A novel inducible transactivation domain in the androgen receptor: implications for PRK in prostate cancer (original) (raw)
Abstract
In addition to the classical activation by ligands, nuclear receptor activity is also regulated by ligand-independent signalling. Here, we unravel a novel signal transduction pathway that links the RhoA effector protein kinase C-related kinase PRK1 to the transcriptional activation of the androgen receptor (AR). Stimulation of the PRK signalling cascade results in a ligand-dependent superactivation of AR. We show that AR and PRK1 interact both in vivo and in vitro. The transactivation unit 5 (TAU-5) located in the N-terminus of AR suffices for activation by PRK1. Thus, TAU-5 defines a novel, signal-inducible transactivation domain. Furthermore, PRK1 promotes a functional complex of AR with the co-activator TIF-2. Importantly, PRK signalling also stimulates AR activity in the presence of adrenal androgens, which are still present in prostate tumour patients subjected to testicular androgen ablation therapy. Moreover, PRK1 activates AR even in the presence of the AR antagonist cyproterone acetate that is used in the clinical management of prostate cancer. Since prostate tumours strongly overexpress PRK1, our data support a model in which AR activity is controlled by PRK signalling.
Keywords: androgen receptor/nuclear hormone receptor/prostate cancer/protein kinase C-related kinase/transcriptional co-activator
Introduction
The androgen receptor (AR) is a member of the steroid hormone receptor family of ligand-activated transcription factors that regulate diverse biological functions including cell growth and differentiation, development, homeostasis and various organ functions in the adult (Mangelsdorf et al., 1995; Cato and Peterzierl, 1998). The AR shares a common modular structure with other nuclear receptors and is composed of several domains that mediate DNA binding, dimerization, ligand binding and transcriptional activity (Mangelsdorf et al., 1995). The ligand binding domain (LBD) performs a number of functions including transcriptional repression or activation by generating the proper interaction surface for multiple partners, including co-repressors and co-activators (Renaud and Moras, 2000). Like other steroid receptors the AR contains a C-terminal ligand-dependent activation function 2 (AF-2) and an additional N-terminal ligand-independent activation function 1 (AF-1). The AR also harbours a less well-defined transcription activation unit termed TAU-5 (Jenster et al., 1995; Jenster, 1999). The TAU-5 is located between amino acids 360 and 528 and is required for full activation of the AR (Jenster et al., 1995; Bevan et al., 1999). However, signals that control TAU-5, and thereby AR activity, are unknown. Recent data show agonist-induced interaction between the N-terminal domain (NTD) and the LBD of the AR, supporting the view that both the AF-1 and the AF-2 are required for transcriptional activation (Ikonen et al., 1997; He et al., 2000). It has been suggested that the ligand-induced conformational changes of the LBD in concert with physical interactions with the NTD render the AR fully active (Ikonen et al., 1997; Bevan et al., 1999).
Androgens and the AR are essential for the differentiation, development and maintenance of male reproductive functions and non-reproductive organs (Trapman and Brinkmann, 1996; Jenster, 1999). In the prostate, the AR is expressed in secretory epithelial cells that respond to androgens. According to the current model, the AR plays an important role in the development of prostate cancer that mainly originates from epithelial cells. Growth and survival of primary prostate cancer cells is critically dependent on androgens (Gregory et al., 1998). Consequently, androgen ablation therapy is the standard clinical procedure used to inhibit prostate tumour growth. However, despite reduced circulating androgen levels and even in the presence of AR antagonists, most prostate cancers recur and progress to a terminal stage. The molecular mechanisms responsible for tumour recurrence are not well defined, but one possible scenario might be the stimulation of AR activity by cross-talk with other signalling pathways (Culig et al., 1994).
In addition to the classical ligand-induced activation mechanisms, nuclear receptors use alternative strategies to transmit extracellular signals into altered gene expression programs. Growth factors or stimulation of protein kinases A (PKA) or C (PKC) activate the AR even in the absence of ligand, or show a synergistic effect with ligand (Trapman and Brinkmann, 1996). Growth factor-regulated signalling pathways also control the activity of nuclear receptor co-activators. Recent data show that co-activators of the p160 family such as SRC-1 or AIB1 are direct targets of mitogen activated protein (MAP) kinases, which modulate their transcriptional activity (Font de Mora and Brown, 2000; Rowan et al., 2000). An alternative strategy to regulate co-factor function is employed by Rho GTPases. RhoA induces translocation of the co-activator FHL2 from the cell membrane to the nucleus, leading to transcriptional activation of FHL2- and AR-dependent genes (Müller et al., 2002).
By affecting multiple signalling pathways Rho family members regulate cell cycle progression, cellular transformation, metastasis and have been implicated in transcriptional regulation (Bar-Sagi and Hall, 2000). The Rho family GTPases work as molecular switches, being inactive when bound to GDP and active when bound to GTP. The exchange of GTP for GDP induces a conformational change that allows the GTPases to bind effector molecules such as the protein kinase C-related kinases (PRKs), which initiate downstream signalling events (Hall, 1998; Bishop and Hall, 2000). The PRKs constitute a subfamily of serine/threonine kinases that comprises PRK1 (also called PKN), PRK2 and PKNβ (Mukai et al., 1995; Palmer et al., 1995; Oishi et al., 1999). PRKs contain three highly-conserved regions: (i) an N-terminal regulatory domain spanning three repeats of charged amino acids with leucine zipper-like sequences (designated CZ region); (ii) a C-terminal catalytic domain; and (iii) a so-called D region located between the CZ region and the catalytic domain (Mukai et al., 1995). RhoA binds to the CZ region through the RhoA effector motif class I in a GTP-dependent manner thereby enhancing PRK kinase activity. The physical interaction of PRK1 with transcription factors such as NeuroD2 or PCD17, and the nuclear localization suggest a potential role for PRKs in transcriptional regulation (Takanaga et al., 1998; Shibata et al., 1999).
Recently, we demonstrated that stimulation of the Rho signalling pathway induces translocation of the co-activator FHL2 to the nucleus leading to activation of the AR by FHL2 (Müller et al., 2002). We now identify a novel pathway independent of FHL2 that links the RhoA effectors PRK1 and PRK2 to the transcriptional activation of AR. Here we show that stimulation of PRK1 signalling by extracellular stimuli or co-expression of constitutively active RhoA or PRK1 mutants results in ligand-dependent superactivation of AR-regulated genes. AR and PRK1 interact both in vivo and in vitro. Structure–function analyses demonstrate that the TAU-5 in the AR-NTD suffices for activation by PRK1, thus defining the TAU-5 as a novel, signal-inducible transactivation domain. In addition, PRK1 signalling also induces the transcriptional activity of mineralocorticoid receptor (MR), progesterone receptor (PR) and p160 co-activators, further supporting the importance of this signalling pathway for the transcriptional regulation of nuclear receptors. Our data show that prostate tumours highly overexpress PRK1. Importantly, PRK1 activates AR not only in the presence of agonists, but also when adrenal androgens or even the antagonist cyproterone acetate (CPA) are present. Taken together, our results demonstrate that the transcriptional activity of AR is controlled by PRK signalling in addition to ligands, providing an explanation for prostate cancer growth even during androgen ablation therapy.
Results
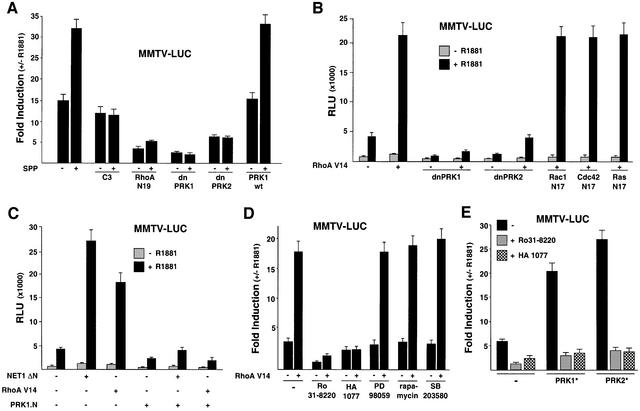
The current dilemma in androgen ablation therapy is the aberrant regulation of AR activity. This clearly points out the relevance of other yet undefined signalling pathways leading to AR-dependent tumour growth. Recently, we showed that Rho family members are overexpressed in human prostate tumours and that stimulation of the Rho signalling pathway leads to an agonist-dependent co-activation of AR-regulated target genes (Müller et al., 2002). To further delineate the mechanisms of Rho signalling to AR, we tested the influence of Rho effectors on AR transcriptional activity. Therefore, we stimulated AR-dependent gene expression with physiological concentrations of the bioactive lipid sphingosine-1-phosphate (SPP) in the presence of AR and an MMTV luciferase reporter (MMTV-LUC). The serum component SPP activates small GTPases of the Rho family via G protein-coupled receptors (Pyne and Pyne, 2000). SPP stimulation of endogenous Rho GTPases increases the agonist-dependent activation of the MMTV-LUC reporter (Figure 1A; Müller et al., 2002). Both the agonist and SPP-induced reporter activity is blocked by either the Rho-specific inhibitor Clostridium botulinum C3 exoenzyme (Busch and Aktories, 2000) or the dominant-negative RhoA mutant RhoA N19 (Figure 1A). In addition, the dominant-negative mutants of the Rho effectors PRK1 (dnPRK1) and PRK2 (dnPRK2) potently inhibit agonist and SPP-induced AR activity (Figure 1A). Wild-type PRK1 (PRK1wt) does not influence AR transcriptional activity (Figure 1A). In the absence of either AR or ligand the MMTV-LUC reporter is not influenced (our unpublished data). The dominant-negative PRK mutants also block ligand-dependent stimulation of AR by the constitutively active RhoA mutant RhoA V14 (Figure 1B), whereas the dominant-negative mutants Rac1 N17, Cdc42 N17 or Ras N17 fail to show any inhibitory effect (Figure 1B). All dominant-negative mutants are functional in our assay system and down-regulate the v-Src-induced activity of a SRE-LUC reporter (Chiariello et al., 2001; our unpublished data). Furthermore, neither the various signal transduction factors nor the chemical inhibitors used in this study alter AR protein levels (see Supplementary figure 1, available at The EMBO Journal Online). Taken together, these data show for the first time an involvement of PRKs in RhoA-mediated activation of AR.
Fig. 1. The RhoA effectors PRK1 and PRK2 mediate transcriptional superactivation of the AR. 293 (A–E and G) and PC3-AR cells (F) were transfected with MMTV-LUC reporter and AR expression plasmids with or without 10–10 M R1881. (A) Stimulation of the endogenous Rho signalling pathway by SPP leads to the activation of AR. Activation is blocked either by C3 exoenzyme, or by dominant-negative forms of RhoA (RhoA N19), PRK1 (dnPRK1), PRK2 (dnPRK2), but not wild-type PRK1 (PRK1wt). Cells were treated with either vehicle or 1.2 µM SPP. (B) Dominant-negative PRK1 (dnPRK1) and PRK2 (dnPRK2), but not the dominant-negative forms of Rac1 (Rac1 N17), Cdc42 (Cdc42 N17) or Ras (Ras N17) block AR activation by constitutively active RhoA (RhoA V14). (C) The dominant-negative acting N-terminus of PRK1 (PRK1.N) blocks AR activation by RhoA V14 or by the RhoA-specific constitutively active GEF NET1 (NET1 ΔN). (D) Ligand-dependent activation of AR by RhoA V14 is blocked by the PRK inhibitors Ro31-8220 or HA 1077 but not by the MEK1 inhibitor PD 98059, the S6 kinase/TOR inhibitor rapamycin or the p38 MAP kinase inhibitor SB 203580. As indicated, cells were transfected with RhoA V14 expression plasmids and treated with either 5 × 10–7 M Ro31-8220, 3 × 10–5 M HA 1077, 50 ng/ml rapamycin, 10–5 M PD 98059 or 10–5 M SB 203580. (E) AR is blocked by the PRK inhibitors Ro31-8220 or HA 1077. Cells co-transfected with the constitutively active forms of PRK1 (PRK1*) or PRK2 (PRK2*) were cultured in the absence or presence of the PRK inhibitors Ro31-8220 (5 × 10–7 M) or HA 1077 (3 × 10–5 M). (F) The PRK inhibitor Ro31-8220 blocks endogenous AR activity in PC3-AR prostate tumour cells. Cells were treated with 7 × 10–7 M Ro31-8220. (G) Influence of Rho effector signalling molecules on AR activity. Wild-type LIMK1 and LIMK2, constitutively active Rho-kinase α (ROCK*), MKK3b [MKK3E(b)], or MKK6b [MKK6E(b)] and dominant-negative p38γ (dnp38γ) or protein kinase Cα (dnPKCα) were analysed for their ability to modulate AR activity. Constitutively active PRK1 (PRK1*) was co-expressed as indicated.
To examine the Rho→PRK signalling pathway leading to AR activation in more detail, we tested whether expression of GDP–GTP exchange factors (GEFs) is able to promote AR-dependent gene expression. GEFs bind small GTPases and are involved in promoting nucleotide exchange (Cerione and Zheng, 1996). The activated form of the Rho-specific GEF NET1 (NET1 ΔN) stimulates the AR- and agonist-dependent activity of MMTV-LUC in 293 cells to a similar extent as the constitutively active RhoA V14 (Figure 1C). We then used the N-terminus of PRK1 (PRK1.N), which acts as a dominant-negative mutant in RhoA- and NET1 signalling by binding to RhoA–GTP (Sahai et al., 1998). When tested in our assay system PRK1.N blocks NET1 ΔN and RhoA V14 stimulated agonist-dependent AR activation (Figure 1C), further indicating the importance of PRK for AR activation. Accordingly, the PRK inhibitors Ro31-8220 or HA 1077 (Davies et al., 2000) abolished agonist-dependent AR activation by RhoA V14 (Figure 1D). In contrast, blocking the MAP kinase pathway by the MEK1 inhibitor PD 98059, inhibiting S6 kinase/TOR with rapamycin, or using the p38 MAP kinase inhibitor SB 203580 does not interfere with the transcriptional activation by RhoA V14 (Figure 1D). The effectiveness of the inhibitors (our unpublished data) was confirmed according to Müller et al. (2002). Next, we analysed constitutively active mutants of PRK1 (PRK1*) and PRK2 (PRK2*). As demonstrated in Figure 1E, AR is strongly activated by both constitutively active mutants of PRK in an agonist-dependent manner and both PRK inhibitors Ro31-8220 or HA 1077 block this activation. Furthermore, Ro31-8220 also blocks endogenous AR transcriptional activity in PC3-AR prostate tumour cells (Figure 1F). As a control, we analysed thyroid hormone receptor α-dependent reporter activity, which is neither influenced by co-expression of PRKs nor by treatment with Ro31-8220 (our unpublished data). Since Ro31-8220 is known to also inhibit the kinases PKCα, RSK, MSK, S6K and GSK3β (Davies et al., 2000), we verified that these kinases do not influence AR activity (Figure 1G; our unpublished data). To further validate PRK as the downstream effector of Rho, we assayed several molecules involved in Rho signalling (Bishop and Hall, 2000). Neither wild-type LIM kinase1 (LIMK1) or LIM kinase2 (LIMK2), nor constitutively active Rho–kinase α (ROCK*), MKK3b [MKK3E(b)], or MKK6b [MKK6E(b)] are able to induce AR-dependent reporter expression (Figure 1G). In addition, neither dominant-negative mutants of p38γ (dnp38γ) or PKCα (dnPKCα) (Figure 1G), nor DMPK, PAK, CRIK or MRCKα (our unpublished data), show any effect. Taken together, these results demonstrate that blocking the endogenous Rho→PRK signalling pathway by PRK inhibitors or dominant-negative PRK mutants severely impairs agonist-dependent AR transactivation. In addition, our data show that constitutively active mutants of the Rho effector PRK stimulate agonist-dependent transcriptional activity of AR.
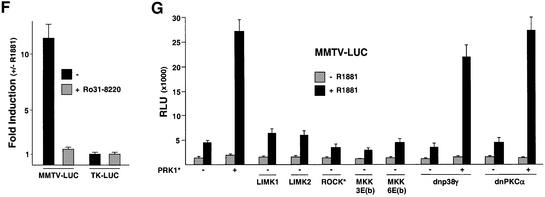
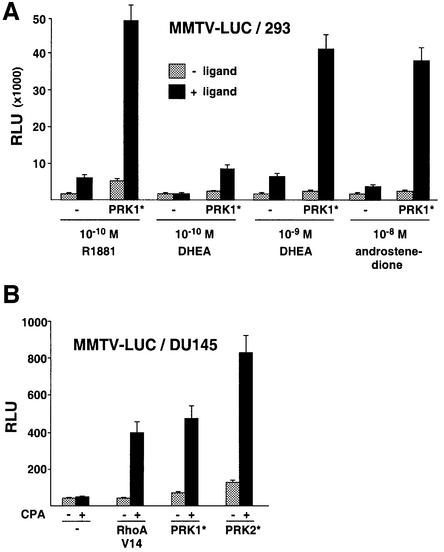
To examine the potential regulation of other steroid hormone receptors by PRK1 signalling the MMTV promoter was chosen because the receptors for glucocorticoids (GR), PR, MR and AR are known to regulate its expression in a ligand-dependent fashion (Schüle et al., 1990). Co-expression of constitutively active PRK1 and MR or PR results in a ligand-dependent superactivation (Figure 2A), whereas the ligand-dependent transcriptional activity of GR is only marginally influenced. The same results were obtained when the activation assay was performed with different agonist concentrations for the various receptors (our unpublished data). When tested on the prostate-specific probasin promoter or on MMTV-LUC, PRK signalling stimulates agonist-dependent AR activity in 293 or DU145 human prostate tumour cells (Figure 2B). These results show that stimulation of AR by PRKs is functional in different cell types and not restricted to a special promoter context.
Fig. 2. Analysis of the PRK signalling. (A) PRK1 signalling mediates activation of AR, MR and PR, but not of GR. 293 cells were co-transfected with MMTV-LUC reporter and expression plasmids coding for either AR, MR, PR or GR in combination with or without PRK1*. Cells were treated with the corresponding agonists (10–10 M R1881, 10–9 M aldosterone, 10–9 M R5020 or 10–10 M DEX). (B) AR superactivation by PRK is independent of a particular promoter context or cell type. 293 and DU145 cells were stimulated with 10–10 M R1881 as indicated. Cells were co-transfected with AR, constitutively active RhoA V14, PRK1* or PRK2*, and either MMTV-LUC or PB-LUC.
Using immunofluorescence analyses we investigated the subcellular distribution of AR and PRK proteins. AR and the constitutively active mutant PRK1* are present in the cytoplasm in the human 293 cell line in the absence of ligand, whereas addition of the AR agonist R1881 results in nuclear AR and triggers PRK1* nuclear localization (Table I). Equally, CPA promotes nuclear localization of both proteins. In contrast, the agonist-loaded mutant AR (ARΔNLS) that is defective in nuclear transloca tion (Poukka et al., 2000) does not support nuclear redistribution of PRK1* (Table I). In agreement with the transactivation data, agonist-loaded MR but not GR leads to enhanced nuclear translocation of PRK1* (Table I).
Table I. Subcellular localization of PRK1.
| Transfection | Ligand | PRK1 staining (%) | |
|---|---|---|---|
| C | C + N and N | ||
| PRK1* | – | 79 | 21 |
| PRK1* | R1881 | 78 | 22 |
| PRK1* + AR | – | 77 | 23 |
| PRK1* + AR | R1881 | 45 | 55 |
| PRK1* + AR | CPA | 52 | 48 |
| PRK1wt + AR | – | 100 | 0 |
| PRK1wt + AR | R1881 | 97 | 3 |
| PRK1* + AR ΔNLS | – | 67 | 33 |
| PRK1* + AR ΔNLS | R1881 | 72 | 28 |
| PRK1* + GFP-MR | – | 77 | 23 |
| PRK1* + GFP-MR | aldosterone | 56 | 44 |
| PRK1* + GR-GFP | – | 86 | 14 |
| PRK1* + GR-GFP | DEX | 79 | 21 |
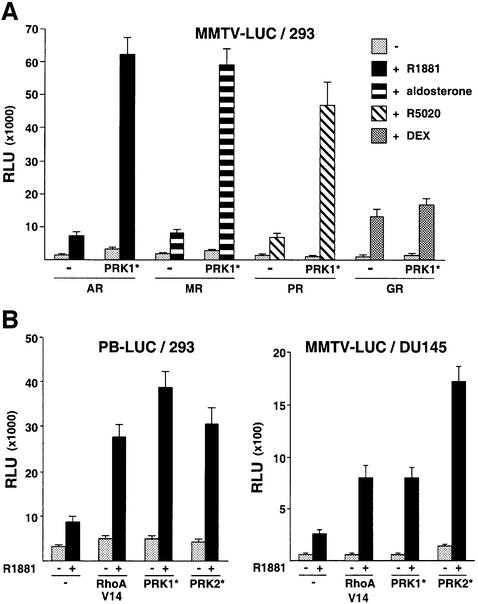
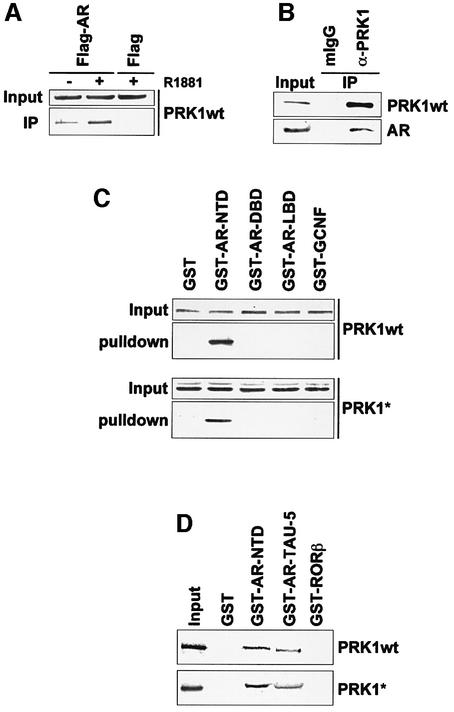
To demonstrate interaction between AR and PRK1, extracts from 293 cells transfected with expression plasmids for PRK1 and Flag epitope-tagged AR were immunoprecipitated using an α-Flag antibody (Figure 3A). Western blot analysis shows that AR and PRK1 interact weakly in the absence of ligand, whereas the Flag-AR– PRK1 complex is efficiently precipitated in the presence of the AR agonist R1881. No PRK1 was found in immunoprecipitated complexes using Flag transfected control extracts (Figure 3A), thus demonstrating specificity. Similar results were obtained with constitutively active PRK1 (our unpublished data). More importantly, AR and PRK1 endogenously expressed in primary human prostate tumours associate in vivo (Figure 3B). Extracts from primary human prostate tumours treated with R1881 were immunoprecipitated using an α-PRK1 antibody. As shown in Figure 3B, the endogenous AR is efficiently co-immunoprecipitated with the α-PRK1 antibody, thus demonstrating the relevance of this interaction in vivo. To further analyse the interaction, we performed in vivo GST pull-down experiments (Figure 3C). The fusion proteins GST–AR-NTD, GST–AR-DBD and GST–AR- LBD were co-expressed with either myc epitope-tagged constitutively active PRK1 (PRK1*) or wild-type PRK1 (PRK1wt) in 293 cells. Protein complexes were affinity purified from whole-cell extracts on glutathione– Sepharose and subsequently detected using either α-myc or α-PRK1 antibodies. As illustrated in Figure 3C, both PRK1wt and PRK1* specifically interact with the AR-NTD. In contrast, the AR-DBD or AR-LBD fails to do so. As expected, no interaction is observed with the control proteins GST or GST–GCNF. The AR-NTD also physically interacts with PRK1wt and PRK1* in vitro (Figure 3D). Pull-down experiments with bacterially expressed GST–AR-NTD fusion protein and [35S]methio nine-labelled in vitro translated PRK1wt and PRK1* show interaction with the AR-NTD or the AR-TAU-5 respectively, but not with the control proteins GST or GST–RORβ. In conclusion, this set of experiments demonstrates association of AR and PRK1 both in vivo and in vitro.
Fig. 3. PRK1 interacts with the AR in vivo and in vitro. (A) PRK1 co-immunoprecipitates with the AR. 293 cells transfected with either expression plasmids for wild-type PRK1 (PRK1wt), Flag-AR or Flag alone in the absence or presence of 10–10 M R1881. Extracts were immunoprecipitated with an α-Flag antibody. Five per cent of the extract used for immunoprecipitation was loaded as input. Western blots were decorated with an α-PRK1 antibody. (B) AR co-immunoprecipitates with PRK1. Extracts from primary human prostate tumours were immunoprecipitated with an α-PRK1 antibody or mouse IgG (control) in the presence of 10–10 M R1881. Five per cent of the extract used for immunoprecipitation was loaded as input. Western blots were decorated with α-AR and PRK1 antibodies. (C) PRK1 associates with the NTD of AR. In vivo GST pull-down assays were performed using lysates from 293 cells transfected with expression plasmids for either PRK1wt or myc-tagged PRK1* and GST fusion proteins of the indicated AR domains. Five per cent of the extract used for immunoprecipitation was loaded as input. Western blots were decorated with either α-PRK1 or α-myc antibodies. GST or GST–GCNF proteins were used as controls. (D) PRK1 interacts with the AR-NTD or the TAU-5 in vitro. GST pull-down assays were performed with [35S]methionine-labelled PRK1wt or PRK1* with the corresponding bacterially expressed GST–AR fusion proteins. GST and GST–RORβ proteins were used as controls.
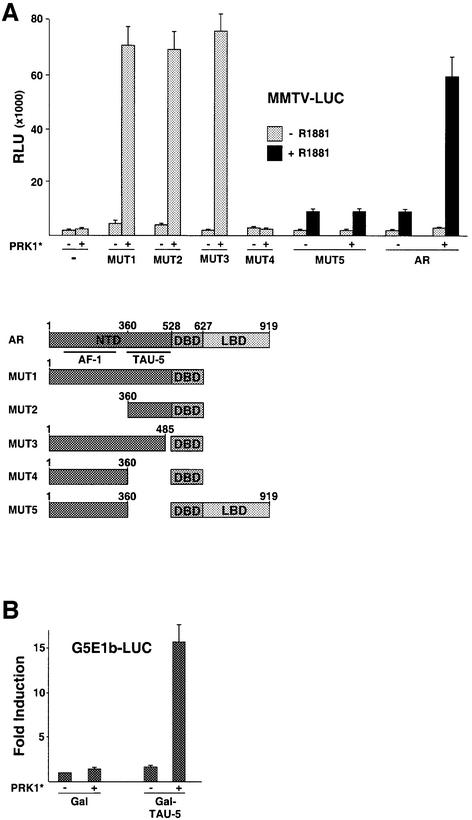
To delineate the domains in the AR that suffice for PRK1 signalling, we tested AR deletion mutants in transactivation assays. Deletion of the entire LBD creates the ligand-independent, constitutively active mutant AR1–627 (Jenster et al., 1995). This mutant (MUT1) is strongly activated by PRK1 signalling (Figure 4A) thus demonstrating that a yet undescribed PRK-inducible transactivation function is located in the AR-NTD. Surprisingly, further analyses revealed that not the AF-1 located between residues 150–350, but the TAU-5 spanning residues 360–528 is responsible for the stimulation by PRK1* (Figure 4A). Conversely, when fused to a heterologous Gal4 DNA binding domain the resulting mutant Gal–TAU-5 suffices for potent transcriptional activation by constitutively active PRK1 (Figure 4B). Taken together, these results show that the AR-TAU-5 is the first PRK-inducible transactivation function described.
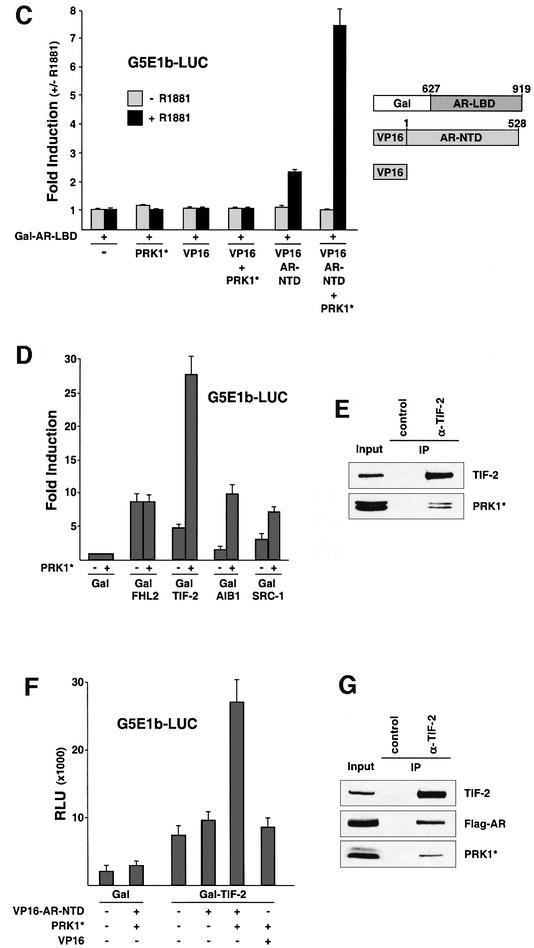
Fig. 4. Activation mode of PRK1. (A) The AR N-terminal TAU-5 domain suffices for transcriptional activation by PRK1. The schematically depicted AR deletion mutants were analysed on the MMTV-LUC reporter in 293 cells with or without co-expression of PRK1*. Deletion mutants containing the LBD of AR were analysed with or without 10–10 M R1881. (B) The activity of Gal–TAU-5 was analysed on the Gal-dependent reporter G5E1b-LUC in the absence or presence of PRK1*. Relative Gal activity was set to 1. (C) PRK1 signalling enhances the N- and C-terminal interaction of AR in an agonist-dependent manner. 293 cells co-transfected with the G5E1b-LUC reporter and Gal–AR-LBD expression plasmids were stimulated with 10–10 M R1881. As indicated, PRK1*, VP16 and VP16–AR-NTD were co-expressed. (D) Co-factor activation by PRK1. Gal fusion proteins of the co-activators FHL2, TIF-2, AIB1 and SRC-1 were tested on G5E1b-LUC in 293 cells with PRK1* as indicated. Relative Gal activity was set to 1. (E) PRK1 associates with the transcriptional co-activator TIF-2 in vivo. 293 cells were transfected with expression plasmids for myc-tagged constitutively active PRK1 (PRK1*). Extracts were immunoprecipitated with α-TIF-2 or control antibodies. Five per cent of the extract was loaded as input. Western blots were decorated with α-TIF-2 or α-myc antibodies. (F) PRK1 signalling enhances the interaction of TIF-2 and AR-NTD. 293 cells were transfected with the G5E1b-LUC reporter. As indicated, Gal, Gal–TIF-2, PRK1*, VP16 and VP16–AR-NTD were co-expressed. (G) AR and PRK1 co-immunoprecipitate with TIF-2. 293 cells were transfected with expression plasmids for myc-tagged constitutively active PRK1 (PRK1*) and AR (Flag-AR). Extracts were treated with α-TIF-2 or control antibodies to immunoprecipitate endogenous TIF-2. Five per cent of the extract was loaded as input. Western blots were decorated with α-TIF-2, α-Flag or α-myc antibodies.
Recent data demonstrate that ligand-induced conformational changes of the AR-LBD in concert with physical interactions with the AR-NTD result in transcriptional activation of the AR whereby p160 co-activators promote these functional interactions (Ikonen et al., 1997; Bevan et al., 1999). To further analyse the functional consequences of PRK1 signalling, we performed mammalian two-hybrid assays with Gal–AR-LBD and VP16–AR- NTD fusion proteins. In agreement with previous data (Ikonen et al., 1997) the LBD and the NTD interact in a ligand-dependent manner, which is reflected in agonist-induced reporter activity (Figure 4C). In the presence of constitutively active PRK1 the functional interaction of the AR-LBD with the NTD is greatly enhanced, thus providing a mechanistic explanation for the stimulation of AR activity (Figure 4C). Since PRK1 neither contains an autonomous transactivation domain nor phosphorylates the AR, nor enhances the physical interaction between the AR-LBD and NTD in GST pull-down assays (our unpublished data), we tested whether PRK1 signalling might stimulate AR by modulating the transcriptional activity of AR-associated co-factors (Figure 4D). The co-activator FHL2 does not respond to PRK1*, clearly demonstrating that PRK1 signalling is independent of FHL2 (Figure 4D). In contrast, the transcriptional activity of the p160 co-activators TIF-2, AIB1 and SRC-1 is significantly stimulated by PRK1* (Figure 4D). Co-immunoprecipitation experiments performed with an α-TIF-2 antibody and cell extracts from 293 cells transfected with PRK1* show that endogenously expressed TIF-2 interacts efficiently with PRK1* in vivo (Figure 4E). Since PRK1* interacts with both AR and TIF-2, we then asked whether PRK1* would be able to promote a functional interaction between the two proteins. Mammalian two-hybrid assays with Gal–TIF-2 and VP16–AR-NTD fusion proteins show low reporter activity reflecting weak interaction (Figure 4F). In the presence of limited amounts of PRK1* Gal–TIF-2 activity is only weakly stimulated, whereas the functional interaction between Gal–TIF-2 and VP16–AR-NTD is significantly enhanced (Figure 4F). In addition, immunoprecipitation of endogenously expressed TIF-2 with an α-TIF-2 antibody results in the co-precipitation of AR and PRK1* (Figure 4G). This data further suggest the formation of a functional complex consisting of TIF-2, AR and PRK1*.
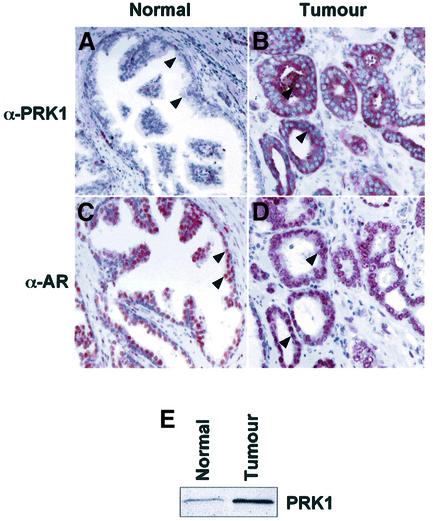
Since p160 co-activators and Rho family members are overexpressed in human prostate tumours (Gregory et al., 2001; Müller et al., 2002), we analysed the expression of PRK1 in human prostate in normal and pathologic situations. Immunohistochemical analyses of PRK1 expression were performed in tissue sections obtained from radical prostatectomies, which contained areas of benign prostate as well as prostate carcinoma. Neither in the secretory epithelium of normal prostate tissue nor in the cells of the normal basal compartment was significant PRK1 immunoreactivity detected (Figure 5A, arrowheads). All cancer specimens obtained from ten different radical prostatectomies revealed a robust increase in PRK1 immunoreactivity (Figure 5B, arrowheads). We also compared AR expression in normal prostate versus prostate tumours in the same cancer specimens used above. As indicated for the majority of prostate tumours (Leav et al., 2001), AR expression is not significantly altered (Figure 5C and D, arrowheads). In addition, western blot analysis from resected human prostate tissue shows that PRK1 is overexpressed in prostate tumours compared with normal prostate (Figure 5E). Taken together, our data demonstrate strong overexpression of PRK1 in prostate carcinoma and co-expression of AR and PRK1 in vivo.
Fig. 5. Overexpression of PRK1 in human prostate cancer. Little PRK1 immunoreactivity is detected in the secretory epithelium of normal prostate (A, arrowheads), but strong PRK1 immunoreactivity is detected in prostate tumours (B, arrowheads). AR immunostaining reveals the presence of AR in benign secretory cells of the gland (C, arrowheads), as well as in carcinoma cells (D, arrowheads). All sections were taken from the same radical prostatectomy specimen. Magnification: ×250. (E) Normal human prostate and prostate tumour extracts were analysed in western blots using an α-PRK1 antibody.
Finally, we analysed whether stimulation of AR transcriptional activity by Rho→PRK signalling is regulated by clinically relevant AR ligands. During testicular androgen ablation therapy, adrenal androgens present in the peripheral blood play an important role in AR-dependent prostate cancer progression, since adrenal glands continue to supply the adrenal androgens dehydroepiandrosterone (DHEA) and androstenedione. These androgen metabolites exhibit weak agonist activity, but are metabolized into potent agonists such as dihydrotestosterone in the prostate (Straub et al., 1998; Gregory et al., 2001). Thus, we tested whether PRK1 signalling would be able to stimulate AR activity at physiological concentrations of the adrenal androgens DHEA and androstenedione in 293 cells that do not metabolize androgens. At concentrations of 10–10 M DHEA or 10–8 M androstenedione, respectively, AR is barely activated by these weak androgens (Figure 6A). Importantly, however, PRK1 signalling strongly induces AR activity in the presence of the adrenal androgens (Figure 6A). We then tested the anti-androgen CPA, which is commonly used during androgen ablation therapy to block the effects of androgens (Leewansangtong and Crawford, 1998). As expected, CPA at 10–5 M only marginally activates the AR (Figure 6B). However, PRK signalling potently stimulates AR activity in the presence of the antagonist CPA. Combined with the observed overexpression of PRK in prostate tumours (Figure 5) these results might, at least in part, provide an explanation for the clinically well-documented observation (Jenster, 1999) that during prostate cancer progression AR- dependent gene expression is upregulated in the absence of testicular androgens and even in the presence of antagonists.
Fig. 6. Ligand-selective stimulation of AR transcriptional activity by PRK. (A) Super-activation of the AR by PRK1 signalling in the presence of adrenal androgens. 293 cells were co-transfected with AR and MMTV-LUC in the presence or absence of the indicated androgens. (B) PRK signalling activates the AR in the presence of the anti-androgen CPA. DU145 cells were stimulated with or without 10–5 M CPA and co-transfected with the indicated expression plasmids.
Discussion
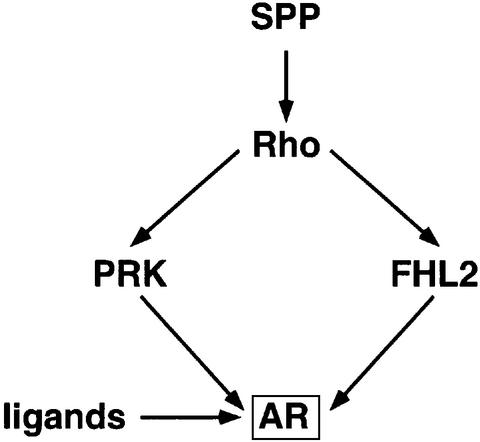
Here we have identified a novel signalling pathway that links the RhoA effectors PRK1 and PRK2 to the transcriptional activation of the AR (Figure 7). The combined use of either the PRK inhibitors Ro31-8220 or HA 1077, or dominant-negative and constitutively active mutants of PRK1 show that PRK is the Rho effector leading to AR-dependent activation. Since the PRK inhibitor Ro31-8220 also blocks GSK3β, S6K, RSK, MSK and PKCα activity (Davies et al., 2000), we excluded that these kinases mediate the regulation of AR by PRK signalling. Furthermore, by ruling out other major signalling pathways we demonstrate specificity for PRKs (Figure 1D, E and G).
Fig. 7. Model of the Rho signalling pathway controlling AR transcriptional activity.
So far, only the transcription factors ATF-2, MEF-2A and GATA-4 are known to be regulated by Rho→PRK signalling (Charron et al., 2001; Marinissen et al., 2001). Our results clearly demonstrate that the mechanism of AR activation is distinct from the p38γ-dependent Rho→PRK signalling that activates these transcription factors, since we excluded the upstream activators MKK3/6 of p38 kinases as regulators of AR (Figure 1G). Furthermore, neither dominant-negative mutants of p38 nor the p38 kinase inhibitor SB 203580 blocked stimulation of AR activity by PRK, demonstrating that p38α/β are also not involved (Figure 1D and G).
In this manuscript we demonstrate that constitutively active PRK1 stimulates the p160 co-activators TIF-2, AIB1 and SRC-1 (Figure 4D). In contrast, PRKs do not stimulate FHL2 transcriptional activity unravelling an alternative, FHL2-independent pathway for Rho→PRK signalling to the AR (Figures 4D and 7; Müller et al., 2002). In addition, PRK robustly modulates AR activity in FHL2-deficient cells further supporting a FHL2-independent pathway (our unpublished data). At this point, the relative importance of FHL2-dependent versus FHL2-independent RhoA signalling is not defined. The relative abundance of FHL2 and PRK1 might determine how Rho signalling acts on AR. In cells that express little or no functional FHL2, PRK1 signalling might be dominant.
Structure–function analyses identified that the yet ill-defined TAU-5 region suffices for PRK1-mediated activation of AR. So far TAU-5 was considered as a constitutively active transactivation domain necessary for maximal AR activity only (Jenster et al., 1995; Bevan et al., 1999). In contrast, our results establish that the TAU-5 is a novel, PRK-inducible transactivation domain. Therefore, AR activity is not only mediated by the N-terminal AF-1 but in addition is also controlled by the signal-regulated TAU-5. As shown by immunoprecipitation analyses and pull-down experiments, PRK1 and AR associate in vivo and in vitro. Indeed, the TAU-5 represents not only a novel PRK-inducible transactivation domain but also suffices for physical association with PRK1.
PRK1 signalling enhances the transcriptional activity of SRC-1, AIB1 and TIF-2 (Figure 4D). TIF-2 and PRK1* interact in vivo (Figure 4E and G), but phosphorylation of TIF-2 by PRK1* is not detected (our unpublished data). As shown in mammalian two-hybrid assays PRK1* supports the formation of both transcriptionally active NTD–LBD and NTD–TIF-2 complexes (Figure 4). This would suggest that binding of PRK1* to TIF-2 and the TAU-5 of AR results in a complex that contains all three proteins and is mediating PRK1 signalling. Since TIF-2 is able to activate MR and PR (Voegel et al., 1996; Fuse et al., 2000) the association of TIF-2 and PRK1* might explain our observation that these receptors are also stimulated by PRK1*. The reason for the unresponsiveness of GR are unknown.
We analysed the expression of the RhoA effector PRK1 in normal human prostate and prostate cancer and revealed strong overexpression in tumours. Our data corroborate earlier results that the PRK isoform PKNβ is barely detected in adult tissue but strongly upregulated in tumours (Oishi et al., 1999). In addition, we recently showed a dramatic increase in Rho expression in poorly differentiated human prostate tumours (Müller et al., 2002). Therefore, prostate tumours not only overexpress Rho GTPases, but also Rho effectors such as PRK1, thus indicating that overexpression of Rho signalling molecules might be a common feature in prostate tumourigenesis.
Of special importance is our observation that PRK signalling stimulates AR not only in the presence of agonists but also in the presence of CPA. The anti-androgen CPA is frequently used in prostate cancer therapy to block AR-dependent tumour growth, though the undesired, partial agonistic function of CPA is well known (Wong et al., 1993). Importantly, even at ligand concentrations where the antagonist CPA exhibits no partial agonistic function PRK signalling potently stimulates AR transcriptional activity (Figure 6B). During testicular androgen ablation therapy the concentrations of adrenal androgens such as DHEA and androstenedione in the peripheral blood of human males is of importance to control the growth of prostate tumours. Our data now show that AR is strongly stimulated by PRK1 signalling in the presence of physiological concentrations of DHEA and androstenedione. Prostate tumours not only overexpress co-activators such as TIF-2 and FHL2, and Rho GTPases, but also show a dramatic increase in PRK expression (Figure 5; Gregory et al., 2001; Müller et al., 2002). Therefore, our results provide, at least in part, an explanation for the clinically well documented observation that during prostate cancer progression AR-dependent gene expression is upregulated in the absence of testicular androgens or even in the presence of antagonists such as CPA. Consequently, PRK might be a promising therapeutic target and inhibitors of PRK signalling may turn out to be beneficial in the treatment of prostate cancer.
Materials and methods
Plasmids
The following plasmids were described previously: AR, PR, MR, GR, Gal-TIF-2, Gal-SRC-1, Gal-FHL2, CMX-Flag, CMX-GST, MMTV-LUC, PB-LUC, TK-LUC and G5E1b-LUC (Müller et al., 2000); RhoA V14, Rac1 N17, C3, Cdc42 N17, Ras N17, LIMK1, LIMK2, RhoA N19, PRK2* (tr.PRK2wt), NET1 ΔN and Gal-AIB1 (Müller et al., 2002); PRK1.N (PKN.N) (Sahai et al., 1998); PRK1* (mycΔNPRK1) and PRK1wt (Flynn et al., 1998); dnPRK1(PRK1 K644E) (Takahashi et al., 1998); dnPKCα (PKCαK368R) (Überall et al., 1997); dnp38γ [p38γ(AF)], MKK3E(b) and MKK6E(b) (Wang et al., 2000); ROCK* (ROKα1–543) (Leung et al., 1996); GST-AR-NTD (Alen et al., 1999); MUT1 to MUT5 (pAR5, 106, 126, 99 and 113), Gal-TAU-5 (G106) (Jenster et al., 1995); VP16-AR-NTD, Gal-AR-LBD and pFlag-AR (Ikonen et al., 1997). dnPRK2 (PRK2 KD1) was kindly provided by B.L.Quillian, Indianapolis; GST-TAU-5 (GST–AR 360–546), GFP-MR, GR-GFP and ARΔNLS (pSG5-ARΔ612–633) by A.C.Cato, Karlsruhe; GST-RORβ by E.Greiner, Heidelberg; GST-GCNF by H.Greschik, Strasbourg. To construct CMX-GST-AR-DBD, CMX-GST-AR-LBD, and pGEX4T1-AR-LBD the corresponding fragments (AR-DBD: amino acids 539–623; AR-LBD: amino acids 624–919) were PCR amplified and inserted at the _Bam_HI site of CMX-GST and pGEX4T1 (Pharmacia). All plasmids were verified by double-stranded sequencing.
Transfections
DU145 and 293 cells were cultured in DMEM and PC3-AR in Ham’s F-12 supplemented with 10% double-stripped fetal calf serum (dsFCS). Transient transfection assays were carried out in 12-well plates (1 × 105 cells per well) as described previously (Müller et al., 2002). After transfection, cells were cultured in DMEM or Ham’s F-12 supplemented with 0.5% dsFCS. The total amount of transfected DNA was kept constant (4 µg) by adding the corresponding amounts of empty expression plasmids and pUC18. The following amounts per well were used: 500 ng reporter plasmids MMTV-LUC, PB-LUC or G5E1b-LUC; 25 ng expression plasmids for AR, PR, ARΔNLS, GFP-MR, GR-GFP, GR, MR, dnPKCα, PRK1*, PRK2*, PRK1wt, VP16-AR-NTD or Gal-AR-LBD; 10 ng expression plasmids for Gal-AIB1, Gal-SRC-1, Gal-TIF-2 or Gal-FHL2; 12.5 ng expression plasmids for MKK3E(b), MKK6E(b), or ROCK*. One hundred nanogrammes of all other expression plasmids were transfected per well. Chemicals were obtained as indicated: PD 98059, SB 203580, SPP (BioMol); Ro31-8220 (Alexis Biochemicals); HA 1077 (Calbiochem); R5020, aldosterone, dexamethasone, DHEA and androstenedione (Sigma); R1881 and CPA (Schering AG, Berlin); rapamycin (ICN Biomedicals Inc.). All chemicals were applied for a total of 20 h except PC3-AR cells, which were treated for 8 h. Luciferase activity was assayed as described previously (Müller et al., 2002). All experiments were repeated at least five times.
Immunofluorescence
Transfected cells were analysed essentially as described previously (Müller et al., 2002). Cells were seeded on coverslips coated with fibronectin and gelatin. Primary antibody staining was performed with the indicated dilutions: α-AR (N-20) (1:4000; Santa Cruz), α-myc (1:10 000; Santa Cruz) and α-PRK1 (1:2000; Transduction Lab). Subcellular localization was visualized using secondary Alexa Fluor 488- and 546-labelled antibodies (1:4000; Molecular Probes). Nuclei were stained with 1 µg/ml DAPI (Roche).
Co-immunoprecipitation assays and western blot analyses
293 cells were transfected in 15 cm dishes with 10 µg of PRK1wt, PRK1*, Flag-AR or CMX-Flag and cultured in the presence of 10–10 M R1881 as indicated. Total cell extract was prepared in the presence or absence of 10–10 M R1881 in IP buffer (50 mM Tris–HCl pH 8.0, 170 mM NaCl, 0.1% NP-40, 20% glycerol, 50 mM NaF, 2 mM NaV, 0.2 mM DTT, 1 µg/ml BSA, 0.1 mM Pefabloc). Extracts from normal human prostate and prostate tumour tissue were prepared in the presence of 10–10 M R1881 in IP buffer. Following centrifugation, pre-cleared supernatants were incubated for 2 h with M2 α-Flag antibody (Sigma), α-TIF-2 (Transduction Lab), or α-PRK1 antibody (Transduction Lab) and GammaBind™–Sepharose 4B (Pharmacia) in IP buffer. Precipitated protein complexes were subsequently washed four times either in the presence or absence of 10–10 M R1881 and subsequently analysed on 10% SDS polyacrylamide gels. Western blots were decorated with M2, α-AR 441 (Santa Cruz), α-TIF-2, α-PRK1 or α-cyclinA (control) antibodies. Secondary antibody and chemoluminescence procedure was performed according to the manufacturer’s instructions (Amersham).
In vivo GST pull-down assays
For in vivo GST pull-down experiments, 293 cells grown in 15 cm dishes were co-transfected with 10 µg of expression vectors for GST, GST-AR-NTD, GST-AR-DBD, GST-AR-LBD, GST-GCNF, myc-tagged PRK1* or PRK1wt. Cells were grown for 18 h in DMEM containing 0.5% dsFCS with or without 10–10 M R1881. Whole-cell extracts were prepared by repeated freeze and thaw cycles either in NENT75-Mo buffer (20 mM Tris pH 6.8, 75 mM NaCl, 1 mM EDTA, 0.1% NP-40, 25% glycerol, 20 mM Na-molybdate) for cells transfected with myc-ΔNPRK1 or in buffer B1 (20 mM HEPES–KOH pH 7.7, 500 mM KCl, 25 mM MgCl2, 100 µM EDTA pH 8.0, 10 mM DTT, 0.15% NP-40) for cells transfected with PRK1wt. GST fusion proteins were immobilized on glutathione–Sepharose (Pharmacia) for 1 h at 4°C. After washing, bound proteins were analysed by SDS–PAGE, followed by immunodetection with α-myc or α-PRK1 antibodies. Equal expression of GST fusion proteins was controlled in western blots using an α-GST antibody. All antibodies were obtained from Santa Cruz.
In vitro GST pull-down assays
Expression of GST fusion proteins (Pharmacia) and the coupled in vitro transcription–translation reaction (Promega) were performed according to the manufacturer’s instructions. GST pull-down assays were performed as previously described (Müller et al., 2000) using buffer containing 250 mM KCl. Ten per cent of the in vitro translated proteins were loaded as input.
Immunohistochemistry
Stainings were performed using a protocol for antigen retrieval and indirect immunoperoxidase as described previously (Müller et al., 2000). α-PRK1 antibody (Transduction Lab) was used at a dilution of 1:50, anti-mouse IgG (1:500; Dako) was used as secondary antibody and immunoreactions were visualized with the ABC-complex diluted 1:50 in PBS (Vectastain, Vector).
Supplementary data
Supplementary data are available at The EMBO Journal Online.
Acknowledgments
Acknowledgements
This work was supported by grants from the Deutsche Forschungsgemeinschaft (SFB 388) and the Schering AG, Berlin to RS. We thank G.Baier, A.O.Brinkmann, M.Brown, A.C.Cato, F.Claessens, J.Han, K.S.Erdmann, O.A.Janne, L.Lim, Y.Ono, J.Palvimo, P.J.Parker, B.L.Quilliam, A.Soler, R.Treisman and U.Wetterauer for generously providing reagents and support, the members of the Schüle lab especially Philip Hublitz and Thomas Günther for fruitful discussion. Special thanks to Ellen Paggen for excellent technical assistance.
References
- Alen P., Claessens,F., Verhoeven,G., Rombauts,W. and Peeters,B. (1999) The androgen receptor amino-terminal domain plays a key role in p160 coactivator-stimulated gene transcription. Mol. Cell. Biol., 19, 6085–6097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Sagi D. and Hall,A. (2000) Ras and Rho GTPases: a family reunion. Cell, 103, 227–238. [DOI] [PubMed] [Google Scholar]
- Bevan C.L., Hoare,S., Claessens,F., Heery,D.M. and Parker,M.G. (1999) The AF1 and AF2 domains of the androgen receptor interact with distinct regions of SRC1. Mol. Cell. Biol., 19, 8383–8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop A.L. and Hall,A. (2000) Rho GTPases and their effector proteins. Biochem. J., 348, 241–255. [PMC free article] [PubMed] [Google Scholar]
- Busch C. and Aktories,K. (2000) Microbial toxins and the glycosylation of rho family GTPases. Curr. Opin. Struct. Biol., 10, 528–535. [DOI] [PubMed] [Google Scholar]
- Cato A.C. and Peterzierl,H. (1998) The androgen receptor as mediator of gene expression and signal transduction pathways. Trends Endocrinol. Metab., 9, 150–154. [DOI] [PubMed] [Google Scholar]
- Cerione R.A. and Zheng,Y. (1996) The Dbl family of oncogenes. Curr. Opin. Cell Biol., 8, 216–222. [DOI] [PubMed] [Google Scholar]
- Charron F., Tsimiklis,G., Arcand,M., Robitaille,L., Liang,Q., Molkentin,J.D., Meloche,S. and Nemer,M. (2001) Tissue-specific GATA factors are transcriptional effectors of the small GTPase RhoA. Genes Dev., 15, 2702–2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiariello M., Marinissen,M.J. and Gutkind,J.S. (2001) Regulation of c-myc expression by PDGF through Rho GTPases. Nat. Cell Biol., 3, 580–586. [DOI] [PubMed] [Google Scholar]
- Culig Z., Hobisch,A., Cronauer,M.V., Radmayr,C., Trapman,J., Hittmair,A., Bartsch,G. and Klocker,H. (1994) Androgen receptor activation in prostatic tumor cell lines by insulin-like growth factor-I, keratinocyte growth factor, and epidermal growth factor. Cancer Res., 54, 5474–5478. [PubMed] [Google Scholar]
- Davies S.P., Reddy,H., Caivano,M. and Cohen,P. (2000) Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem. J., 351, 95–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn P., Mellor,H., Palmer,R., Panayotou,G. and Parker,P.J. (1998) Multiple interactions of PRK1 with RhoA. Functional assignment of the Hr1 repeat motif. J. Biol. Chem., 273, 2698–2705. [DOI] [PubMed] [Google Scholar]
- Font de Mora J. and Brown,M. (2000) AIB1 is a conduit for kinase-mediated growth factor signaling to the estrogen receptor. Mol. Cell. Biol., 20, 5041–5047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuse H., Kitagawa,H. and Kato,S. (2000) Characterization of transactivational property and coactivator mediation of rat mineralocorticoid receptor activation function-1 (AF-1). Mol. Endocrinol., 14, 889–899. [DOI] [PubMed] [Google Scholar]
- Gregory C.W., Hamil,K.G., Kim,D., Hall,S.H., Pretlow,T.G., Mohler,J.L. and French,F.S. (1998) Androgen receptor expression in androgen-independent prostate cancer is associated with increased expression of androgen-regulated genes. Cancer Res., 58, 5718–5724. [PubMed] [Google Scholar]
- Gregory C.W., He,B., Johnson,R.T., Ford,O.H., Mohler,J.L., French,F.S. and Wilson,E.M. (2001) A mechanism for androgen receptor-mediated prostate cancer recurrence after androgen deprivation therapy. Cancer Res., 61, 4315–4319. [PubMed] [Google Scholar]
- Hall A. (1998) Rho GTPases and the actin cytoskeleton. Science, 279, 509–514. [DOI] [PubMed] [Google Scholar]
- He B., Kemppainen,J.A. and Wilson,E.M. (2000) FXXLF and WXXLF sequences mediate the NH2-terminal interaction with the ligand binding domain of the androgen receptor. J. Biol. Chem., 275, 22986–22994. [DOI] [PubMed] [Google Scholar]
- Ikonen T., Palvimo,J.J. and Janne,O.A. (1997) Interaction between the amino- and carboxyl-terminal regions of the rat androgen receptor modulates transcriptional activity and is influenced by nuclear receptor coactivators. J. Biol. Chem., 272, 29821–29828. [DOI] [PubMed] [Google Scholar]
- Jenster G. (1999) The role of the androgen receptor in the development and progression of prostate cancer. Semin. Oncol., 26, 407–421. [PubMed] [Google Scholar]
- Jenster G., van der Korput,H.A., Trapman,J. and Brinkmann,A.O. (1995) Identification of two transcription activation units in the N-terminal domain of the human androgen receptor. J. Biol. Chem., 270, 7341–7346. [DOI] [PubMed] [Google Scholar]
- Leav I., Lau,K.M., Adams,J.Y., McNea,J.E., Taplin,M.E., Wang,J., Singh,H. and Ho,S.M. (2001) Comparative studies of the estrogen receptors β and α and the androgen receptor in normal human prostate glands, dysplasia, and in primary and metastatic carcinoma. Am. J. Pathol., 159, 79–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leewansangtong S. and Crawford,E.D. (1998) Maximal androgen withdrawal for prostate cancer therapy: current status and future potential. Endocr.-Related Cancer, 5, 325–339. [Google Scholar]
- Leung T., Chen,X.Q., Manser,E. and Lim,L. (1996) The p160 RhoA-binding kinase ROK α is a member of a kinase family and is involved in the reorganization of the cytoskeleton. Mol. Cell. Biol., 16, 5313–5327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangelsdorf D.J. et al. (1995) The nuclear receptor superfamily: the second decade. Cell, 83, 835–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinissen M.J., Chiariello,M. and Gutkind,J.S. (2001) Regulation of gene expression by the small GTPase Rho through the ERK6 (p38γ) MAP kinase pathway. Genes Dev., 15, 535–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukai H., Mori,K., Takanaga,H., Kitagawa,M., Shibata,H., Shimakawa,M., Miyahara,M. and Ono,Y. (1995) Xenopus PKN: cloning and sequencing of the cDNA and identification of conserved domains. Biochim. Biophys. Acta, 1261, 296–300. [DOI] [PubMed] [Google Scholar]
- Müller J.M. et al. (2000) FHL2, a novel tissue-specific coactivator of the androgen receptor. EMBO J., 19, 359–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller J.M., Metzger,E., Greschik,H., Bosserhoff,A.K., Mercep,L., Buettner,R. and Schüle,R. (2002) The transcriptional coactivator FHL2 transmits Rho signals from the cell membrane into the nucleus. EMBO J., 21, 736–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi K., Mukai,H., Shibata,H., Takahashi,M. and Ona,Y. (1999) Identification and characterization of PKNβ, a novel isoform of protein kinase PKN: expression and arachidonic acid dependency are different from those of PKNα. Biochem. Biophys. Res. Commun., 261, 808–814. [DOI] [PubMed] [Google Scholar]
- Palmer R.H., Ridden,J. and Parker,P.J. (1995) Cloning and expression patterns of two members of a novel protein-kinase-C-related kinase family. Eur. J. Biochem., 227, 344–351. [DOI] [PubMed] [Google Scholar]
- Poukka H., Karvonen,U., Yoshikawa,N., Tanaka,H., Palvimo,J.J. and Janne,O.A. (2000) The RING finger protein SNURF modulates nuclear trafficking of the androgen receptor. J. Cell Sci., 113, 2991–3001. [DOI] [PubMed] [Google Scholar]
- Pyne S. and Pyne,N. (2000) Sphingosine 1-phosphate signalling via the endothelial differentiation gene family of G-protein-coupled receptors. Pharmacol. Ther., 88, 115–131. [DOI] [PubMed] [Google Scholar]
- Renaud J.P. and Moras,D. (2000) Structural studies on nuclear receptors. Cell. Mol. Life Sci., 57, 1748–1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowan B.G., Garrison,N., Weigel,N.L. and O‘Malley,B.W. (2000) 8-Bromo-cyclic AMP induces phosphorylation of two sites in SRC-1 that facilitate ligand-independent activation of the chicken progesterone receptor and are critical for functional cooperation between SRC-1 and CREB binding protein. Mol. Cell. Biol., 20, 8720–8730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahai E., Alberts,A.S. and Treisman,R. (1998) RhoA effector mutants reveal distinct effector pathways for cytoskeletal reorganization, SRF activation and transformation. EMBO J., 17, 1350–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schüle R., Rangarajan,P., Kliewer,S., Ransone,L.J., Bolado,J., Yang,N., Verma,I.M. and Evans,R.M. (1990) Functional antagonism between oncoprotein c-Jun and the glucocorticoid receptor. Cell, 62, 1217–1226. [DOI] [PubMed] [Google Scholar]
- Shibata H., Oda,H., Mukai,H., Oishi,K., Misaki,K., Ohkubo,H. and Ono,Y. (1999) Interaction of PKN with a neuron-specific basic helix–loop–helix transcription factor, NDRF/NeuroD2. Brain Res. Mol. Brain Res., 74, 126–134. [DOI] [PubMed] [Google Scholar]
- Straub R.H., Konecna,L., Hrach,S., Rothe,G., Kreutz,M., Scholmerich,J., Falk,W. and Lang,B. (1998) Serum dehydroepiandrosterone (DHEA) and DHEA sulfate are negatively correlated with serum interleukin-6 (IL-6), and DHEA inhibits IL-6 secretion from mononuclear cells in man in vitro: possible link between endocrinosenescence and immunosenescence. J. Clin. Endocrinol. Metab., 83, 2012–2017. [DOI] [PubMed] [Google Scholar]
- Takahashi M., Mukai,H., Toshimori,M., Miyamoto,M. and Ono,Y. (1998) Proteolytic activation of PKN by caspase-3 or related protease during apoptosis. Proc. Natl Acad. Sci. USA, 95, 11566–11571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takanaga H., Mukai,H., Shibata,H., Toshimori,M. and Ono,Y. (1998) PKN interacts with a paraneoplastic cerebellar degeneration-associated antigen, which is a potential transcription factor. Exp. Cell Res., 241, 363–372. [DOI] [PubMed] [Google Scholar]
- Trapman J. and Brinkmann,A.O. (1996) The androgen receptor in prostate cancer. Pathol. Res. Pract., 192, 752–760. [DOI] [PubMed] [Google Scholar]
- Überall F., Giselbrecht,S., Hellbert,K., Fresser,F., Bauer,B., Gschwendt,M., Grunicke,H.H. and Baier,G. (1997) Conventional PKC-α, novel PKC-ε and PKC-τ, but not atypical PKC-λ are MARCKS kinases in intact NIH 3T3 fibroblasts. J. Biol. Chem., 272, 4072–4078. [DOI] [PubMed] [Google Scholar]
- Voegel J.J., Heine,M.J., Zechel,C., Chambon,P. and Gronemeyer,H. (1996) TIF2, a 160 kDa transcriptional mediator for the ligand-dependent activation function AF-2 of nuclear receptors. EMBO J., 15, 3667–3675. [PMC free article] [PubMed] [Google Scholar]
- Wang X., McGowan,C.H., Zhao,M., He,L., Downey,J.S., Fearns,C., Wang,Y., Huang,S. and Han,J. (2000) Involvement of the MKK6-p38γ cascade in γ-radiation-induced cell cycle arrest. Mol. Cell. Biol., 20, 4543–4552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong C.I., Zhou,Z.X., Sar,M. and Wilson,E.M. (1993) Steroid requirement for androgen receptor dimerization and DNA binding. Modulation by intramolecular interactions between the NH2-terminal and steroid-binding domains. J. Biol. Chem., 268, 19004–19012. [PubMed] [Google Scholar]