Smad-dependent GADD45β expression mediates delayed activation of p38 MAP kinase by TGF-β (original) (raw)
Abstract
Transforming growth factor-β (TGF-β), when bound to its specific receptor, activates the transcription factor Smad by phosphorylation. TGF-β also activates the p38 MAPK pathway, but there seem to be disparate mechanisms for the early p38 activation and delayed p38 activation. In this report, we demonstrate that Smad-dependent expression of GADD45β is responsible for the delayed activation of p38 by TGF-β. The GADD45β protein binds and activates MTK1 (= MEKK4), which is a member of the MAPKKK family kinases and an upstream activator of the p38 MAPK cascade. Both TGF-β-induced GADD45β expression and the delayed p38 activation require functional Smad proteins. Antisense inhibition of GADD45β expression suppresses the TGF-β-induced delayed p38 activation, whereas overexpression of GADD45β activates the p38 MAPK via MTK1. Expression of the angiogenesis inhibitor thrombospondin-1 (TSP-1) is induced by TGF-β via Smad-dependent p38 activation. Thus TGF-β-induced p38 activation, mediated by GADD45β expression, may play an important role in the biological effects of TGF-β.
Keywords: GADD45β/p38/Smad/TGF-β/thrombospondin-1
Introduction
Transforming growth factor-β (TGF-β) belongs to a family of multifunctional cytokines that regulate essential cellular functions, such as proliferation, differentiation, apoptosis, extracellular matrix production and angiogenesis (Derynck and Feng, 1997; Massagué, 1998). An altered cellular response to TGF-β has been postulated as a mechanism whereby cells undergo neoplastic transformation, because many cancers of epithelial and lymphoid origins are resistant to the negative growth-regulatory effects of TGF-β (Markowitz et al., 1995; Hahn et al., 1996; Thiagalingam et al., 1996; Derynck et al., 2001). TGF-β exerts its biological effects by binding to a cell surface receptor complex composed of type I (TβRI) and type II (TβRII) receptor serine/threonine kinases (Wrana et al., 1994). Upon ligand binding, TβRII phosphorylates and activates TβRI, which subsequently phosphorylates the cytoplasmic Smad2 and Smad3 proteins. Phosphoryl ated Smad proteins form stable complexes with a common partner, Smad4 (also known as DPC4). The activated Smad2/4 and Smad3/4 complexes translocate into the nucleus, where the Smad dimers induce transcriptional activation (or inhibition) of specific target genes in co operation with other transcription factors (Macias-Silva et al., 1996; Zhang et al., 1996; Nakao et al., 1997; Massagué and Wotton, 2000; ten Dijke et al., 2000). Underscoring the importance of Smad proteins in TGF-β signaling, disruption of Smad pathway components abated transcriptional responses to TFG-β. Furthermore, Smad4 mutations are observed frequently (∼50%) in human pancreatic carcinomas (Hahn et al., 1996; Schutte et al., 1996; Villanueva et al., 1998).
Beside the Smad proteins, TGF-β also activates the p38 MAPK pathway, which may play an important role in TGF-β-induced gene expression (Hanafusa et al., 1999; Sano et al., 1999). In this regard, previous studies indicate that TAK1, a member of the MAPKKK family, is involved in TGF-β-induced p38 activation (Yamaguchi et al., 1995; Shibuya et al., 1996). TβRI interacts indirectly with TAK1 via bridging proteins XIAP and TAB1 (Yamaguchi et al., 1999; Reffey et al., 2001). It has been suggested further that TGF-β activates p38 MAPK via TAK1 without any involvement of the Smad transcription factors (Sano et al., 1999; Yamaguchi et al., 1999; Reffey et al., 2001). Activation of TAK1 alone, however, seems insufficient to account for the in vivo activation of p38 in response to TGF-β. TGF-β induces TAK1 activity rapidly but only transiently; TAK1 activity peaks at ∼10 min following TGF-β stimulation, and declines to basal level by 30 min (Yamaguchi et al., 1995; Zhang et al., 2000). Rapid activation of p38 by TGF-β, consistent with the kinetics of TAK1 activity, has been reported in some cell lines (Hannigan et al., 1998; Hanafusa et al., 1999; Sano et al., 1999). In many other cell types (e.g. pancreatic acinar cells, keratinocytes, osteoblasts, gingival fibroblasts and rat hepatocytes), however, maximal p38 activation occurs only 1–2 h following TGF-β stimulation, and the activity persists for several hours (Ravanti et al., 1999; Johansson et al., 2000; Karsdal et al., 2001; Herrera et al., 2001; Simeone et al., 2001; Schiffer et al., 2001; Schrantz et al., 2001). These findings suggest that there is an additional, TAK1-independent, signaling mechanism that mediates TGF-β-induced p38 activation.
We previously have demonstrated that the stress- and cytokine-inducible GADD45-family proteins (GADD45α, β and γ) function as specific activators of MTK1 (also known as MEKK4), a MAPKKK upstream in the p38 pathway (Takekawa and Saito, 1998; Mita et al., 2002). In this report, we provide evidence that GADD45β is an important mediator of TGF-β-induced delayed activation of p38. TGF-β induces GADD45β expression and p38 activation in pancreatic carcinoma cells, both in a Smad-dependent manner. Antisense inhibition of GADD45β expression suppresses p38 activation induced by TGF-β, whereas ectopic GADD45β expression activates p38 MAPK via MTK1 MAPKKK, demonstrating that Smad-induced GADD45β is an essential upstream activator of p38 induced by TGF-β. We will demonstrate further that TGF-β-induced expression of thrombospondin-1 (TSP-1), a potent inhibitor of tumor cell growth and angiogenesis, is mediated by the Smad–GADD45β–p38 pathway. TSP-1 secreted by TGF-β-stimulated pancreatic cancer cells has an anti-angiogenic activity in vitro. Based on these observations, we propose that TGF-β-induced p38 activation is mediated by Smad-dependent GADD45β expression, and that this process may play an important role in tumor suppression by TGF-β.
Results
Smad4 is required for TGF-β-induced p38 activation in pancreatic cell lines
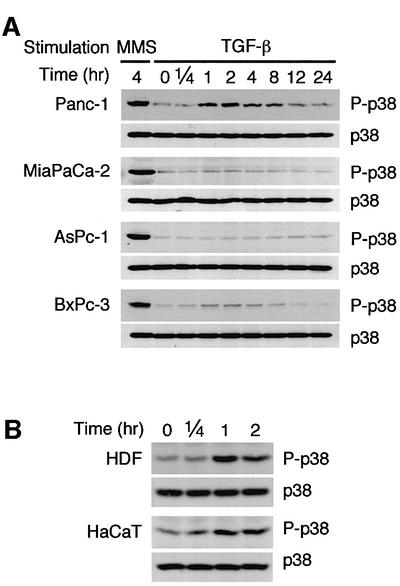
In order to clarify the molecular mechanism of p38 activation by TGF-β, we first examined the time course of p38 MAPK activation in TGF-β-responsive (Panc-1) and TGF-β-unresponsive (MiaPaCa-2, AsPc-1 and BxPc-3) pancreatic cancer cell lines (Freeman et al., 1995; Schutte et al., 1996; Grau et al., 1997; Villanueva et al., 1998; Giehl et al., 2000). MiaPaCa-2 does not express TβRII, whereas AsPc-1 and BxPc-3 are null for Smad4 (Freeman et al., 1995; Schutte et al., 1996; Villanueva et al., 1998; Barbera et al., 2000). These cell lines were treated with either methyl methanesulfonate (MMS) or TGF-β, and the activity of p38 MAPK was monitored by immunoblotting using an antibody specific to the phosphorylated (activated) p38. Robust activation of p38 by MMS was observed in all the cell lines, indicating that there is no defect in the p38 MAPK per se (Figure 1A).
Fig. 1. Activation of p38 MAPK by TGF-β. (A) TGF-β-induced p38 activation in pancreatic carcinoma cells. TGF-β-responsive (Panc-1) and -unresponsive (MiaPaCa-2, AsPc-1 and BxPc-3) pancreatic carcinoma cells were treated with TGF-β (10 ng/ml) or MMS (100 µg/ml), and cell extracts were prepared at the indicated time points. The phosphorylation status of endogenous p38 was analyzed by immunoblotting using an antibody specific to phosphorylated p38 (P-p38). The total level of p38 in cell lysates is also indicated (p38). (B) TGF-β-induced delayed activation of p38. Primary cultures of human dermal fibroblasts (HDFs) and an immortalized human keratinocyte cell line, HaCaT cells, were treated with TGF-β (10 ng/ml) and subjected to immunoblot analyses as in (A).
With TGF-β, however, p38 activation was observed only in the TGF-β-responsive Panc-1 cells: p38 was active at 1 h following TGF-β stimulation, and reached its maximum level by 2 h (Figure 1A). We call this a delayed response, because the p38 response is much faster in certain other cells (see Figure 2D). The total amount of p38 did not change during the period of observation. The delayed response was not limited to the pancreatic carcinoma cells, because a similar time course was observed in primary cultures of human dermal fibroblasts and in the immortalized human keratinocytes, HaCaT (Figure 1B).
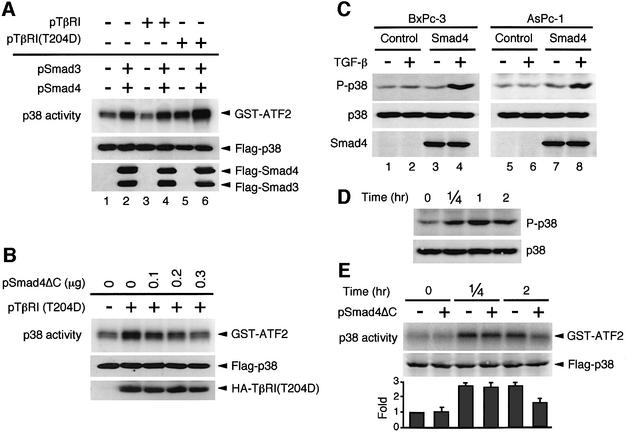
Fig. 2. Smad-dependent activation of p38 MAPK. (A) Overexpression of Smad proteins activates p38 MAPK. HeLa cells were co-transfected with expression vectors for Flag-p38 (0.05 µg/plate) and, where indicated, HA-TβRI (TβRI; 0.2 µg/plate), constitutively active HA-TβRI [TβRI(T204D); 0.2 µg/plate], Flag-Smad3 (0.1 µg/plate) and Flag-Smad4 (0.1 µg/plate). The total amount of transfected plasmid DNA was kept constant by including appropriate amounts of pcDNA3. After 36 h, kinase activity of immunoprecipitated Flag-p38 was measured in vitro using an exogenous substrate GST–ATF2 (upper panel). The expression levels of Flag-p38 (middle panel) and Flag-Smad3/4 (lower panel) proteins in the total cell lysates were monitored by immunoblotting using an anti-Flag antibody. (B) Dominant-negative Smad4 (Smad4ΔC) inhibits p38 activation induced by the constitutively active TβRI(T204D). HeLa cells were transfected with expression vectors for Flag-p38 (0.05 µg/plate), together with HA-TβRI(T204D) (0.1 µg/plate) and Smad4ΔC as indicated. Kinase activity of Flag-p38 was assayed in vitro as in (A). The middle and lower panels show the amounts of the Flag-p38 and HA-TβRI(T204D) proteins in the total cell lysates. (C) Reintroduction of Smad4 into Smad4-null cells restores TGF-β-induced p38 activation. Smad4-deficient BxPc-3 and AsPc-1 cells were infected with a recombinant adenovirus expressing Smad4 or a control GFP adenovirus (Control). At 24 h post-infection, the cells were treated with TGF-β (10 ng/ml) for 2 h and cell extracts were prepared. The phosphorylation status of endogenous p38 was analyzed by immunoblotting using an antibody specific to phosphorylated p38 (upper panel). The total level of p38 (middle panel) and Smad4 (lower panel) in cell lysates is also shown. These experiments (A–C) were repeated at least three times with similar results. (D) Rapid and sustained activation of p38 MAPK by TGF-β in C2C12 cells. TGF-β-induced p38 activation in a mouse myoblast cell line, C2C12, was analyzed by immunoblotting as in Figure 1. (E) Smad4ΔC selectively inhibits delayed activation of p38 by TGF-β. C2C12 cells were transfected with expression vectors for Flag-p38 (0.1 µg/plate), together with 0.3 µg of either the pSmad4ΔC expression vector (+) or the empty vector pcDNA3 (–). After 36 h, cells were treated with TGF-β (10 ng/ml). Kinase activity of Flag-p38 was assayed in vitro as in (A). The fold activation of p38 was determined by FLA-3000 imageanalyzer (Fujifilm) from three independent experiments and is indicated below each lane. Error bars indicate the SEM.
In contrast, p38 activation was not observed in the TGF-β-non-responsive cells. It might be expected that TβRII-deficient MiaPaCa-2 cells did not activate p38 upon TGF-β treatment. More importantly, however, TGF-β also failed to activate p38 in the Smad4-null cell lines, AsPc-1 and BxPc-3, which express functional TGF-β receptors, suggesting that TGF-β-induced p38 activation requires the Smad proteins in these pancreatic cell lines.
To confirm the involvement of Smad proteins in the TGF-β-induced p38 activation, we exploited the previous observation that overexpression of Smad proteins can induce gene expression even in the absence of exogenous TGF-β (Zhang et al., 1996; Nakao et al., 1997). HeLa cells were transiently transfected with expression plasmids for Smad3 and Smad4 together with Flag-tagged p38. Some cells were also transfected with either wild-type TβRI or constitutively active TβRI(T204D). The Flag-p38 protein was immunoprecipitated from cell lysates, and its kinase activity was measured in vitro using GST–ATF2 as a specific substrate. Figure 2A, lane 1 shows the basal Flag-p38 activity when neither TβRI nor Smad vector was co-transfected. When cells were co-transfected with expression plasmids for Smad3 and Smad4, a significant increase in p38 kinase activity was observed (lane 2). Expression of the constitutively active TβRI(T204D), but not wild-type TβRI, also increased p38 activity (lanes 3 and 5). Finally, co-expression of the Smad3 and Smad4 proteins with the constitutively active TβRI(T204D) synergistically activated p38 (lane 6).
We also examined the role of the Smad proteins in TGF-β-induced p38 activation using a dominant-negative Smad4 mutant (Smad4ΔC) (Zhang et al., 1996). For this experiment, HeLa cells were co-transfected with TβRI(T204D) and Flag-p38 expression plasmids, together with increasing amounts of the dominant-negative Smad4ΔC plasmid, and the activity of immunoprecipitated Flag-p38 was assayed in vitro. As seen in Figure 2B, Smad4ΔC suppressed TβRI(T204D)-induced p38 activity in a dose-dependent manner. These results corroborate the notion that Smad proteins are required for the TGF-β-induced p38 activation.
We next employed gene transfer experiments to test whether the lack of the Smad4 protein alone accounts for the unresponsiveness of BxPc-3 and AsPc-1 (Figure 2C). An efficient gene transfer into these cell lines was achieved by a recombinant adenovirus encoding wild-type Smad4 (Fujii et al., 1999). A recombinant virus encoding green fluorescent protein (GFP) was used as a control. The expression of Smad4 in infected cells was confirmed by western blot analysis (bottom panels). Consistent with earlier results, TGF-β did not activate p38 in the control GFP-expressing cells (lanes 2 and 6). In contrast, re-establishment of Smad4 expression in the same cell lines restored TGF-β-induced activation of p38 (lanes 4 and 8), thus proving that a lack of Smad4 protein is the cause of unresponsiveness in these cell lines.
To test whether Smad proteins are also involved in the early activation of p38 by TGF-β, we assessed the effect of the dominant-negative Smad4ΔC in C2C12 cells, a mouse myoblast cell line. As reported (Hanafusa et al., 1999), TGF-β induced p38 activation in C2C12 cells by as early as 15 min, which was sustained for at least 2 h (Figure 2D). Expression of Smad4ΔC significantly suppressed p38 activation at 2 h following TGF-β stimulation, but not at 15 min (Figure 2E). These findings suggest that Smad-dependent signaling mediates TGF-β-induced delayed p38 activation, while the rapid p38 activation occurs independently of Smad. We thus conclude that delayed, but not early, p38 activation induced by TGF-β involves Smad in different cell types.
TGF-β induces GADD45β expression in a Smad-dependent manner
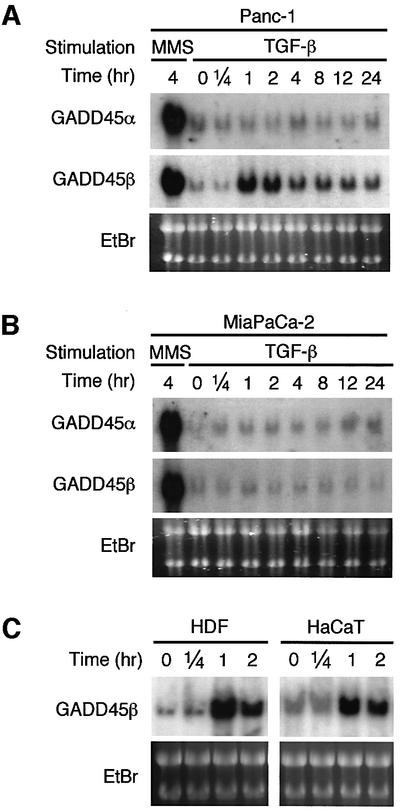
Because the Smad proteins function primarily in transcriptional regulation, it is likely that TGF-β activates p38 in these cells through Smad-induced gene expression. We previously found that the GADD45 family proteins (GADD45α, β and γ) directly bind to the N-terminal regulatory domain of MTK1, a human MAPKKK involved in the p38 pathway, and thereby activate its kinase domain (Takekawa and Saito, 1998; Mita et al., 2002). Expression of GADD45 family genes is induced by environmental stresses (e.g. UV, MMS and high osmolarity), as well as by certain cytokines [e.g. interleukin (IL)-18 and IL-6] (Zhan et al., 1994; Takekawa and Saito, 1998; Lu et al., 2001; Yang et al., 2001). To test the possible involvement of the GADD45 proteins in Smad-mediated p38 activation, we analyzed GADD45 expression following TGF-β stimulation. MMS, a potent GADD45 inducer, was used as a positive control. As shown in Figure 3, TGF-β induced GADD45β mRNA in the TGF-β-responsive Panc-1 cells (Figure 3A), but not in the TβRII-deficient MiaPaCa-2 cells (Figure 3B). Because MMS induced GADD45β mRNA expression in both cell lines, there is no intrinsic defect in the GADD45β gene itself. It should be noted that the time course of the GADD45β mRNA expression in Panc-1 cells approximates that of TGF-β-induced p38 activation. TGF-β-induced neither GADD45α nor GADD45γ in these cells (Figure 3, and data not shown). TGF-β induction of GADD45β mRNA expression was also observed in human dermal fibroblasts and immortalized human keratinocytes, HaCaT cells (Figure 3C). Thus, TGF-β-induced expression of GADD45β was observed in a number of different cell types we examined.
Fig. 3. Expression of the GADD45-family genes in various cell lines. Panc-1 (A), MiaPaCa-2 (B), human dermal fibroblasts (HDFs) (C; left panel) or HaCaT cells (C; right panel) were treated with TGF-β or MMS for various times, as indicated. Total RNA (20 µg/lane) was separated by agarose gel electrophoresis and hybridized with either a 32P-labeled GADD45α or a β cDNA probe. Ethidium bromide (EtBr)-stained gels of the same samples are shown at the bottom to indicate the amounts of applied RNA.
If Smad is responsible for the TGF-β-induced GADD45β expression, no induction of GADD45β mRNA expression would be expected in Smad4-null cell lines, such as BxPc-3 and AsPc-1. Indeed, in these cell lines, no significant GADD45β expression was induced by TGF-β (Figure 4A). To exclude the possibility that these cell lines harbor additional mutation(s) that are in fact responsible for their failure to induce GADD45β expression, these cells were infected with a recombinant adenovirus encoding wild-type Smad4. While the control, GFP virus-infected cells did not respond to TGF-β at all, the Smad4 virus-infected cells induced strong GADD45β expression upon TGF-β stimulation (Figure 4B). Thus, in these Smad4-deficient cells, expression of Smad4 restores both TGF-β-induced p38 activation (Figure 2C) and GADD45β expression (Figure 4B).
Fig. 4. TGF-β-induced GADD45β expression requires Smad4. (A) The absence of TGF-β-stimulated GADD45β mRNA induction in Smad4-deficient BxPc-3 and AsPc-1 cells. (B) Re-expression of Smad4 in the same Smad4-null cell lines restores TGF-β-induced GADD45β expression. Cells were infected with a recombinant adenovirus expressing Smad4 or a control GFP adenovirus (Control). At 24 h post-infection, the cells were treated for 2 h with TGF-β, and total RNA was prepared for northern blot analysis, as in Figure 5.
TGF-β-induced GADD45β expression activates the p38 MAPK via MTK1
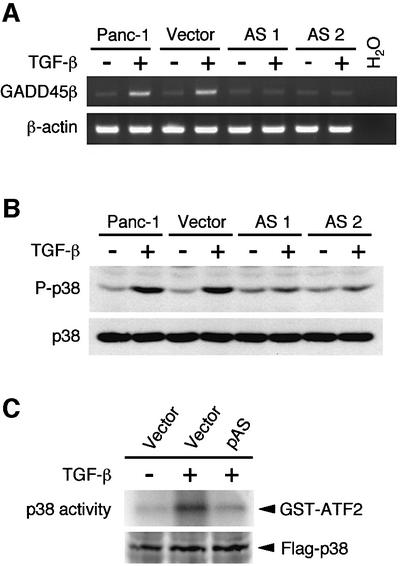
The results in the previous section are consistent with either of the following two models. Model 1: the activated Smad complex induces GADD45β expression and, concomitantly but independently, activates p38 MAPK. Model 2: the activated Smad complex induces GADD45β expression, which subsequently activates p38 MAPK. Model 2 is more attractive in the light of our previous observation that GADD45β activates MTK1, a MAPKKK upstream in the p38 MAPK pathway (Takekawa and Saito, 1998; Mita et al., 2002). In order to determine which is the correct model, we first tested whether GADD45β expression is required for TGF-β-induced p38 activation. For this purpose, TFG-β-responsive Panc-1 cells were stably transfected with antisense (AS) GADD45β cDNA, thereby blocking expression of the endogenous GADD45β gene. As a control, the same cells were transfected with the empty vector. In each transfectant, GADD45β mRNA levels, before and after TGF-β stimulation, were assayed by RT–PCR. The antisense GADD45β effectively suppressed the endogenous GADD45β expression, as seen in Figure 5A. We then assessed the p38 activity in these cells using anti-phospho-p38 immunoblot analysis. Figure 5B shows that GADD45β antisense RNA significantly suppressed p38 activation in response to TGF-β stimulation, indicating a requirement for GADD45β expression in TGF-β-induced p38 activation. We further tested the effect of reduced GADD45β expression on p38 activation in the TGF-β-responsive HaCaT cells. A Flag-p38 expression plasmid was transiently co-transfected with either an empty vector or an antisense GADD45β expression vector into HaCaT cells. Kinase activity of immunoprecipitated Flag-p38 was then assayed in vitro. As shown in Figure 5C, expression of GADD45β antisense RNA suppressed p38 activation induced by TGF-β. We conclude, therefore, that model 2 is correct.
Fig. 5. Antisense GADD45β inhibition suppresses TGF-β-induced p38 activation. (A) RT–PCR detection of GADD45β sense transcripts in the parental Panc-1 cells, and the Panc-1 cells stably transfected with either an empty vector (Vector) or an antisense GADD45β expression vector (AS1 and AS2). Following 2 h incubation of the cells with (+) or without (–) TGF-β, total RNA was prepared and subjected to RT–PCR using primers specific to the GADD45β sense transcript. PCR products were analyzed by agarose gel electrophoresis. (B) GADD45β antisense RNA suppresses p38 activation in response to TGF-β. After 2 h incubation of the same cells as in (A) with (+) or without (–) TGF-β, the phosphorylation status of the endogenous p38 was analyzed by immunoblotting using the anti-phospho p38 antibody (upper panel). The level of p38 in cell lysates is also shown (lower panel). (C) Effects of antisense GADD45β on TGF-β-induced p38 activation in HaCaT cells. HaCaT cells were transiently transfected with Flag-p38 expression plasmid together with empty vector pcDNA3 (vector) or antisense GADD45β expression vector (pAS). After 36 h, cells were treated with TGF-β (+) or left untreated (–). After 2 h, the kinase activity of Flag-p38 was determined as in Figure 2A.
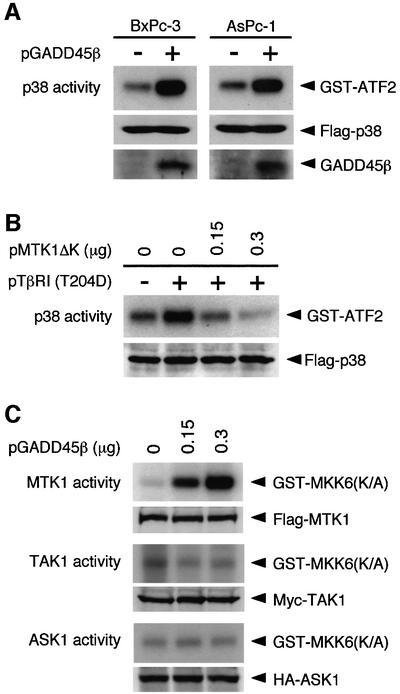
A corollary of this model is that ectopic expression of GADD45β should activate p38 even in Smad4-null cells. To test this prediction, Smad4-deficient cells (AsPc-1 and BxPc-3) were transiently transfected with a GADD45β expression plasmid together with a Flag-tagged p38 construct. Kinase activity of immunoprecipitated Flag-p38 was assayed in vitro. In both the Smad4-null cell lines, p38 MAPK was activated by expression of GADD45β (Figure 6A), as predicted by the model. We then tested another prediction of the model, namely that the MTK1 MAPKKK is involved in TGF-β-induced activation of p38. Previously it was shown that a C-terminally truncated MTK1 mutant (MTK1ΔK) has a dominant-inhibitory effect on p38 activation induced by the GADD45 family proteins (Takekawa and Saito, 1998; Yang et al., 2001). Using the dominant-inhibitory MTK1ΔK, we tested whether the activation of p38 by the constitutively active TβRI(T204D) requires MTK1 activity. Thus HeLa cells were co-transfected with a TβRI(T204D) and a Flag-p38 expression plasmid together with increasing amounts of the MTK1ΔK expression plasmid. As seen in Figure 6B, expression of MTK1ΔK suppressed TβRI(T204D)-induced p38 activity in a dose-dependent manner, supporting the notion that GADD45β–MTK1 signaling mediates the activation of p38 in response to TGF-β.
Fig. 6. GADD45β mediates TGF-β-induced p38 activation via MTK1. (A) Ectopic expression of GADD45β activates the p38 pathway in Smad4-null cell lines. The Smad4-defective BxPc-3 and AsPc-1 cells were transiently transfected with 10 µg of pFlag-p38, together with 20 µg of either pGADD45β or control pcDNA3. After 24 h, the kinase activity of Flag-p38 was determined as described in Figure 2. The middle and bottom panels show the amounts of the Flag-p38 and GADD45β proteins in the total cell lysates, respectively. (B) Dominant-inhibitory MTK1ΔK suppresses p38 activation induced by the constitutively active TβRI(T204D). HeLa cells were transfected with expression vectors for Flag-p38 (0.05 µg/plate), HA-TβRI(T204D) (0.1 µg/plate) and MTK1ΔK as indicated. The total amount of transfected plasmid DNA was kept constant by including appropriate amounts of pcDNA3. After 36 h, the kinase activity of immunoprecipitated Flag-p38 was measured by in vitro kinase assay. (C) GADD45β selectively activates the MTK1 MAPKKK. COS-7 cells were transfected with 0.1 µg of expression vectors encoding Flag-MTK1, Myc-TAK1 or HA-ASK1, together with the indicated amounts of pGADD45β. Activities of the epitope-tagged MAPKKKs were measured in immune complex kinase assays using a specific exogenous substrate GST–MKK6(K/A). The expression levels of the MAPKKKs in cell lysates were monitored by immunoblotting. These experiments (A–C) were repeated at least three times with similar results.
To test whether other MAPKKKs that have been implicated in p38 activation are involved in the GADD45β response, we transfected COS-7 cells with various MAPKKK constructs (Flag-MTK1, Myc-TAK1 or HA-ASK1) together with increasing amounts of the GADD45β expression vector (Yamaguchi et al., 1995; Ichijo et al., 1997). The epitope-tagged MAPKKKs were immunoprecipitated from cell extracts, and their kinase activities assayed in vitro using a common substrate [GST–MKK6(K/A)]. As shown in Figure 6C, the catalytic activity of MTK1, but not those of TAK1 and ASK1, was stimulated by GADD45β in a dose-dependent manner.
Smad-dependent activation of p38 is required for TSP-1 expression
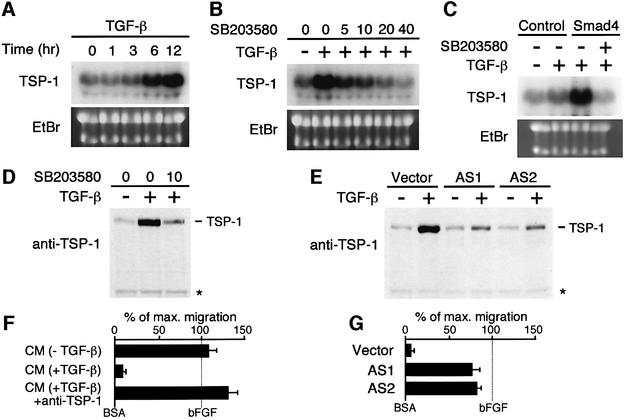
Finally, we examined the role of p38 MAPK in the cellular response to TGF-β. TGF-β affects the expression of numerous genes via Smad activation. TGF-β may also influence gene expression through activation of p38, which modifies a number of transcription factors. In order to identify the subset of genes whose expression is regulated by TGF-β via p38 activation, we examined the effect of SB203580, a specific inhibitor of p38 (Lee et al., 1994). Panc-1 cells were treated with TGF-β in the presence or absence of SB203580, and the gene expression pattern at three time points (0, 2 and 12 h) was profiled by differential cDNA array hybridization. Initially we identified genes whose expression is regulated (either up or down) by TGF-β. Among these TGF-β-responsive genes, we then identified genes whose TGF-β response is diminished by SB203580. From ∼40 genes that met these criteria, we chose TSP-1, a potent inhibitor of tumor cell growth and angiogenesis (Lawler, 2000; Bornstein, 2001), for further studies. It has been reported recently that Smad4-mediated suppression of tumorigenicity in human pancreatic adenocarcinoma cells is due primarily to repression of tumor angiogenesis (Schwarte-Waldhoff et al., 2000).
Northern blot analysis of the total RNA isolated from Panc-1 cells confirmed the cDNA array data that TSP-1 mRNA was strongly induced by TGF-β (Figure 7A). Furthermore, SB203580 reduced the TGF-β-induced TSP-1 expression in a dose-dependent manner (Figure 7B). The TSP-1 protein level is regulated similarly, as determined by western blotting with an anti-TSP-1 monoclonal antibody. The amounts of the TSP-1 protein (which is secreted) in cell culture supernatants significantly increased following TGF-β stimulation, and this increase was suppressed by SB203580 (Figure 7D). Thus, p38 activity is required for TSP-1 expression in response to TGF-β. To evaluate whether TGF-β-stimulated Panc-1 cells secrete a significant amount of active TSP-1, we examined the anti-angiogenic activity of cell culture supernatants using an in vitro endothelial cell migration assay (Figure 7F). Conditioned medium of TGF-β-stimulated Panc-1 cells, which contains secreted TSP-1, strongly inhibited the basic fibroblast growth factor (bFGF)-induced endothelial cell migration. This inhibition was abolished when a neutralizing antibody to TSP-1 was included in the assay, indicating that TSP-1 secreted by TGF-β-stimulated Panc-1 cells inhibits angiogenesis.
Fig. 7. TGF-β-induced thrombospondin 1 expression is regulated by Smad-mediated p38 activation. (A–C) Northern blot analyses of TGF-β-induced TSP-1 expression. Total RNA samples extracted from Panc-1 cells at the indicated times following TGF-β treatment were analyzed in (A). Ethidium bromide (EtBr)-stained gels of the same samples are also shown to indicate the amounts of RNA applied. In (B), the p38-specific inhibitor SB203580 was added to Panc-1 cells at the indicated concentrations (µM) 1 h before TGF-β stimulation. Total RNA was prepared 12 h later. In (C), Smad4-deficient BxPc-3 cells were infected with recombinant adenovirus expressing Smad4, or with GFP (Control). At 24 h post-infection, cells were treated for an additional 12 h with TGF-β in the presence or absence of 10 µM SB203580, before preparation of total RNA. (D and E) Western blot analyses of TSP-1 expression in cell culture supernatants. Proteins in conditioned media (8 µg/lane) of parental Panc-1 cells treated with (+) or without (–) TGF-β in the presence or absence of SB203580 (10 µM) (D), or Panc-1 cells stably transfected with either an empty vector (Vector) or an antisense GADD45β expression vector (AS1 and AS2) treated with (+) or without (–) TGF-β (E), were separated by SDS–PAGE, and TSP-1 was detected using a specific monoclonal antibody. A non-specific background band of immunoblotting was shown as a loading control (asterisk). (F and G) Anti-angiogenic activity of conditioned media. In (F), conditioned media (CM) from Panc-1 cells treated with or without TGF-β were tested in the presence or absence of neutralizing anti-TSP-1 antibody (20 µg/ml) for their ability to block the migration of microvascular endothelial cells induced by bFGF (20 ng/ml). All samples were tested in quadruplicate. Data are reported as percentage of maximum migration. In (G), conditioned media were prepared from TGF-β-stimulated Panc-1 cells transfected with either an empty vector (Vector) or an antisense GADD45β expression vector (AS1 and AS2).
Because TGF-β-induced activation of p38 is mediated by Smad-dependent GADD45β expression in these cell lines, TSP-1 expression should also be dependent on Smad and GADD45β. To test this prediction, we first analyzed TSP-1 mRNA levels in the Smad4-null BxPc-3 cells. TSP-1 mRNA expression was strongly induced by TGF-β in BxPc-3 cells infected with Smad4 recombinant adenovirus, but not in the control (GFP virus-infected) cells. In the Smad4-transfected cells, TGF-β-mediated TSP-1 expression was completely inhibited by SB203580 (Figure 7C). We also found that antisense suppression of GADD45β expression inhibits TGF-β-induced TSP-1 expression in Panc-1 cells (Figure 7E). This is consistent with the reduced p38 activity by GADD45β antisense inhibition (Figure 5B). Finally, conditioned medium from TGF-β-stimulated Panc-1 cells expressing GADD45β antisense RNA did not significantly inhibit the bFGF-induced endothelial cell migration in vitro (Figure 7G). Thus we conclude that TGF-β induces TSP-1 expression through the p38 activation mediated by Smad-dependent GADD45β expression. We further suggest that the p38 pathway plays an important role in tumor inhibition by inducing TSP-1 expression.
Discussion
In this study, we explored the mechanism of TGF-β-induced p38 activation using several types of cells including pancreatic carcinoma cells. The pancreas, whose cancer has a high incidence of mutations in the TGF-β signaling pathway, is one of the most important tissues in which to define this pathway. Our results provide evidence that TGF-β activates p38 through Smad-mediated induction of GADD45β gene expression, at least in pancreatic cells.
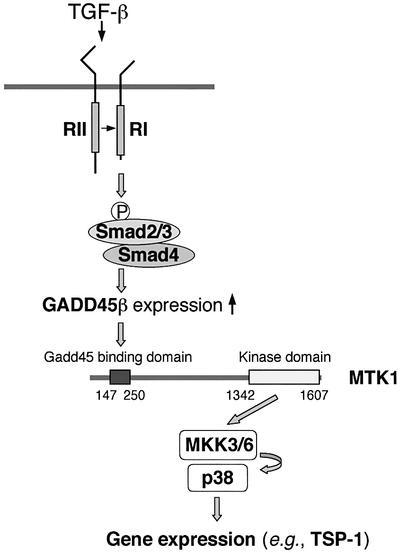
We found that TGF-β activates p38 in TGF-β-responsive pancreatic cells, but not in Smad4-deficient cells. In parallel, we also found that TGF-β induces expression of GADD45β in TGF-β-responsive cells, but not in Smad4-deficient cells. Reestablishment of Smad4 expression in the Smad4-deficient cells restores both p38 activation and GADD45β expression in response to TGF-β. We also showed that inhibition of GADD45β expression by antisense RNA significantly suppresses p38 activation by TGF-β, whereas ectopic expression of GADD45β is sufficient to activate the p38 pathway even in Smad4-null cells. GADD45β expression selectively activates MTK1, but not other MAPKKKs such as TAK1 and ASK1. These observations, together with previous findings, indicate the following scenario for TGF-β-induced p38 activation (Figure 8). TGF-β activates its receptor which phosphorylates Smad3 (or Smad2), resulting in the formation of active Smad3/4 or Smad2/4 complexes. The active Smad complexes translocate to the nucleus and induce GADD45β expression. GADD45β then activates the MTK1 MAPKKK. Activated MTK1 activates its cognate MAPKKs (MKK3 or MKK6), which eventually activate p38 MAPK.
Fig. 8. Model of signal flow from TGF-β to p38-dependent gene expression. Upon TGF-β binding, TβRII phosphorylates and activates TβRI, which subsequently phosphorylates the cytoplasmic Smad2 or Smad3, resulting in formation of a stable heterodimer with Smad4. The active Smad2/4 or Smad3/4 complex translocates to the nucleus and induces GADD45β expression. The GADD45β protein binds and activates the MTK1 MAPKKK, which leads to activation of the p38 MAPK. Active p38 translocates into the nucleus and regulates genes (including TSP-1) via phosphorylation of transcription factors.
It has been reported that TGF-β activates the p38 pathway through the TAK1 MAPKKK, which associates with TβRI (Sano et al., 1999; Yamaguchi et al., 1999; Reffey et al., 2001). Unlike our current observations, the TAK1 model does not involve the Smad proteins. This apparent discrepancy is probably due to cell- or tissue-specific differences. It has been shown that TGF-β activates the p38 MAPK pathway with different time courses in different cells. TAK1 is activated very rapidly, within 10 min, by TGF-β through TβRI (Yamaguchi et al., 1995; Zhang et al., 2000). In certain cell types (e.g. human neutrophils, HEK-293 cells and C2C12 cells), TGF-β activates p38 with very rapid kinetics that are consistent with the TAK1 mechanism (Hannigan et al., 1998; Hanafusa et al., 1999; Sano et al., 1999). In other cell types (e.g. pancreatic acinar cells, keratinocytes, osteoblasts, gingival fibroblasts, podocytes and hepatocytes), however, a significant delay is observed between exposure to TGF-β and p38 activation (Ravanti et al., 1999; Johansson et al., 2000; Herrera et al., 2001; Karsdal et al., 2001; Schiffer et al., 2001; Schrantz et al., 2001; Simeone et al., 2001). Indeed, TGF-β takes as much as 1 h to stimulate p38 activity in pancreatic carcinoma cells, HaCaT cells and human dermal fibroblasts (see Figure 1). It seems likely that the rapid activation of p38 in certain cells is mediated primarily by TAK1, while the delayed-type activation of p38 in other cells is mediated mainly by Smad-dependent GADD45β–MTK1 signaling. Indeed, in C2C12 cells, the dominant-negative Smad4ΔC mutant suppressed only delayed p38 activation, but not the early activation phase (Figure 2E).
We have demonstrated previously that the GADD45 family proteins bind to and activate the MTK1 MAPKKK, leading to activation of p38 MAPK (Takekawa and Saito, 1998; Mita et al., 2002). Interestingly, recent studies have reported that, in Th1 (T helper type 1) cells, cytokine- and T-cell receptor (TCR)-induced activation of p38 MAPK, which is essential for interferon-γ production, is mediated by GADD45β and GADD45γ, respectively (Lu et al., 2001; Yang et al., 2001), showing functional distinction among the GADD45 isoforms. Our current study suggests that GADD45β is more important than the other isoforms in TGF-β signaling.
To investigate the role of p38 activation in TGF-β-mediated gene regulation, we searched, by cDNA array analysis, for genes that are induced by TGF-β in a p38-dependent manner. One such gene encodes TSP-1, a potent inhibitor of tumor cell growth and angiogenesis (Lawler, 2000; Bornstein, 2001). Interestingly, it has been reported recently that restoration of Smad4 to Smad4-deficient pancreatic carcinoma cells increased the steady-state mRNA levels of TSP-1, and suppressed tumor formation in vivo by repressing tumor angiogenesis (Schwarte-Waldhoff et al., 2000). Nonetheless, TSP-1 mRNA expression is unlikely to be mediated directly by the Smad proteins, because the TSP-1 promoter region is devoid of any known Smad-binding element (Schwarte-Waldhoff et al., 2000). Our finding that TSP-1 expression requires Smad- and GADD45β-dependent p38 activation offers a mechanism for TGF-β-induced TSP-1 expression. It is likely that TSP-1 expression is only indirectly dependent on the Smad proteins, and that direct gene induction is mediated by transcription factors that are activated by p38, such as ATF2. We can speculate further that TSP-1 expression, in normal pancreatic cells, might contribute to the tumor-suppressive effect of TGF-β. Indeed, the TSP-1 protein secreted by TGF-β-stimulated cells showed an anti-angiogenic effect in vitro (Figure 7F and G). If so, inefficient activation of p38 in Smad4-deficient tumor cells, and a resulting deficit of TSP-1, might contribute to the etiology of invasive pancreatic cancer. We should note, however, that the role of p38 in the cellular response to TGF-β is by no means limited to TSP-1 expression.
Materials and methods
Media and buffers
Lysis buffer, kinase buffer and SDS loading buffer have been described previously (Takekawa et al., 2000).
Expression constructs
Mammalian expression plasmids pCMV-Flag-p38, pFlag-MTK1, pMTK1ΔK and pGADD45β, and the GST fusion constructs GST–ATF2 and GST–MKK6(K/A) have been described previously (Takekawa and Saito, 1998). pSmad4ΔC was constructed by deletion of the coding sequence of human Smad4 from Lys515 to the C-terminus of the human Smad4-coding sequence. To generate an antisense GADD45β expression vector (pBCMG-AS-GADD45β), GADD45β cDNA containing the complete coding sequence was subcloned in the antisense orientation into pBCMG-hygro (Karasuyama et al., 1990). Expression vectors for Flag-Smad3 and Flag-Smad4 were provided by M.Kurokawa. Myc-TAK1 and HA-ASK1 expression vectors were provided by K.Matsumoto and H.Ichijo, respectively (Yamaguchi et al., 1995; Ichijo et al., 1997). Mammalian expression vectors for wild-type TβRI and constitutively activated TβRI(T204D) were from J.Massagué (Wieser et al., 1995). Recombinant adenovirus expressing Smad4 was provided by K.Miyazono (Fujii et al., 1999).
Tissue culture and gene transfer
For transient transfection assays, HeLa, COS-7 and C2C12 cells, grown in 35 mm dishes, were transfected with the appropriate expression plasmids using the Effectene Transfection Reagent (Qiagen). The total amount of plasmid DNA was adjusted to 0.4 µg per plate with vector DNA (pcDNA3). AsPc-1, BxPc-3 and HaCaT (Boukamp et al., 1988) cells were suspended at a density of 2 × 107 cells/ml and transfected by electroporation using the Bio-Rad Gene Pulser II at 0.25 kV and 975 µF. For adenovirus-mediated transient transfection, cells in serum-free RPMI-1640 were infected with recombinant adenovirus at a multiplicity of infection (m.o.i.) of 20 p.f.u./cell as described (Fujii et al., 1999) and, after 1 h, were refed with medium containing 10% serum. For both plasmid and viral transfection experiments, cells were harvested 36 h after transfection and, where indicated, treated with TGF-β1 (10 ng/ml; Sigma) or MMS (100 µg/ml). To establish transfectants stably expressing antisense GADD45β, Panc-1 cells were transfected using the Effectene Reagent, selected with hygromycin (0.2 mg/ml), and stable transfectants were isolated by ring cloning.
Immunoblotting
Immunoblot analyses were performed as described previously (Takekawa and Saito, 1998). For TSP-1 immunoblots, cells were incubated with TGF-β (10 ng/ml) in the presence or absence of 10 µM SB203580 for 24 h in serum-free Dulbecco’s modified Eagle’s medium (DMEM), cell culture supernatants were concentrated with Centricon-100 tubes, and 8 µg of total protein per lane was electrophoresed under reducing conditions as described (Schwarte-Waldhoff et al., 2000). The following antibodies were used: anti-Flag monoclonal antibody (mAb) M2 (Sigma); anti-HA mAb 12CA5 (Roche Molecular Biochemicals); anti-Myc mAb 9E10 (Santa Cruz); anti-Smad4 mAb (Transduction Laboratories); anti-TSP-1 mAb A6.1 (Oncogene Research Products); rabbit polyclonal antibodies to p38 (Santa Cruz); rabbit polyclonal antisera specific to phosphorylated p38 (Cell Signaling); and goat polyclonal antisera to GADD45β (Santa Cruz).
Immune-complex protein kinase assay
To assess the kinase activity of epitope-tagged MAPKKKs, transfected cells were lysed in lysis buffer plus 0.5% deoxycholate. Cell lysates were incubated with an appropriate antibody for 2 h at 4°C. Immunocomplexes were recovered with the aid of protein G–Sepharose beads, washed twice with lysis buffer containing 500 mM NaCl, twice with lysis buffer, and twice again with kinase buffer. Immunoprecipitates were resuspended in 30 µl of kinase buffer containing 3 µg of GST–MKK6(K/A). The kinase reaction was initiated by addition of 20 µCi of [γ-32P]ATP and 20 µM ATP. Following 15 min incubation at 30°C, reactions were terminated by the addition of SDS loading buffer. Samples were boiled, separated by SDS–PAGE, dried and visualized by autoradiography. The kinase activity of precipitated Flag-p38 was measured in a similar in vitro kinase assay using the specific substrate GST–ATF2 as described previously (Takekawa and Saito, 1998).
Northern blot analysis and RT–PCR
Cells were treated with either TGF-β1 (10 ng/ml) or MMS (100 µg/ml). A 20 µg aliquot per lane of total RNA, prepared using Trizol (Gibco-BRL), was fractionated by denaturing agarose gel electrophoresis, and transferred onto a nylon membrane. The blot was hybridized with a probe specific to either GADD45α, β, γ or TSP-1, as described (Takekawa et al., 2000). To assess the presence of sense transcripts in antisense transfectants, one-step RT–PCR was carried out with total RNA (1.0 µg) and primers specific to the sense transcript, using the Platinum Quantitative RT–PCR ThermoScript One-Step System (Life Technologies).
cDNA expression array
The Gene Navigator cDNA cancer array (Toyobo) was used to examine differential gene expression in Panc-1 cells stimulated with TGF-β (10 ng/ml) in the presence or absence of 10 µM SB203580. The array comprises sequences of 550 annotated cancer-associated genes and 11 housekeeping genes. Isolation of poly(A) RNA, cDNA synthesis, labeling of cDNA with chemiluminescence and hybridization of cDNA probes to the array filters were performed according to the manufacturer’s protocol. Following hybridization and washing, the array filters were scanned in a FluorImager (Amersham Pharmacia Biotech), and were data-processed with Array-Pro Analyzer software (Toyobo). As the arrays were double-spotted, the intensity value for each gene was calculated as the average of the two spots, and normalized to hybridization signals from the 11 housekeeping genes.
Endothelial cell migration assays
Endothelial cell migration was assayed in a modified Boyden chamber as described (Schwarte-Waldhoff et al., 2000). Briefly, human microvascular endothelial cells (HMVECs) were attached to the undersurface of a gelatinized membrane (8-µm pore size) in the lower wells. Concentrated serum-free conditioned medium (CM) (20 µg/ml) in DMEM with 0.1% bovine serum albumin (BSA) and bFGF (20 ng/ml; Sigma) was added to the upper wells. Where noted, neutralizing antibody to TSP-1 (A4.1; Oncogene Research Products) was added at 20 µg/ml. After 4 h, the filters were stained and the number of cells migrating to the top of the membrane per 10 high-power fields was counted. Results are reported as the percentage of the maximum migration induced by bFGF (20 ng/ml) after subtraction of background migration toward the vehicle (0.1% BSA). All samples were tested in quadruplicate. All assays contained neutralizing antibody to TGF-β (15 µg/ml; Genzyme-Techne) to eliminate the effects of exogenous TGF-β.
Acknowledgments
Acknowledgements
We thank M.Kitamura and K.Fujii for their excellent technical assistance, J.Massagué (Memorial Sloan-Kettering Cancer Center), K.Matsumoto (Nagoya University). K.Miyazono (University of Tokyo), H.Ichijo (Tokyo Medical and Dental University), M.Kurokawa (University of Tokyo) and T.Takahashi (Aichi Cancer Center Research Institute) for cDNAs, N.Fusenig (German Cancer Research Center) for HaCaT cells, and P.O’Grady for critical reading of the manuscript. This work was supported in part by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan, and grants from PRESTO (JST), the Welfide Medicinal Research Foundation and the Takeda Science Foundation.
References
- Barbera V.M., Martin,M., Marinoso,L., Munne,A., Carrato,A., Real,F.X. and Fabre,M. (2000) The 18q21 region in colorectal and pancreatic cancer: independent loss of DCC and DPC4 expression. Biochim. Biophys. Acta, 1502, 283–296. [DOI] [PubMed] [Google Scholar]
- Bornstein P. (2001) Thrombospondins as matricellular modulators of cell function. J. Clin. Invest., 107, 929–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boukamp P., Petrussevska,R.T., Breitkreutz,D., Hornung,J., Markham, A. and Fusenig,N.E. (1988) Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J. Cell Biol., 106, 761–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derynck R. and Feng,X.-H. (1997) TGF-β receptor signaling. Biochim. Biophys. Acta, 1333, F105–F150. [DOI] [PubMed] [Google Scholar]
- Derynck R., Akhurst,R.J. and Balmain,A. (2001) TGF-β signaling in tumor suppression and cancer progression. Nat. Genet., 29, 117–129. [DOI] [PubMed] [Google Scholar]
- Freeman J.W., Mattingly,C.A. and Strodel,W.E. (1995) Increased tumorigenicity in the human pancreatic cell line MIA PaCa-2 is associated with an aberrant regulation of an IGF-1 autocrine loop and lack of expression of the TGF-β type RII receptor. J. Cell Physiol., 165, 155–163. [DOI] [PubMed] [Google Scholar]
- Fujii M. et al. (1999) Roles of bone morphogenetic protein type I receptors and Smad proteins in osteoblast and chondroblast differentiation. Mol. Biol. Cell, 10, 3801–3813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giehl K., Seidel,B., Gierschik,P., Adler,G. and Menke,A. (2000) TGF-β1 represses proliferation of pancreatic carcinoma cells which correlates with Smad4-independent inhibition of ERK activation. Oncogene, 19, 4531–4541. [DOI] [PubMed] [Google Scholar]
- Grau A.M., Zhang,L., Wang,W., Ruan,S., Evans,D.B., Abbruzzese,J.L. Zhang,W. and ChiaoP.J. (1997) Induction of p21waf1 expression and growth inhibition by transforming growth factor β involve the tumor suppressor gene DPC4 in human pancreatic adenocarcinoma cells. Cancer Res., 57, 3929–3934. [PubMed] [Google Scholar]
- Hahn S.A. et al. (1996) DPC4, a candidate tumor suppressor gene at human chromosome 18q21.1. Science, 271, 350–353. [DOI] [PubMed] [Google Scholar]
- Hanafusa H., Ninomiya-Tsuji,J., Masuyama,N., Nishita,M., Fujisawa,J., Shibuya,H., Matsumoto,K. and Nishida,E. (1999) Involvement of the p38 mitogen-activated protein kinase pathway in transforming growth factor-β-induced gene expression. J. Biol. Chem., 274, 27161–27167. [DOI] [PubMed] [Google Scholar]
- Hannigan M., Zhan,L., Ai,Y. and Huang,C.-K. (1998) The role of p38 MAP kinase in TGF-β1-induced signal transduction in human neutrophils. Biochem. Biophys. Res. Commun., 246, 55–58. [DOI] [PubMed] [Google Scholar]
- Herrera B., Fernadez,M., Roncero,C., Ventura,J.J., Porras,A., Valladares,A., Benito,A. and Fabregat,I. (2001) Activation of p38MAPK by TGF-β in fetal rat hepatocytes requires radical oxygen production, but is dispensable for cell death. FEBS Lett., 499, 225–229. [DOI] [PubMed] [Google Scholar]
- Ichijo H. et al. (1997) Induction of apoptosis by ASK1, a mammalian MAPKKK that activates SAPK/JNK and p38 signaling pathways. Science, 275, 90–94. [DOI] [PubMed] [Google Scholar]
- Johansson N., Ala-aho,R., Uitto,V., Grenman,R., Fusenig,N.E., Lopez-Otin,C. and Kahari,V.M. (2000) Expression of collagenase-3 (MMP-13) and collagenase-1 (MMP-1) by transformed keratinocytes is dependent on the activity of p38 mitogen-activated protein kinase. J. Cell Sci., 113, 227–235. [DOI] [PubMed] [Google Scholar]
- Karasuyama H., Kudo,A. and Melchers,F. (1990) The proteins encoded by the VpreB and λ5 pre-B cell-specific genes can associate with each other and with µ heavy chain. J. Exp. Med., 172, 969–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karsdal M.A., Fjording,M.S., Foged,N.T, Delaisse,J.-M. and Lochter,A. (2001) Transforming growth factor-β-induced osteoblast elongation regulates osteoclastic bone resorption through a p38 mitogen-activated protein kinase- and matrix metalloproteinase-dependent pathway. J. Biol. Chem., 276, 39350–39358. [DOI] [PubMed] [Google Scholar]
- Labbé M.A., Silvestri,C., Hoodless,P.A., Wrana,J.L. and Attisano,L. (1998) Smad2 and Smad3 positively and negatively regulate TGFβ-dependent transcription through the forkhead DNA-binding protein FAST2. Mol. Cell, 2, 109–120. [DOI] [PubMed] [Google Scholar]
- Lawler J. (2000) The functions of thrombospondin-1 and -2. Curr. Opin. Cell Biol., 12, 634–640. [DOI] [PubMed] [Google Scholar]
- Lee J.C. et al. (1994) A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature, 372, 739–746. [DOI] [PubMed] [Google Scholar]
- Lu B., Yu,H., Chow,C.-W., Li,B., Zheng,W.-P., Davis,R.J. and Flavell,R.A. (2001) GADD45γ mediates the activation of the p38 and JNK MAP kinase pathways and cytokine production in effector TH1 cells. Immunity, 14, 583–590. [DOI] [PubMed] [Google Scholar]
- Macias-Silva M., Abdollah,S., Hoodless,P.A., Pirone,R., Attisano,L. and Wrana,J.L. (1996) MADR2 is a substrate of the TGFβ receptor and its phosphorylation is required for nuclear accumulation and signaling. Cell, 87, 1215–1224. [DOI] [PubMed] [Google Scholar]
- Markowitz S. et al. (1995) Inactivation of the type II TGF-β receptor in colon cancer cells with microsatellite instability. Science, 268, 1336–1338. [DOI] [PubMed] [Google Scholar]
- Massagué J. (1998) TGF-β signal transduction. Annu. Rev. Biochem., 67, 753–791. [DOI] [PubMed] [Google Scholar]
- Massagué J. and Wotton,D. (2000) Transcriptional control by the TGF-β/Smad signaling system. EMBO J., 19, 1745–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mita H., Tsutsui,J., Takekawa,M., Witten,E.A. and Saito,H. (2002) Regulation of MTK1/MEKK4 kinase activity by its N-terminal auto-inhibitory domain and GADD45 binding. Mol. Cell. Biol., 22, 4544–4555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao A. et al. (1997) TGF-β receptor-mediated signalling through Smad2, Smad3 and Smad4. EMBO J., 16, 5353–5362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravanti L., Häkkinen,L., Larjava,H., Saarialho-Kere,U., Foschi,M., Han,J. and Kähäri,V.-M. (1999) Transforming growth factor-β induces collagenase-3 expression by human gingival fibroblasts via p38 mitogen-activated protein kinase. J. Biol. Chem., 274, 37292–37300. [DOI] [PubMed] [Google Scholar]
- Reffey S.B., Wurthner,J.U., Parks,W.T., Roberts,A.B. and Duckett,C.S. (2001) X-linked inhibitor of apoptosis protein functions as a cofactor in transforming growth factor-β signaling. J. Biol. Chem., 276, 26542–26549. [DOI] [PubMed] [Google Scholar]
- Sano Y., Harada,J., Tashiro,S., Gotoh-Mandeville,R., Maekawa,T. and IshiiS. (1999) ATF-2 is a common nuclear target of Smad and TAK1 pathways in transforming growth factor-β signaling. J. Biol. Chem., 274, 8949–8957. [DOI] [PubMed] [Google Scholar]
- Schiffer M., Bitzer,M., Roberts,I.S.D., Kopp,J.B., ten Dijke,P., Mundel,P. and Böttinger,E.P. (2001) Apoptosis in podocytes induced by TGF-β and Smad7. J. Clin. Invest., 108, 807–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrantz N., Bourgeade,M.-F., Mouhamad,S., Leca,G., Sharma,S. and Vazquez,A. (2001) p38-mediated regulation of an Fas-associated death domain protein-independent pathway leading to caspase-8 activation during TGFβ-induced apoptosis in human Burkitt lymphoma B cells BL41. Mol. Biol. Cell, 12, 3139–3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutte M. et al. (1996) DPC4 gene in various tumor types. Cancer Res., 56, 2527–2530. [PubMed] [Google Scholar]
- Schwarte-Waldhoff I. et al. (2000) Smad4/DPC4-mediated tumor suppression through suppression of angiogenesis. Proc. Natl Acad. Sci. USA, 97, 9624–9629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuya H., Yamaguchi,K., Shirakabe,K., Tonegawa,A., Gotoh,Y., Ueno,N., Irie,K., Nishida,E. and Matsumoto,K. (1996) TAB1: an activator of the TAK1 MAPKKK in TGF-β signal transduction. Science, 272, 1179–1182. [DOI] [PubMed] [Google Scholar]
- Simeone D.M., Zhang,L., Graziano,K., Nicke,B., Pham,T., Schaefer,C. and Logsdon,C.D. (2001) Smad4 mediates activation of mitogen-activated protein kinases by TGF-β in pancreatic acinar cells. Am. J. Physiol. Cell Physiol., 281, C311–C319. [DOI] [PubMed] [Google Scholar]
- Takekawa M. and Saito,H. (1998) A family of stress-inducible GADD45-like proteins mediate activation of the stress-responsive MTK1/MEKK4 MAPKKK. Cell, 95, 521–530. [DOI] [PubMed] [Google Scholar]
- Takekawa M., Adachi,M., Nakahata,A., Nakayama,I., Itoh,F., Tsukuda, H., Taya,Y. and Imai,K. (2000) p53-inducible Wip1 phosphatase mediates a negative feedback regulation of p38 MAPK–p53 signaling in response to UV radiation. EMBO J., 19, 6517–6526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ten Dijke P., Miyazono,K. and Heldin,C.H. (2000) Signaling inputs converge on nuclear effectors in TGF-β signaling. Trends Biochem. Sci., 25, 64–70. [DOI] [PubMed] [Google Scholar]
- Thiagalingam S. et al. (1996) Evaluation of candidate tumor suppressor genes on chromosome 18 in colorectal cancers. Nat. Genet., 13, 343–346. [DOI] [PubMed] [Google Scholar]
- Villanueva A. et al. (1998) Disruption of the antiproliferative TGF-β signaling pathways in human pancreatic cancer cells. Oncogene, 17, 1969–1978. [DOI] [PubMed] [Google Scholar]
- Wieser R., Wrana,J.L. and Massagué,J. (1995) GS domain mutations that constitutively activate TβR-1, the downstream signaling component in the TGF-β receptor complex. EMBO J., 14, 2199–2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrana J.L, Attisano,L., Wieser,R., Ventura,F. and Massagué,J. (1994) Mechanism of activation of the TGF-β receptor. Nature, 370, 341–347. [DOI] [PubMed] [Google Scholar]
- Yamaguchi K., Shirakabe,K., Shibuya,H., Irie,K., Oishi,I., Ueno,N., Taniguchi,T., Nishida,E. and Matsumoto,K. (1995) Identification of a member of the MAPKKK family as a potential mediator of TGF-β signal transduction. Science, 270, 2008–2011. [DOI] [PubMed] [Google Scholar]
- Yamaguchi K. et al. (1999) XIAP, a cellular member of the inhibitor of apoptosis protein family, links the receptors to TAB1–TAK1 in the BMP signaling pathway. EMBO J., 18, 179–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Zhu,H., Murphy,T.L., Ouyang,W. and Murphy,K.M. (2001) IL-18-stimulated GADD45β required in cytokine-induced, but not TCR-induced, IFN-γ production. Nat. Immunol., 2, 157–164. [DOI] [PubMed] [Google Scholar]
- Zhan Q. et al. (1994) The gadd and MyD genes define a novel set of mammalian genes encoding acidic proteins that synergistically suppress cell growth. Mol. Cell. Biol., 14, 2361–2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D., Gaussin,V., Taffet,G.E., Belaguli,N.S., Yamada,M., Schwartz, R.J., Michael,L.H., Overbeek,P.A. and Schneider,M.D. (2000) TAK1 is activated in the myocardium after pressure overload and is sufficient to provoke heart failure in transgenic mice. Nat. Med., 6, 556–563. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Feng,X.-H., Wu,R.-Y. and Derynck,R. (1996) Receptor-associated Mad homologues synergize as effectors of the TGF-β response. Nature, 383, 168–172. [DOI] [PubMed] [Google Scholar]