IL-1 receptor-associated kinase 1 is critical for latent membrane protein 1-induced p65/RelA serine 536 phosphorylation and NF-κB activation (original) (raw)
Abstract
Epstein–Barr virus latent infection integral membrane protein 1 (LMP1) mimics a constitutively active TNF receptor (TNFR). LMP1 has two C-terminal cytosolic domains, transformation effector sites (TES)1 and -2, that engage TNFR-associated factors (TRAFs) and the TNFR-associated death domain protein, respectively, and activate NF-κB. NF-κB activation is critical for Epstein–Barr virus-infected lymphoblast survival. TES1- and TES2-mediated NF-κB activations are IL-1 receptor-associated kinase 1 (IRAK1)-dependent. Because IRAK1 is upstream of TRAF6 in IL-1 activation of NF-κB, the potential role of IRAK1 in LMP1-mediated NF-κB activation through TRAF6 and inhibitor of κB (IκB) kinase (IKK) was initially investigated. Surprisingly, LMP1 expression activated TRAF6 ubiquitination, IKKβ induction of IκBα phosphorylation, and p65 nuclear translocation in both WT and IRAK1-deficient I1A 293 cells. LMP1 also induced IKKα-mediated p100 processing and p52 nuclear localization in WT and IRAK1-deficient I1A 293 cells. Further, LMP1 TES1 and TES2 induced p65, p50, and p52 NF-κB DNA binding in WT and IRAK1-deficient I1A 293 cells. However, LMP1 induced p65/RelA S536 phosphorylation only in WT 293 cells or in IRAK1 kinase point mutant reconstituted I1A 293 cells but not in IRAK1-deficient I1A 293 cells. IRAK1 was also required for LMP1 activation of p38, one of the kinases that can mediate p65/RelA S536 phosphorylation and activate NF-κB-dependent transcription. Thus, the critical IRAK1 role in LMP1-induced NF-κB activation is in mediating p65/RelA S536 phosphorylation through an effect on p38 or other p65 S536 kinases.
Epstein–Barr virus (EBV) is causally associated with lymphoid and epithelial malignancies such as B cell lymphoproliferative diseases in severely immune-compromised people, Burkitt’s lymphoma, T cell lymphoma, Hodgkin’s Disease, and Nasopharyngeal Carcinoma (1, 2). EBV-encoded latent infection membrane protein 1 (LMP1) is critical for EBV-infected B lymphocyte conversion to proliferating lymphoblasts and is expressed in most EBV-associated malignancies (1, 2). LMP1 expression causes established rodent fibroblasts to lose contact inhibition and form tumors in severe combined immunodeficient mice (3). LMP1 expression in human B lymphoblasts alters the growth phenotype and induces an antiapoptotic state (4, 5), whereas expression in transgenic mice results in B cell lymphomas (6).
The experiments reported here investigate molecular mechanisms that underlie LMP1 effects. LMP1 consists of a 24-amino acid cytosolic N terminus, 162 amino acids in six hydrophobic transmembrane domains, and a 200-amino acid cytosolic C terminus. The LMP1 N terminus and six transmembrane domains aggregate and thereby enable constitutive clustering and signaling from the cytosolic C terminus. The LMP1 C terminus first and last 35 residues constitute domains that engage TNF receptor (TNFR)-associated factors (TRAFs) and the TNFR-associated death domain protein (TRADD), respectively. The TRAFs and TRADD-binding domains activate NF-κB, C-Jun N-terminal kinase, and p38 (7–22) The two domains are referred to as C-terminal activation regions and transformation effector sites (TES)1 and -2. TES1 interacts with TRAF1, 2, 3, and 5 through a consensus PXQXT motif similar to that in the CD40 TNFR. CD40 TRAF binding is critical for B lymphocyte survival in germinal centers after antigen stimulation (23). TES2 binds TRADD and receptor-interacting protein through the LMP1 C-terminal 11 residues. LMP1 TES2 mutants that do not interact with TRADD fail to activate NF-κB (13, 20). TES1 and 2 are critical for NF-κB activation and EBV conversion of primary B lymphocytes to proliferating lymphoblasts (LCLs). NF-κB activation is critical for EBV LCL survival (24, 25).
NF-κB is a family of transcription factors whose activity is regulated by transcription, post-translational processing, retention in the cytoplasm by inhibitors [inhibitor of κB (IκB)], IκB serine/threonine phosphorylations, ubiquitinations and degradations that release NF-κB heterodimers to the nucleus, and serine/threonine phosphorylations that activate transcription (reviewed in refs. 26 and 27). In the first delineated or canonical NF-κB pathway, TNFR signaling induces activation of an IKKα, -β, and -γ complex, IKKβ phosphorylation of IκBα, IκBα ubiquitination and degradation, p65/p50 translocation to the nucleus, p65/p50 binding to cognate DNA sites, and specific promoter up-regulation (26, 27). p65-mediated transcription is regulated by S536 phosphorylation in the transactivation domain (TAD) (reviewed in ref. 28). TRAF family member-associated NF-κB activator (TANK)-binding kinase (TBK), IKKε, and p38 can phosphorylate p65 S536 and activate NF-κB-mediated transcription downstream of TNFα and IL-1β (29–34). CD40, lymphotoxin β receptor (LTβR), and B cell-activating factor activate NK-κB-dependent promoters through a noncanonical pathway that involves TRAFs, NF-κB-inducing kinase (NIK), IKKα, p100 processing to p52, and translocation of p52/Rel heterodimers (26, 27).
Experiments using cells deficient in Toll-IL-1 receptor (TIR) signaling components have indicated that IL-1 receptor-associated kinase 1 (IRAK1) and TRAF6 are critical for LMP1-induced NF-κB activation (35). These experiments were undertaken to investigate the role of IRAK1 in LMP1-mediated NF-κB activation.
Results
LMP1 Activation of NF-κB Does Not Require IRAK1 Kinase Activity.
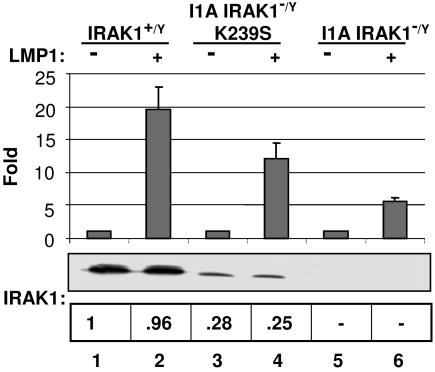
As reported (35), LMP1 expression-mediated NF-κB activation was 75% reduced in IRAK1-deficient I1A 293 cells (Fig. 1, lane 6). The potential importance of IRAK1 kinase activity for LMP1-induced NF-κB activation was investigated by using IRAK1-deficient I1A that had been reconstituted with a kinase-negative IRAK1 K239S point mutant (I1A-K239S) (35, 36). IRAK1 K239S restored 60% of WT NF-κB responsiveness to IRAK1-deficient I1A cells, despite only 25–28% of WT IRAK1 expression (Fig. 1, lane 4). Thus, IRAK1 kinase activity is dispensable for LMP1-induced NF-κB activation.
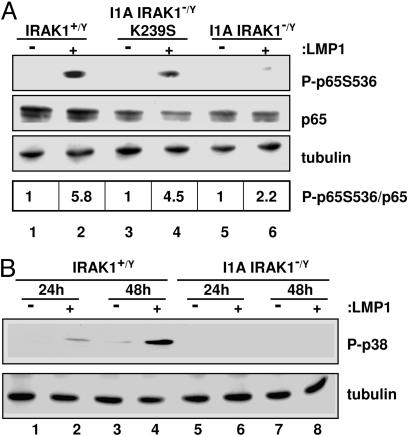
Fig. 1.
IRAK1 kinase activity is not required for LMP1-induced NF-κB activation. Control WT (lanes 1 and 2), IRAK1 null (I1A) 293 cells (lanes 5 and 6), or I1A 293 cells stably expressing kinase-dead IRAK1 (I1A-K239S) (lanes 3 and 4) were transfected with either pSG5 (−) or pSG5- FLMP1 (+). After 24 h, NF-κB luciferase reporter activity was measured. Luciferase activity was normalized for transfection efficiency by phosphoglycerate kinase β-gal activity. The level of IRAK1 in cells described above was determined by Western blot analysis. The intensity of each band was measured by using a Molecular Imaging Station from Kodak (Rochester, NY).
LMP1 Induces TRAF6 Polyubiquitylation in IRAK1-Deficient Cells.
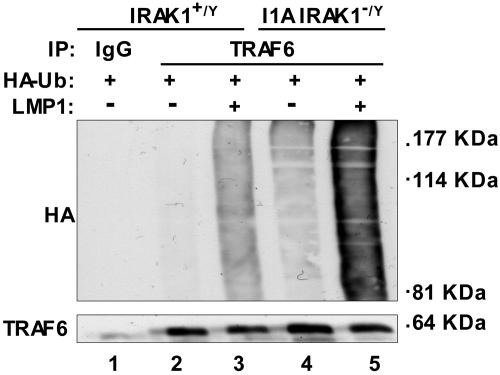
Downstream of IL-1, IRAK1 associates with TRAF6 and affects TRAF6 polyubiquitination, TAK1 activation, IKKβ activation, IκBα degradation and NF-κB activation (37, 38). To investigate whether IRAK1 has a similar role in TRAF6 polyubiquitination downstream of LMP1, TRAF6 ubiquitination was assayed in WT and IRAK1-deficient I1A 293 cells after cotransfection with LMP1 and hemagglutinin (HA)-ubiquitin expression vectors, immunoprecipitation of TRAF6, and anti-HA Western blot. In WT 293 cells, LMP1 significantly induced TRAF6 polyubiquitination (Fig. 2, compare lanes 3 with 2). Surprisingly, background TRAF6 polyubiquitination was higher in IRAK1-deficient I1A 293 cells and increased substantially in response to LMP1 expression (Fig. 2, compare lane 5 with lanes 4 and 3). Thus, IRAK1 deficiency results in increased basal and LMP1-induced TRAF6 polyubiquitination. Clearly, TRAF6 polyubiquitination does not require IRAK1. Enhanced TRAF6 polyubiquitination in I1A cells is likely due to the low level of LMP1-mediated NF-κB activation in IRAK1-deficient I1A cells. In WT cells, NF-κB activation induces expression of A20, which deubiquitinates TRAF6-conjugated K63 polyubiquitin linkages and conjugates TRAF6 with K48 polyubiquitin linkages, resulting in TRAF6 degradation and down-regulation of NF-κB activation (39–42).
Fig. 2.
LMP1-induced TRAF6 polyubiquitination in control and IRAK null 293 cells. Control WT (lanes 2 and 3) or IRAK null (I1A) 293 cells (lanes 4 and 5) were transfected with either pSG5 (−) or pSG5-FLMP1 (+) and pcDNA-HA-Ub. After 24 h, TRAF6 was immunoprecipitated, and the immunoprecipitates were subjected to Western blot analysis with either anti-HA antibody (top) or anti-TRAF6 antibody (bottom).
LMP1 Induces IκBα Phosphorylation and Nuclear p65 Translocation in IRAK1-Deficient I1A 293 Cells.
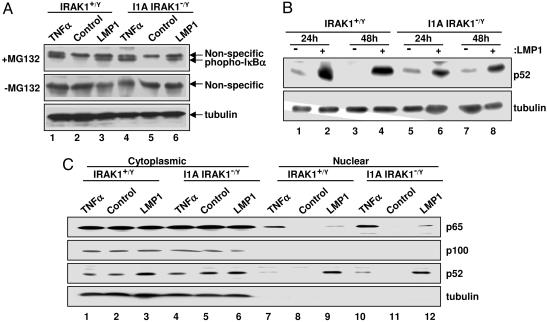
To more directly assay the effect of IRAK1 deficiency on LMP1-mediated IKKβ activation and IκBα phosphorylation, WT or I1A 293 cells were transfected with LMP1 and treated or not with MG132 for 3 h to inhibit phospho-IκBα degradation. TNFα treatment for 30 min in the presence or absence of MG132 served as a positive control. LMP1 and TNFα induced similar levels of IκBα phosphorylation in MG132-treated WT or I1A 293 cells. IκBα phosphorylation was not detected in either cell type in the absence of LMP1 or MG132, indicating that LMP1 and TNFα similarly activate IKKβ to phosphorylate IκBα in WT and IRAK1-deficient I1A 293 cells (Fig. 3A). The requirement for MG132 for observation of phospho-IκBα is consistent with active ubiquitination and degradation of phosphorylated IκBα in the absence of MG132. Furthermore, p65 localized to the nucleus of LMP1 expressing or TNFα-treated WT and IRAK1-deficient I1A 293 cells (Fig. 3C, lanes 9, 7, 10, and 12), whereas the vector control did not induce p65 nuclear localization (Fig. 3C, lanes 8 and 11). Thus, LMP1 induction of IκBα phosphorylation and p65 nuclear localization was similar in WT and IRAK1-deficient I1A 293 cells.
Fig. 3.
LMP1-induced IKK activation in IRAK1 null 293 cells. (A) Control WT (lanes 1–3) or IRAK1 null (I1A) 293 cells (lanes 4–6) were transfected with either pSG5 (Control, lanes 2 and 5) or pSG5-FLMP1 (LMP1, lanes 3 and 6). A parallel untransfected culture was treated with TNFα for 30 min (lanes 1 and 4). Cells were treated with or without MG132 for 30 min before harvest. Equal amounts of cell extracts were subjected to Western blot analysis with either antiphospho-IκBα antibody or antitubulin antibody. (B) Control WT (lanes 1–4) or IRAK null (I1A) 293 cells (lanes 5–8) were transfected with either pSG5 (−) or pSG5-FLMP1 (+). After 24 or 48 h, equal amounts of cell extracts were subjected to Western blot analysis with either anti-p100/p52 antibody or antitubulin antibody. (C) Control WT (lanes 1–3 and 7–9) and IRAK1 null (I1A) 293 cells (lanes 4–6 and 10–12) were transfected with pSG5 (Control) or pSG5-FLMP1 (LMP1). After 48 h, cytoplasmic (lanes 1–6) or nuclear (lanes 7–12) extracts were subjected to Western blot analysis with either anti-p65/RelA antibody or anti-p100/p52 antibody; p100 and tubulin were controls for cytoplasmic contamination of the nuclear fraction. As another control, untransfected WT 293 cells were treated with TNFα for 30 min before harvest.
Because LMP1 TES1- and TES2-mediated NF-κB activations are similarly decreased in I1A 293 cells (35) and TES1 effects mostly NIK/IKKα noncanonical NF-κB activation (43–46), the potential role of IRAK1 in noncanonical NF-κB activation was investigated. LMP1 also induced similar levels of p100 processing to p52 in WT and IRAK1-deficient I1A 293 cells at both 24 and 48 h, indicating IRAK1 is not required for LMP1-induced NIK/IKKα activation (Fig. 3B, lanes 2, 4, 6, and 8). Further, LMP1 induced p52 nuclear localization in WT and IRAK1-deficient I1A 293 cells (Fig. 3C, lanes 9 and 12) but not in vector control-transfected cells (Fig. 3C, lanes 8 and 11). These data indicate that IRAK1 is not required for LMP1 activation of NIK/IKKα, p100 to p52 processing, or p52 nuclear translocation.
LMP1 Induces Similar NF-κB Probe DNA-Binding Activities in WT and IRAK1-Deficient I1A 293 Cells.
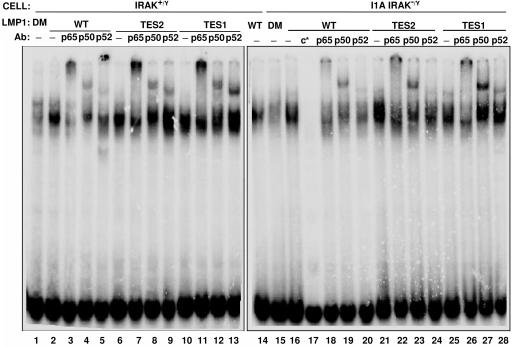
Because LMP1 induced similar IκBα phosphorylation and p65 and p52 nuclear localization in WT and I1A 293 cells, potential differences in LMP1 effects on NF-κB binding to DNA were assessed. LMP1, LMP1 TES2 (null mutant in TES1), LMP1 TES1 (null mutant in TES2), or LMP1 DM (null mutant in TES 1 and TES2) were expressed in WT control or IRAK1-deficient I1A 293 cells. EMSA using a HIV1 LTR NF-κB site revealed that LMP1 (Fig. 4, lanes 2, 14, and 16), LMP1 TES2 (Fig. 4, lanes 6 and 21), and LMP1 TES1 (Fig. 4, lanes 10 and 25) induced similarly intense specific gel shifts in extracts from WT and I1A 293 cells. The LMP1-, LMP1 TES2-, and LMP1 TES1-induced NF-κB complexes were particularly impressive in comparison to LMP1 DM, which was similar to control 293 cell extracts (Fig. 4, lanes 1 and 15 and data not shown). The gel-shift complexes were substantially diminished in the presence of excess unlabeled probe DNA (Fig. 4, lane 17).
Fig. 4.
NF-κB complexes from IRAK1-null I1A 293 cells bind DNA. Control WT (lanes 1–14) or IRAK1 null (I1A) 293 cells (lanes 15–28) were transfected with LMP1 WT (lanes 2–5 and 16–20), LMP1 TES2 (lanes 6–9 and 21–24), LMP1 TES1 (lanes 10–13 and 25–28), or a nonactivating double mutant, LMP1 DM (lanes 1 and 15) (35). Extracts were made 48 h after transfection, and EMSAs used a NF-κB probe derived from the HIV LTR. NF-κB EMSAs were treated with antibodies to p65 (lanes 3, 7, 11, 18, 22, and 26), to p50 (lanes 4, 8, 12, 19, 23, and 27) or to p52 (lanes 5, 9, 13, 20, 24, and 28) to assess NF-κB complex composition. Specificity of the complexes was demonstrated by competition with 100× cold probe (lane 17) (c*, cold competitor probe).
Specific p65, p50, and p52 components in the gel shift were identified by antibody-mediated supershift analyses. The LMP1-, LMP1 TES2-, and LMP1 TES1-induced complexes supershifted extensively in response to p65 antibody, whereas p50 antibody supershifted more rapidly migrating complexes and p52 antibody supershifted more slowly migrating components (Fig. 4, lanes 3–5, 7–9, and 11–13). Antibody to p52 may have supershifted more probe using extracts from TES1-expressing cells than with extracts from LMP1 WT- or TES2-expressing cells (Fig. 4, lane 13 compared with lanes 5 and 9). Most notably, the NF-κB complex was similar with extracts from WT and IRAK1-deficient I1A 293 cells (Fig. 4, lanes 18–20, 22–24, and 26–28). These data indicate that LMP1, TES2, and TES1 induce similar p65, p50, and p52 gel-shift complexes in control and IRAK1-deficient I1A 293 cells. Thus, IRAK1 is not required for LMP1, TES2, or TES1 induction of NF-κB p65, p50, or p52 DNA-binding activities.
IRAK1 Is Required for LMP1-Induced p65/RelA Phosphorylation.
Because IRAK1 is not required for LMP1 TES1- or TES2-induced IKK activation or any step before or including DNA binding, and transcriptional activation also depends on p65 TAD S536 phosphorylation (28), LMP1-induced p65 S536 phosphorylation was assayed in WT, IRAK1-deficient I1A, and IRAK1 kinase-deficient I1A-K239S 293 cells. LMP1 significantly induced p65 S536 phosphorylation in WT 293 and in I1A-K239 293 cells (Fig. 5A, lanes 2 and 4) but did not induce p65 S536 phosphorylation in I1A 293 cells (Fig. 5A, lane 6). Thus, IRAK1 but not IRAK1 kinase activity is required for LMP1-induced p65 TAD S536 phosphorylation.
Fig. 5.
LMP1-induced p65/RelA phosphorylation and p38 mitogen-activated protein kinase activation depend on IRAK1. (A) Control WT (lanes 1 and 2), IRAK1 null (I1A) 293 cells (lanes 5 and 6), or I1A 293 cells stably expressing kinase-dead IRAK1 (I1A-K239S) (lanes 3 and 4) were transfected with either pSG5 (−) or pSG5-FLMP1 (+) and p65-GFP. After 24 h, cell extracts were harvested and subjected to Western blot analysis with either antiphospho-p65 serine 536 antibody, anti-p65 antibody, or antitubulin antibody. (B) Control (lanes 1–4) or I1A (lanes 5–8) 293 cells were transfected with either pSG6(−) or pSG5-FLMP1 (+) and Flag-p38. After 24 and 48 h, cell extracts were harvested and subjected to Western blot analysis with either anti-phospho-p38 at T180/Y182 antibody or antitubulin antibody. The intensity of each band was measured by using a Molecular Imaging Station from Kodak.
IRAK1 Is Required for LMP1-Induced p38 Activation.
Because p38 activation can effect p65 S536 phosphorylation and is required for TNF-induced NF-κB-mediated transcription (32, 33, 47), the role of IRAK1 in LMP1-induced p38 activation was assessed. Using antibody specific for p38 T180/Y182 phosphorylation, LMP1 activated p38 phosphorylation at 24 and 48 h in WT 293 cells but did not activate p38 in IRAK1-deficient I1A 293 cells (Fig. 5B, lanes 2 and 4, versus Fig. 5B, lanes 6 and 8). These data indicate that IRAK1 is required for LMP1 activation of p38 in 293 cells.
Discussion
These investigations were initiated with the hypothesis that, like TIR signaling, the dependence of LMP1-mediated NF-κB activation on IRAK1 reflected a critical role in the recruitment and activation of TRAF6 (37, 38). In TIR signaling, IRAK1 is a scaffold for recruitment of TRAF6 to Myd88 and subsequent TRAF6 activation (37, 38). However, our data indicate that IRAK1 is dispensable for LMP1-mediated TRAF6 polyubiquitination, IκBα phosphorylation, p100 processing to p52, p65 and p52 nuclear localization, and NF-κB gel-shift activities. Instead, IRAK1 was critically required for p65/RelA TAD S536 phosphorylation (Fig. 6).
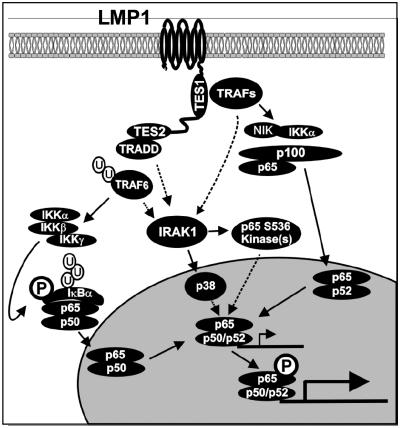
Fig. 6.
Model for LMP1-induced NF-κB activation. LMP1 TES1 binds TRAFs, activates NIK and IKKα, induces p100 processing to p52, and induces nuclear translocation of p65/p52 NF-κB complexes. LMP1 TES2 binds TRADD and receptor-interacting protein and stimulates TRAF6 ubiquitination, IKKβ activation, IκBα phosphorylation and degradation, and nuclear translocation of p65/p50 NF-κB complexes. IRAK1 is essential for NF-κB transactivation induced by both TES1 and TES2. IRAK1 is not required for IKK activation but is required for phosphorylation of the p65 transactivation domain at Serine 536 and subsequent p65/p50- or p65/p52-mediated transcriptional activation. LMP1-mediated p38 activation is IRAK1-dependent. IRAK1 may be a scaffold for p38 or another p65 S536 kinase such as TBK1 or IKKε.
In I1A 293 cells, both TES1- and TES2-mediated NF-κB activation is decreased (35). Indeed, p65 TAD S536 phosphorylation could be the underlying IRAK1-dependent step in both TES1- and LMP1 TES2-mediated NF-κB activation. Our data indicate that TES1 and TES2 induce similar levels of p65 gel-shift activity, consistent with the previous observation that TES1 activates noncanonical p65/p52 complexes in C33 cells (48). Also, lymphotoxin β induces NIK- and IKKα-mediated p65 transcriptional activity through p65 S536 phosphorylation (29). Furthermore, phorbol 12-myristate 13-acetate and ionomycin-induced p65 S536 phosphorylation is not inhibited by IκBα expression, which is characteristic of the noncanonical pathway (49). Thus, the IRAK1 dependence of TES1-mediated NF-κB activation is likely because of a need for p65 phosphorylation for efficient transcriptional enhancement by p65/p52 complexes. IRAK1 may also be required for RelB phosphorylation by LMP1 TES1, although the significance of RelB phosphorylation is not understood (28).
TBK1, IKKε, and p38 mitogen-activated protein kinase can activate NF-κB-mediated transcription by phosphorylating the p65 TAD S536 in response to TIR signaling (31–34). Phosphorylation of p65 S536 enhances transactivation by recruitment of histone acetyltransferases. Interestingly, our data indicate that IRAK1 kinase activity is not required for p65 S536 phosphorylation.
In the experiments reported here, IRAK1 was required for LMP1-mediated p38 activation. LMP1 TES1 and TES2 contribute to p38 activation (18). Furthermore, p38 is required for TNFR-induced NF-κB-mediated transcription (47) and for LPS- and IL-1β-mediated p65 S536 phosphorylation (32). Also, IRAK1-dependent p38 activation downstream of LMP1 could have a role in p38-mediated histone H3 phosphorylation and -increased NF-κB transcriptional activity (50). Although our experiments indicate that IRAK1 is critical for LMP1-induced p38 activation in 293 cells, we have not shown that p38 activation is critical for LMP1-induced NF-κB-dependent transcription in these cells.
In addition to p38, TBK1 and IKKε could have critical roles in LMP1-induced IRAK1-dependent p65 S536 phosphorylation and activation of NF-κB-mediated transcription. Indeed, a dominant-negative TBK1 kinase point mutant partially inhibited LMP1-induced NF-κB activation in 293 cells (data not shown). TRAF3 can associate with TBK1 and IRAK1 in TIR signaling (51, 52). Perhaps TRAF3 can mediate IRAK1/TBK1 recruitment to LMP1 TES1.
IRAK1 may also mediate p65 S536 phosphorylation in response to TNFα. The signaling molecule that interacts with pelle-like kinase (SIMPL) binds IRAK1 and is required for TNFα-mediated NF-κB activation in 293 cells (53). In response to TNFα, SIMPL localizes into the nucleus, binds to p65, and induces p65-mediated transcription (53). In the absence of SIMPL, p65 translocates to the nucleus but fails to induce transcription. Thus, SIMPL may function as a scaffold to recruit a kinase(s) that phosphorylates p65 S536. LMP1 signaling may parallel TNFR signaling in using an IRAK1-SIMPL cascade that culminates in p65 S536 phosphorylation.
While this manuscript was in preparation, LMP1-mediated IKKβ activation and NF-κB DNA binding were found to be intact in 293 cells depleted for IRAK1 with small interference RNA, leading to the incorrect conclusion that IRAK1 is not required for LMP1-induced NF-κB activation (54).
Materials and Methods
Cells, Plasmids, Antibodies, and Transfections.
I1A 293 cells stably transfected with kinase-dead IRAK1 (K239S) were kindly provided by Xiaoxia Li (Cleveland Clinic Foundation, Cleveland). A vector expressing GFP-p65 was kindly provided by D. Ballard (Vanderbilt University, Nashville, TN). Antibodies to IRAK1, TRAF6, p65/RelA, and HA were purchased from Santa Cruz Biotechnology. An antibody to p100/p52 was a kind gift from Warner Greene (University of California, San Francisco). Antibodies to phospho-IκBα, phospho-p65/RelA at serine 536, and phospho-p38 at threonine 180/tyrosine 182 were purchased from Cell Signaling Technology (Beverly, MA). Antibody to tubulin was purchased from Sigma Aldrich. For transient transfection, effectene transfection reagents were used according to the manufacturer’s directions (Qiagen, Valencia, CA).
Immunoprecipitation.
Five million cells were lysed with radioimmunoprecipitation assay (RIPA) buffer (25 mM Tris·HCl, pH 7.5/150 mM NaCl/2 mM EDTA/1% Nonidet P-40/0.1% SDS/0.5% sodium deoxycholate) containing 50 mM sodium fluoride and 1 mM sodium orthovanadate and protease inhibitor mixture (Roche, Indianapolis). Cell lysates were precleared with protein A/G agarose beads (Santa Cruz Biotechnology) and incubated at 4°C for overnight with anti-TRAF6 antibody (Santa Cruz Biotechnology). Immune complexes were collected on protein A/G agarose beads and washed three times with RIPA buffer. Immunoprecipitates were eluted by addition of an equal volume of 2× SDS/PAGE loading buffer (100 mM Tris·HCl, pH 6.8, containing 4% SDS, 0.02% bromophenol blue, and 2% 2-mercaptoethanol) and subjected to Western blot analysis with either anti-HA antibody or anti-TRAF6 antibody (Santa Cruz Biotechnology).
EMSA.
EMSAs (24) used 2 μg of antibodies to p65/RelA and p50 from Santa Cruz Biotechnology or to p52 from Upstate Technology (Charlottesville, VA).
Acknowledgments
We thank members of the laboratory for helpful discussion. We are grateful to Micah Luftig, Kevin Hall, and Daniela Böhm for technical support and discussion. This work was supported by National Institutes of Health Grants R01 CA85180 and T32 AI07061 (Y.-J.S.).
Abbreviations
EBV
Epstein–Barr virus
LMP1
latent membrane protein 1
TES
transformation effector site
IκB
inhibitor of κB
IKK
IκB kinase
TNFR
TNF receptor
TRAF
TNFR-associated factor
IRAK1
IL-1 receptor-associated kinase 1
TES
transformation effector site
HA
hemagglutinin
TRADD
TNFR-associated death domain protein
TAD
transactivation domain
TIR
TBK, TRAF family member-associated NF-κB activator-binding kinase
NIK
NF-κB-inducing kinase
TIR
Toll-IL-1 receptor
SIMPL
signaling molecule that interacts with pelle-like kinase.
Footnotes
Conflict of interest statement: No conflicts declared.
References
- 1.Rickinson A. B., Kieff E. In: Fields Virology. Knipe D. M., Howley P. M., editors. Vol. 2. Philadelphia: Lippincott Williams & Wilkins; 2001. pp. 2575–2628. [Google Scholar]
- 2.Kieff E., Rickinson A. B. In: Fields Virology. Knipe D. M., Howley P. M., editors. Vol. 2. Philadelphia: Lippincott Williams & Wilkins; 2001. pp. 2511–2574. [Google Scholar]
- 3.Wang D., Liebowitz D., Kieff E. Cell. 1985;43:831–840. doi: 10.1016/0092-8674(85)90256-9. [DOI] [PubMed] [Google Scholar]
- 4.Wang D., Liebowitz D., Wang F., Gregory C., Rickinson A., Larson R., Springer T., Kieff E. J. Virol. 1988;62:4173–4184. doi: 10.1128/jvi.62.11.4173-4184.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Henderson S., Rowe M., Gregory C., Croom-Carter D., Wang F., Longnecker R., Kieff E., Rickinson A. Cell. 1991;65:1107–1115. doi: 10.1016/0092-8674(91)90007-l. [DOI] [PubMed] [Google Scholar]
- 6.Kulwichit W., Edwards R. H., Davenport E. M., Baskar J. F., Godfrey V., Raab-Traub N. Proc. Natl. Acad. Sci. USA. 1998;95:11963–11968. doi: 10.1073/pnas.95.20.11963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mosialos G., Birkenbach M., Yalamanchili R., VanArsdale T., Ware C., Kieff E. Cell. 1995;80:389–399. doi: 10.1016/0092-8674(95)90489-1. [DOI] [PubMed] [Google Scholar]
- 8.Kaye K. M., Izumi K. M., Mosialos G., Kieff E. J. Virol. 1995;69:675–683. doi: 10.1128/jvi.69.2.675-683.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mitchell T., Sugden B. J. Virol. 1995;69:2968–2976. doi: 10.1128/jvi.69.5.2968-2976.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huen D. S., Henderson S. A., Croom-Carter D., Rowe M. Oncogene. 1995;10:549–560. [PubMed] [Google Scholar]
- 11.Devergne O., Hatzivassiliou E., Izumi K. M., Kaye K. M., Kleijnen M. F., Kieff E., Mosialos G. Mol. Cell. Biol. 1996;16:7098–7108. doi: 10.1128/mcb.16.12.7098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Izumi K. M., Kaye K. M., Kieff E. D. Proc. Natl. Acad. Sci. USA. 1997;94:1447–1452. doi: 10.1073/pnas.94.4.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Izumi K. M., Kieff E. D. Proc. Natl. Acad. Sci. USA. 1997;94:12592–12597. doi: 10.1073/pnas.94.23.12592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sandberg M., Hammerschmidt W., Sugden B. J. Virol. 1997;71:4649–4656. doi: 10.1128/jvi.71.6.4649-4656.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kieser A., Kilger E., Gires O., Ueffing M., Kolch W., Hammerschmidt W. EMBO J. 1997;16:6478–6485. doi: 10.1093/emboj/16.21.6478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eliopoulos A. G., Stack M., Dawson C. W., Kaye K. M., Hodgkin L., Sihota S., Rowe M., Young L. S. Oncogene. 1997;14:2899–2916. doi: 10.1038/sj.onc.1201258. [DOI] [PubMed] [Google Scholar]
- 17.Eliopoulos A. G., Blake S. M., Floettmann J. E., Rowe M., Young L. S. J. Virol. 1999;73:1023–1035. doi: 10.1128/jvi.73.2.1023-1035.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eliopoulos A. G., Gallagher N. J., Blake S. M., Dawson C. W., Young L. S. J. Biol. Chem. 1999;274:16085–16096. doi: 10.1074/jbc.274.23.16085. [DOI] [PubMed] [Google Scholar]
- 19.Devergne O., Cahir McFarland E. D., Mosialos G., Izumi K. M., Ware C. F., Kieff E. J. Virol. 1998;72:7900–7908. doi: 10.1128/jvi.72.10.7900-7908.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Izumi K. M., Cahir McFarland E. D., Ting A. T., Riley E. A., Seed B., Kieff E. D. Mol. Cell. Biol. 1999;19:5759–5767. doi: 10.1128/mcb.19.8.5759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaye K. M., Izumi K. M., Li H., Johannsen E., Davidson D., Longnecker R., Kieff E. J. Virol. 1999;73:10525–10530. doi: 10.1128/jvi.73.12.10525-10530.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hatzivassiliou E., Miller W. E., Raab-Traub N., Kieff E., Mosialos G. J. Immunol. 1998;160:1116–1121. [PubMed] [Google Scholar]
- 23.Yasui T., Muraoka M., Takaoka-Shichijo Y., Ishida I., Takegahara N., Uchida J., Kumanogoh A., Suematsu S., Suzuki M., Kikutani H. Int. Immunol. 2002;14:319–329. doi: 10.1093/intimm/14.3.319. [DOI] [PubMed] [Google Scholar]
- 24.Cahir-McFarland E. D., Davidson D. M., Schauer S. L., Duong J., Kieff E. Proc. Natl. Acad. Sci. USA. 2000;97:6055–6060. doi: 10.1073/pnas.100119497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cahir-McFarland E. D., Carter K., Rosenwald A., Giltnane J. M., Henrickson S. E., Staudt L. M., Kieff E. J. Virol. 2004;78:4108–4119. doi: 10.1128/JVI.78.8.4108-4119.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ghosh S., Karin M. Cell. 2002;109:S81–S96. doi: 10.1016/s0092-8674(02)00703-1. [DOI] [PubMed] [Google Scholar]
- 27.Hayden M. S., Ghosh S. Genes Dev. 2004;18:2195–2224. doi: 10.1101/gad.1228704. [DOI] [PubMed] [Google Scholar]
- 28.Viatour P., Merville M. P., Bours V., Chariot A. Trends Biochem. Sci. 2005;30:43–52. doi: 10.1016/j.tibs.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 29.Jiang X., Takahashi N., Matsui N., Tetsuka T., Okamoto T. J. Biol. Chem. 2003;278:919–926. doi: 10.1074/jbc.M208696200. [DOI] [PubMed] [Google Scholar]
- 30.Sakurai H., Chiba H., Miyoshi H., Sugita T., Toriumi W. J. Biol. Chem. 1999;274:30353–30356. doi: 10.1074/jbc.274.43.30353. [DOI] [PubMed] [Google Scholar]
- 31.Buss H., Dorrie A., Schmitz M. L., Hoffmann E., Resch K., Kracht M. J. Biol. Chem. 2004;279:55633–55643. doi: 10.1074/jbc.M409825200. [DOI] [PubMed] [Google Scholar]
- 32.Madrid L. V., Mayo M. W., Reuther J. Y., Baldwin A. S., Jr. J. Biol. Chem. 2001;276:18934–18940. doi: 10.1074/jbc.M101103200. [DOI] [PubMed] [Google Scholar]
- 33.Sizemore N., Leung S., Stark G. R. Mol. Cell. Biol. 1999;19:4798–4805. doi: 10.1128/mcb.19.7.4798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fujita F., Taniguchi Y., Kato T., Narita Y., Furuya A., Ogawa T., Sakurai H., Joh T., Itoh M., Delhase M., et al. Mol. Cell. Biol. 2003;23:7780–7793. doi: 10.1128/MCB.23.21.7780-7793.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luftig M., Prinarakis E., Yasui T., Tsichritzis T., Cahir-McFarland E., Inoue J., Nakano H., Mak T. W., Yeh W. C., Li X., et al. Proc. Natl. Acad. Sci. USA. 2003;100:15595–15600. doi: 10.1073/pnas.2136756100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li X., Commane M., Jiang Z., Stark G. R. Proc. Natl. Acad. Sci. USA. 2001;98:4461–4465. doi: 10.1073/pnas.071054198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Janssens S., Beyaert R. Mol. Cell. 2003;11:293–302. doi: 10.1016/s1097-2765(03)00053-4. [DOI] [PubMed] [Google Scholar]
- 38.Jiang Z., Ninomiya-Tsuji J., Qian Y., Matsumoto K., Li X. Mol. Cell. Biol. 2002;22:7158–7167. doi: 10.1128/MCB.22.20.7158-7167.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boone D. L., Turer E. E., Lee E. G., Ahmad R. C., Wheeler M. T., Tsui C., Hurley P., Chien M., Chai S., Hitotsumatsu O., et al. Nat. Immunol. 2004;5:1052–1060. doi: 10.1038/ni1110. [DOI] [PubMed] [Google Scholar]
- 40.Heyninck K., Beyaert R. Trends Biochem. Sci. 2005;30:1–4. doi: 10.1016/j.tibs.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 41.Lee E. G., Boone D. L., Chai S., Libby S. L., Chien M., Lodolce J. P., Ma A. Science. 2000;289:2350–2354. doi: 10.1126/science.289.5488.2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wertz I. E., O’Rourke K. M., Zhou H., Eby M., Aravind L., Seshagiri S., Wu P., Wiesmann C., Baker R., Boone D. L., et al. Nature. 2004;430:694–699. doi: 10.1038/nature02794. [DOI] [PubMed] [Google Scholar]
- 43.Atkinson P. G., Coope H. J., Rowe M., Ley S. C. J. Biol. Chem. 2003;278:51134–51142. doi: 10.1074/jbc.M304771200. [DOI] [PubMed] [Google Scholar]
- 44.Eliopoulos A. G., Caamano J. H., Flavell J., Reynolds G. M., Murray P. G., Poyet J. L., Young L. S. Oncogene. 2003;22:7557–7569. doi: 10.1038/sj.onc.1207120. [DOI] [PubMed] [Google Scholar]
- 45.Luftig M., Yasui T., Soni V., Kang M. S., Jacobson N., Cahir-McFarland E., Seed B., Kieff E. Proc. Natl. Acad. Sci. USA. 2004;101:141–146. doi: 10.1073/pnas.2237183100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saito N., Courtois G., Chiba A., Yamamoto N., Nitta T., Hironaka N., Rowe M., Yamamoto N., Yamaoka S. J. Biol. Chem. 2003;278:46565–46575. doi: 10.1074/jbc.M302549200. [DOI] [PubMed] [Google Scholar]
- 47.Read M. A., Whitley M. Z., Gupta S., Pierce J. W., Best J., Davis R. J., Collins T. J. Biol. Chem. 1997;272:2753–2761. doi: 10.1074/jbc.272.5.2753. [DOI] [PubMed] [Google Scholar]
- 48.Paine E., Scheinman R. I., Baldwin A. S., Jr., Raab-Traub N. J. Virol. 1995;69:4572–4576. doi: 10.1128/jvi.69.7.4572-4576.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sasaki C. Y., Barberi T. J., Ghosh P., Longo D. L. J. Biol. Chem. 2005;280:34538–34547. doi: 10.1074/jbc.M504943200. [DOI] [PubMed] [Google Scholar]
- 50.Saccani S., Pantano S., Natoli G. Nat. Immunol. 2002;3:69–75. doi: 10.1038/ni748. [DOI] [PubMed] [Google Scholar]
- 51.Oganesyan G., Saha S. K., Guo B., He J. Q., Shahangian A., Zarnegar B., Perry A., Cheng G. Nature. 2005;439:208–211. doi: 10.1038/nature04374. [DOI] [PubMed] [Google Scholar]
- 52.Hacker H., Redecke V., Blagoev B., Kratchmarova I., Hsu L. C., Wang G. G., Kamps M. P., Raz E., Wagner H., Hacker G., et al. Nature. 2005;439:204–207. doi: 10.1038/nature04369. [DOI] [PubMed] [Google Scholar]
- 53.Kwon H. J., Breese E. H., Vig-Varga E., Luo Y., Lee Y., Goebl M. G., Harrington M. A. Mol. Cell. Biol. 2004;24:9317–9326. doi: 10.1128/MCB.24.21.9317-9326.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu L., Nakano H., Wu Z. J. Biol. Chem. 2006;281:2162–2169. doi: 10.1074/jbc.M505903200. [DOI] [PubMed] [Google Scholar]