell types that do not express LFA-1 (and ICAM-1). Similar transmission results were obtained with these virus stocks, including efficient transmission with DC1 cells (Fig. 2B) (results not shown). Furthermore, we measured no difference in virus binding capacity for iDC and different mDC subsets. Thus, the ICAM-1 interaction partner, LFA-1, is required on the T cell to facilitate the DC-T-cell contact. This cell-cell contact may trigger intracellular events in the T cell that favor productive infection. For instance, ICAM-1 binding to LFA-1 has been demonstrated to upregulate the activities of phosphatidylinositol 3-kinase, sphingomyelinase, and c-Jun N-terminal kinase (28).
There is evidence that M-tropic HIV-1 isolates can infect iDC through the CCR5 coreceptor (16, 17, 35). To exclude DC infection in our transmission assay, we used the CXCR4-using LAI primary isolate. CXCR4 is not expressed on iDC, but could be upregulated during maturation, coinciding with increased entry of CXCR4-using HIV-1 (5, 25, 39, 49). We therefore analyzed the cell surface expression of the HIV receptor CD4 and coreceptors CCR5 and CXCR4, but did not measure significant differences among the mDC. Furthermore, we did not observe any virus replication in prolonged DC cultures without T cells, and the presence of antiviral compounds like AZT and SDF-1α in the DC1 cell-virus preincubation step did not affect transmission in the regular assay (results not shown). We therefore exclude the possibility that DC infection plays a major role in this transmission assay.
This study provides new insight into the mechanism of virus transmission from DC to T cells. The in vivo biological significance of the increased transmission that we observed with DC1 cells remains to be elucidated. We speculate that HIV can enhance its own transmission by a selective trigger of maturation of this specific DC1 subset. In this scenario, subepithelial DC-SIGN+ iDC would capture HIV originating from infected epithelial DC-SIGN− DC (Langerhans cells) that are permissive for HIV infection (42). The iDC-virus complex would subsequently start to migrate, coinciding with maturation into DC1 cells. At the time of encounter with T cells in the lymph node, increased ICAM-1 expression on the DC1 surface will facilitate the DC-T-cell contact and thus stimulate the transfer of the virus particle to the T cell. Alternatively, prior or simultaneous infection by bacterial or viral pathogens may enhance the chance of HIV-1 transmission by local enrichment of mature cells of the DC1 type. The highly efficient in vitro transmission assay with DC1 cells that we report in this study provides a framework for further mechanistic studies and for the screening of potential transmission inhibitors.
Acknowledgments
We thank Melissa Pope and John Moore for critical reading of the manuscript and Bill Paxton and Moustapha Chalaby for helpful discussions.
This work was sponsored in part by the Dutch AIDS Fund (Amsterdam).
REFERENCES
- 1.Banchereau, J., and R. M. Steinman. 1998. Dendritic cells and the control of immunity. Nature 392**:**245-252. [DOI] [PubMed] [Google Scholar]
- 2.Blauvelt, A., H. Asada, M. W. Saville, V. Klaus-Kovtun, D. J. Altman, R. Yarchoan, and S. I. Katz. 1997. Productive infection of dendritic cells by HIV-1 and their ability to capture virus are mediated through separate pathways. J. Clin. Investig. 100**:**2043-2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cameron, P. U., P. S. Freudenthal, J. M. Barker, S. Gezelter, K. Inaba, and R. M. Steinman. 1992. Dendritic cells exposed to human immunodeficiency virus type-1 transmit a vigorous cytopathic infection to CD4+ T cells. Science 257**:**383-387. [DOI] [PubMed] [Google Scholar]
- 4.Cameron, P. U., M. G. Lowe, S. M. Crowe, U. O'Doherty, M. Pope, S. Gezelter, and R. M. Steinman. 1994. Susceptibility of dendritic cells to HIV-1 infection in vitro. J. Leukoc. Biol. 56**:**257-265. [DOI] [PubMed] [Google Scholar]
- 5.Canque, B., Y. Bakri, S. Camus, M. Yagello, A. Benjouad, and J. C. Gluckman. 1999. The susceptibility to X4 and R5 human immunodeficiency virus-1 strains of dendritic cells derived in vitro from CD34+ hematopoietic progenitor cells is primarily determined by their maturation stage. Blood 93**:**3866-3875. [PubMed] [Google Scholar]
- 6.Cella, M., M. Salio, Y. Sakakibara, H. Langen, I. Julkunen, and A. Lanzavecchia. 1999. Maturation, activation, and protection of dendritic cells induced by double-stranded RNA. J. Exp. Med. 189**:**821-829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Das, A. T., B. Klaver, and B. Berkhout. 1999. A hairpin structure in the R region of the human immunodeficiency virus type 1 RNA genome is instrumental in polyadenylation site selection. J. Virol. 73**:**81-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Jong, E. C., P. L. Vieira, P. Kalinski, J. H. Schuitemaker, Y. Tanaka, E. A. Wierenga, M. Yazdanbakhsh, and M. L. Kapsenberg. 2002. Microbial compounds selectively induce Th1 cell-promoting or Th2-cell promoting dendritic cells in vitro with diverse Th cell-polarizing signals. J. Immunol. 168**:**1704-1709. [DOI] [PubMed] [Google Scholar]
- 9.Doms, R. W., and J. P. Moore. 2000. HIV-1 membrane fusion: targets of opportunity. J. Cell Biol. 151**:**F9-F14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flynn, S., K. M. Toellner, C. Raykundalia, M. Goodall, and P. Lane. 1998. CD4 T cell cytokine differentiation: the B cell activation molecule, OX40 ligand, instructs CD4 T cells to express interleukin 4 and upregulates expression of the chemokine receptor, Blr-1. J. Exp. Med. 188**:**297-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frank, I., M. Piatak, Jr., H. Stoessel, N. Romani, D. Bonnyay, J. D. Lifson, and M. Pope. 2002. Infectious and whole inactivated simian immunodeficiency viruses interact similarly with primate dendritic cells (DCs): differential intracellular fate of virions in mature and immature DCs. J. Virol. 76**:**2936-2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fujiwara, M., R. Tsunoda, S. Shigeta, T. Yokota, and M. Baba. 1999. Human follicular dendritic cells remain uninfected and capture human immunodeficiency virus type 1 through CD54-CD11a interaction. J. Virol. 73**:**3603-3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gagliardi, M. C., F. Sallusto, M. Marinaro, A. Langenkamp, A. Lanzavecchia, and M. T. De Magistris. 2000. Cholera toxin induces maturation of human dendritic cells and licenses them for Th2 priming. Eur. J. Immunol. 30**:**2394-2403. [DOI] [PubMed] [Google Scholar]
- 14.Geijtenbeek, T. B., D. S. Kwon, R. Torensma, S. J. van Vliet, G. C. van Duijnhoven, J. Middel, I. L. Cornelissen, H. S. Nottet, V. N. KewalRamani, D. R. Littman, C. G. Figdor, and Y. van Kooyk. 2000. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell 100**:**587-597. [DOI] [PubMed] [Google Scholar]
- 15.Geijtenbeek, T. B., R. Torensma, S. J. van Vliet, G. C. van Duijnhoven, G. J. Adema, Y. van Kooyk, and C. G. Figdor. 2000. Identification of DC-SIGN, a novel dendritic cell-specific ICAM-3 receptor that supports primary immune responses. Cell 100**:**575-585. [DOI] [PubMed] [Google Scholar]
- 16.Granelli-Piperno, A., E. Delgado, V. Finkel, W. Paxton, and R. M. Steinman. 1998. Immature dendritic cells selectively replicate macrophagetropic (M-tropic) human immunodeficiency virus type 1, while mature cells efficiently transmit both M- and T-tropic virus to T cells. J. Virol. 72**:**2733-2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Granelli-Piperno, A., V. Finkel, E. Delgado, and R. M. Steinman. 1999. Virus replication begins in dendritic cells during the transmission of HIV-1 from mature dendritic cells to T cells. Curr. Biol. 9**:**21-29. [DOI] [PubMed] [Google Scholar]
- 18.Hioe, C. E., L. Bastiani, J. E. Hildreth, and S. Zolla-Pazner. 1998. Role of cellular adhesion molecules in HIV type 1 infection and their impact on virus neutralization. AIDS Res. Hum. Retrovir. 14(Suppl. 3)**:**S247-S254. [PubMed] [Google Scholar]
- 19.Hu, J., M. B. Gardner, and C. J. Miller. 2000. Simian immunodeficiency virus rapidly penetrates the cervicovaginal mucosa after intravaginal inoculation and infects intraepithelial dendritic cells. J. Virol. 74**:**6087-6095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang, Q., D. Liu, P. Majewski, L. Schulte, J. M. Korn, R. A. Young, E. S. Lander, and N. Hacohen. 2001. The plasticity of dendritic cell responses to pathogens and their components. Science 294**:**870-875. [DOI] [PubMed] [Google Scholar]
- 21.Kalinski, P., C. M. Hilkens, E. A. Wierenga, and M. L. Kapsenberg. 1999. T-cell priming by type-1 and type-2 polarized dendritic cells: the concept of a third signal. Immunol. Today 20**:**561-567. [DOI] [PubMed] [Google Scholar]
- 22.Kalinski, P., J. H. Schuitemaker, C. M. Hilkens, and M. L. Kapsenberg. 1998. Prostaglandin E2 induces the final maturation of IL-12-deficient CD1a+ CD83+ dendritic cells: the levels of IL-12 are determined during the final dendritic cell maturation and are resistant to further modulation. J. Immunol. 161**:**2804-2809. [PubMed] [Google Scholar]
- 23.Kawamura, T., S. S. Cohen, D. L. Borris, E. A. Aquilino, S. Glushakova, L. B. Margolis, J. M. Orenstein, R. E. Offord, A. R. Neurath, and A. Blauvelt. 2000. Candidate microbicides block HIV-1 infection of human immature Langerhans cells within epithelial tissue explants. J. Exp. Med. 192**:**1491-1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kwon, D. S., G. Gregorio, N. Bitton, W. A. Hendrickson, and D. R. Littman. 2002. DC-SIGN-mediated internalization of HIV is required for trans-enhancement of T cell infection. Immunity 16**:**135-144. [DOI] [PubMed] [Google Scholar]
- 25.Lee, B., M. Sharron, L. J. Montaner, D. Weissman, and R. W. Doms. 1999. Quantification of CD4, CCR5, and CXCR4 levels on lymphocyte subsets, dendritic cells, and differentially conditioned monocyte-derived macrophages. Proc. Natl. Acad. Sci. USA 96**:**5215-5220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Le Naour, F., L. Hohenkirk, A. Grolleau, D. E. Misek, P. Lescure, J. D. Geiger, S. Hanash, and L. Beretta. 2001. Profiling changes in gene expression during differentiation and maturation of monocyte-derived dendritic cells using both oligonucleotide microarrays and proteomics. J. Biol. Chem. 276**:**17920-17931. [DOI] [PubMed] [Google Scholar]
- 27.Luksch, C. R., O. Winqvist, M. E. Ozaki, L. Karlsson, M. R. Jackson, P. A. Peterson, and S. R. Webb. 1999. Intercellular adhesion molecule-1 inhibits interleukin 4 production by naive T cells. Proc. Natl. Acad. Sci. USA 96**:**3023-3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ni, H. T., M. J. Deeths, W. Li, D. L. Mueller, and M. F. Mescher. 1999. Signaling pathways activated by leukocyte function-associated Ag-1-dependent costimulation. J. Immunol. 162**:**5183-5189. [PubMed] [Google Scholar]
- 29.Orentas, R. J., and J. E. Hildreth. 1993. Association of host cell surface adhesion receptors and other membrane proteins with HIV and SIV. AIDS Res. Hum. Retrovir. 9**:**1157-1165. [DOI] [PubMed] [Google Scholar]
- 30.Patterson, S., A. Rae, N. Hockey, J. Gilmour, and F. Gotch. 2001. Plasmacytoid dendritic cells are highly susceptible to human immunodeficiency virus type 1 infection and release infectious virus. J. Virol. 75**:**6710-6713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pöhlmann, S., F. Baribaud, B. Lee, G. J. Leslie, M. D. Sanchez, K. Hiebenthal-Millow, J. Münch, F. Kirchhoff, and R. W. Doms. 2001. DC-SIGN interactions with human immunodeficiency virus type 1 and 2 and simian immunodeficiency virus. J. Virol. 75**:**4664-4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pope, M., M. G. Betjes, N. Romani, H. Hirmand, P. U. Cameron, L. Hoffman, S. Gezelter, G. Schuler, and R. M. Steinman. 1994. Conjugates of dendritic cells and memory T lymphocytes from skin facilitate productive infection with HIV-1. Cell 78**:**389-398. [DOI] [PubMed] [Google Scholar]
- 33.Pope, M., S. Gezelter, N. Gallo, L. Hoffman, and R. M. Steinman. 1995. Low levels of HIV-1 infection in cutaneous dendritic cells promote extensive viral replication upon binding to memory CD4+ T cells. J. Exp. Med. 182**:**2045-2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reece, J. C., A. J. Handley, E. J. Anstee, W. A. Morrison, S. M. Crowe, and P. U. Cameron. 1998. HIV-1 selection by epidermal dendritic cells during transmission across human skin. J. Exp. Med. 187**:**1623-1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rowland-Jones, S. L. 1999. HIV: the deadly passenger in dendritic cells. Curr. Biol. 9**:**R248-R250. [DOI] [PubMed] [Google Scholar]
- 36.Sallusto, F., M. Cella, C. Danieli, and A. Lanzavecchia. 1995. Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment: downregulation by cytokines and bacterial products. J. Exp. Med. 182**:**389-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sallusto, F., and A. Lanzavecchia. 1994. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J. Exp. Med. 179**:**1109-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sallusto, F., A. Lanzavecchia, and C. R. Mackay. 1998. Chemokines and chemokine receptors in T-cell priming and Th1/Th2-mediated responses. Immunol. Today 19**:**568-574. [DOI] [PubMed] [Google Scholar]
- 39.Salomon, B., and J. A. Bluestone. 1998. LFA-1 interaction with ICAM-1 and ICAM-2 regulates Th2 cytokine production. J. Immunol. 161**:**5138-5142. [PubMed] [Google Scholar]
- 40.Smits, H. H., E. C. de Jong, J. H. Schuitemaker, T. B. Geijtenbeek, Y. van Kooyk, M. L. Kapsenberg, and E. A. Wierenga. 2002. Intercellular adhesion molecule-1/LFA-1 ligation favors human Th1 development. J. Immunol. 168**:**1710-1716. [DOI] [PubMed] [Google Scholar]
- 41.Stahl-Hennig, C., R. M. Steinman, K. Tenner-Racz, M. Pope, N. Stolte, K. Matz-Rensing, G. Grobschupff, B. Raschdorff, G. Hunsmann, and P. Racz. 1999. Rapid infection of oral mucosal-associated lymphoid tissue with simian immunodeficiency virus. Science 285**:**1261-1265. [DOI] [PubMed] [Google Scholar]
- 42.Steinman, R. M. 2000. DC-SIGN: a guide to some mysteries of dendritic cells. Cell 100**:**491-494. [DOI] [PubMed] [Google Scholar]
- 43.Steinman, R. M., and K. Inaba. 1999. Myeloid dendritic cells. J. Leukoc. Biol. 66**:**205-208. [DOI] [PubMed] [Google Scholar]
- 44.Trinchieri, G. 1995. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu. Rev. Immunol. 13**:**251-276. [DOI] [PubMed] [Google Scholar]
- 45.Tsunetsugu-Yokota, Y., S. Yasuda, A. Sugimoto, T. Yagi, M. Azuma, H. Yagita, K. Akagawa, and T. Takemori. 1997. Efficient virus transmission from dendritic cells to CD4+ T cells in response to antigen depends on close contact through adhesion molecules. Virology 239**:**259-268. [DOI] [PubMed] [Google Scholar]
- 46.Vieira, P. L., E. C. de Jong, E. A. Wierenga, M. L. Kapsenberg, and P. Kalinski. 2000. Development of Th1-inducing capacity in myeloid dendritic cells requires environmental instruction. J. Immunol. 164**:**4507-4512. [DOI] [PubMed] [Google Scholar]
- 47.Weissman, D., Y. Li, J. Ananworanich, L. J. Zhou, J. Adelsberger, T. F. Tedder, M. Baseler, and A. S. Fauci. 1995. Three populations of cells with dendritic morphology exist in peripheral blood, only one of which is infectable with human immunodeficiency virus type 1. Proc. Natl. Acad. Sci. USA 92**:**826-830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Whelan, M., M. M. Harnett, K. M. Houston, V. Patel, W. Harnett, and K. P. Rigley. 2000. A filarial nematode-secreted product signals dendritic cells to acquire a phenotype that drives development of Th2 cells. J. Immunol. 164**:**6453-6460. [DOI] [PubMed] [Google Scholar]
- 49.Zoeteweij, J. P., H. Golding, H. Mostowski, and A. Blauvelt. 1998. Cytokines regulate expression and function of the HIV coreceptor CXCR4 on human mature dendritic cells. J. Immunol. 161**:**3219-3223. [PubMed] [Google Scholar]