Rapid generation of inducible mouse mutants (original) (raw)
Abstract
We have generated an optimized inducible recombination system for conditional gene targeting based on a Cre recombinase–steroid receptor fusion. This configuration allows efficient Cre-mediated recombination in most organs of the mouse upon induction, without detectable background activity. An ES cell line, was established that carries the inducible recombinase and a _loxP_-flanked lacZ reporter gene. Out of this line, completely ES cell-derived mice were efficiently produced through tetraploid blastocyst complementation, without the requirement of mouse breeding. Our findings provide a new concept allowing the generation of inducible mouse mutants within 6 months, as compared to 14 months using the current protocol.
INTRODUCTION
Conditional gene targeting has become increasingly used in reverse mouse genetics, as it facilitates setting the site and time of gene inactivation in the body (1–3). This is achieved by the expression of the site-specific DNA recombinase Cre in conjunction with the target gene containing two Cre recognition sites (loxP). In the conditional mouse mutant, inactivation of the target gene is restricted in a spatial and/or temporal manner, depending on the expression pattern of the recombinase. Since the initial demonstration of this technology in 1994 (4), an increasing number of conditional knockout experiments have been published, most of which employed tissue-specific promoters to control Cre expression. Spatially regulated gene inactivation has proven to be powerful in dissecting the role of different cell types in a physiological process, as demonstrated for example by studies concerning the role of insulin receptor signaling in glucose homeostasis (5). The method of choice for precise gene function analyses in adult mice, however, is the temporal control of gene inactivation as it can prevent impaired embryonic development until the time of induction. In particular, inducible gene targeting in all organs would be a useful tool if the major site of gene action is not precisely defined. Optimally, such a system should permit a tight control of gene inactivation, efficient recombination in every single cell of the body upon induction, and the opportunity to produce sufficient numbers of inducible mouse mutants within a short time.
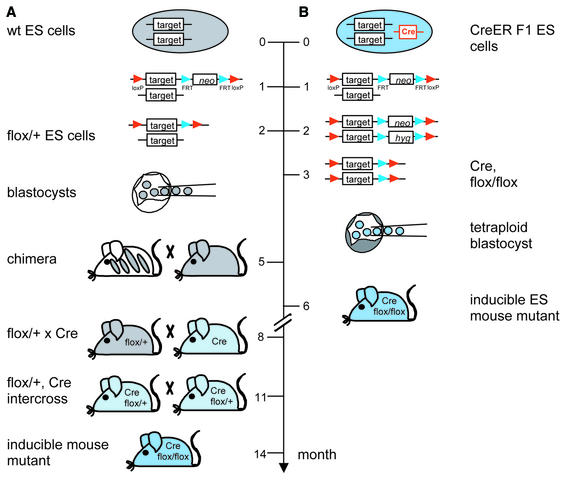
The major drawback of the current strategy is the long time frame required for the derivation of a conditional mouse mutant. Since two copies of the _loxP_-flanked allele must be combined with the recombinase transgene, at least three breeding steps (3 months each) are required to obtain a reasonable number of mice with the desired genotype (see Fig. 5A). While the first conditional mouse mutants can be expected within 14 months, additional time for breeding is usually needed to obtain sufficient numbers of mice for phenotypic analysis. To date, only a few mouse strains permitting ubiquitously inducible gene targeting are available, all of which suffer from incomplete recombination upon induction or background activity in the absence of inducer (6–10). Recently, a mouse strain has been generated by the insertion of a tamoxifen-inducible CreER fusion protein gene into the ubiquitously expressed Rosa26 gene (11). While this strain represented the most efficient tool for ubiquitously inducible gene targeting so far, a low affinity ER domain was used that requires high doses of the inducer 4-hydroxytamoxifen to achieve the maximal rate of recombination. Therefore, the side effects of the inducer may interfere with the phenotypic outcome of target gene inactivation. Taken together, there is a need for improved methods which both enable efficient gene knockout in all organs at non-toxic concentrations of the inducer and which accelerate the production of inducible mouse mutants.
Figure 5.
The generation of inducible mouse mutants via the current protocol and via tetraploid blastocyst complementation. (A) The current protocol includes the generation of targeted ES cells, the FLP-mediated deletion of the neo gene, the production of chimeric mice, the breeding for germline transmission and another two breeding steps to combine two copies of the _loxP_-flanked allele with the recombinase transgene. Following this scheme, the generation of conditional mouse mutants usually takes 14 months. Chimeras can be directly bred to Cre transgenic mice supposing that appropriate Cre strains on pure genetic backgrounds are available that allow the detection of germline transmission by coat color. This would save 3 months required for one breeding step. (B) The new approach uses hybrid ES cells carrying the Cre gene with an appropriate expression pattern for conditional gene inactivation in mice. Following the introduction of loxP sites into both alleles of the target gene, the FLP-mediated deletion of the selection marker genes and the injection of these ES cells into tetraploid blastocysts, conditional mouse mutants are obtained within 6 month. The time required for each step is indicated.
In this paper we describe a novel approach for the rapid generation of inducible mouse mutants, which overcomes the drawbacks of the current technology. The approach is based on a ubiquitously expressed, high affinity CreER fusion protein that allows the efficient inducible inactivation of a _loxP_-flanked target gene in most organs. In addition, we demonstrate that hybrid ES cells tolerate at least three consecutive gene targeting cycles including selection with the antibiotics G418 and hygromycin B without affecting the potency to complement tetraploid blastocysts. Our findings provide a new concept allowing the rapid production of ES cell-derived, inducible knockout mice.
MATERIALS AND METHODS
Construction of vectors
CreER T2. The Cre gene was amplified by PCR (Expand High Fidelity PCR system; Clontech) from PGKCrebpA using the oligos PR-A (TAACTAGCGGCCGCGTCGACCATGTCC AATTTACTGACCGTACAC) and creER5 (TCTCATGTCT CCAGCAGAATCGCCATCTTCCAGCAGGCG). In parallel the ER domain was amplified from the CreED2 vector (12) using the oligos creER4 (CTGGAAGATGGCGATTCTGC TGGAGACATGAGAGCTGCC) and creER1 (TAGTTAG CGGCCGCTCAGACTGTGGCAGGGAAACCCTC). Both PCR products were used as overlapping templates in a PCR with the primers PR-A and creER1. The resulting 2 kb product was digested with _Not_I and inserted into the _Not_I site of pBS. Site-directed mutagenesis (Stratagene) was applied to introduce a G400V mutation using primers ER-G400Vs (CATGGAGCACCCAGTGAAGCTACTGTTTG) and ER-G400Vas (CAAACAGTAGCTTCACTGGGTGCTCCATG). For the introduction of two amino acid changes, M543A/L544A, the primers ER AA2′ s EagI (ATGACCTGCTG CTGGAGGCGGCCGACGCCCACCGCCTAC) and ER AA2 as EagI (GTAGGCGGTGGGCGTCGGCCGCCTCCAGCA GCAGGTCAT) were used in a site-directed mutagenesis. The creER T2 was inserted into an expression vector containing a CMV promoter, an intron and a polyadenylation signal resulting in the vector CMV-CreER.
Rosa targeting vector. A 129 SV/EV-BAC library (Incyte Genomics) was screened with a probe against exon 2 of the Rosa26 locus [amplified from mouse genomic DNA using Rscreen1s (GACAGGACAGTGCTTGTTTAAGG) and Rscreen1as (TGACTACACAATATTGCTCGCAC)]. Out of the identified BAC clone an 11 kb _Eco_RV subfragment was inserted into the _Hin_dII site of pBS. Two fragments (a 1 kb _Sac_II–_Xba_I and a 4 kb _Xba_I fragment) were used as homology arms and inserted into a vector consisting of a FRT-flanked neomycin resistance gene (unpublished) and a splice acceptor site from adenovirus (13).
CreER T2 knock-in vector. The CreER T2 coding sequence and a polyadenylation site was inserted into the Rosa-targeting vector.
Rosa(lacZ reporter) knock-in vector. The neo gene of the Rosa-targeting vector was deleted through transformation of FLP-expressing bacteria (14). In the resulting vector a cassette containing a _loxP_-flanked PGK-hygro-pA followed by lacZ was inserted.
ect2 Targeting vector. The vector was generated by insertion of PCR fragments (with attached restriction sites) amplified from BAC DNA (129SVJ) into a vector consisting of a FRT-flanked neomycin resistance gene flanked by two loxP sites (G. Kauselmann, unpublished results). The 989 bp PCR fragment of the 5′ targeting arm was inserted with _Not_I/_Sgf_I 5′ of the loxP sites and the 4023 bp PCR fragment of the 3′ arm with _Xho_I/_Pme_I 3′ of the loxP sites. The 428 bp PCR fragment of exon 6 and the flanking intronic region were inserted with _Asc_I/_Fse_I between the loxP sites.
Cell culture
Culture and targeted mutagenesis of ES cells were carried out as previously described (15) with ES cell lines derived from both inbred and F1 embryos.
Generation of ect2 flox ES cells. The C57BL/6 cell line Bruce4 was electroporated with the described targeting vector, and out of 480 clones 3 homologous recombined clones were detected.
Mice
All mice were kept in the animal facility at Artemis Pharmaceuticals GmbH in microisolator cages (Tecniplast Sealsave). B6D2F1 mice for the generation of tetraploid blastocysts were obtained from Janvier. The pol_β_flox/rosa(CreER T2) and ect2 flox/rosa(CreER T2) mice were generated by breeding of rosa(CreER T2) ES mice with βT14 (4) and ect2 flox females (unpublished), respectively.
Production of ES mice by tetraploid embryo complementation
The production of mice by tetraploid embryo complementation was essentially performed as described (16).
Ligand administration
An aliquot of 100 mg tamoxifen-free base (T5648; Sigma) was suspended in 100 µl ethanol and dissolved in 1 ml sunflower oil (Sigma). This 10 mg/100 µl tamoxifen solution was sonicated for 1–2 min and then stored at –20°C. For p.o. administration the solution was thawed at 55°C and administrated to 4–8-week-old mice by a feeding needle (18061-20FST; Fine Science Tools GmbH).
Western blot analysis
Western blot analysis was performed using SDS–PAGE (NuPAGE; Invitrogen) and the Breeze Immunodetection System (Invitrogen) according to the manufacturer’s protocols. Immunodetection was done using HC-20 against ER, PRB-106C against cre, I-19 against actin and rabbit polyclonal IgG antibodies (Santa Cruz Biotechnology Inc.).
RESULTS
Design of F1 ES cells carrying an inducible Cre transgene
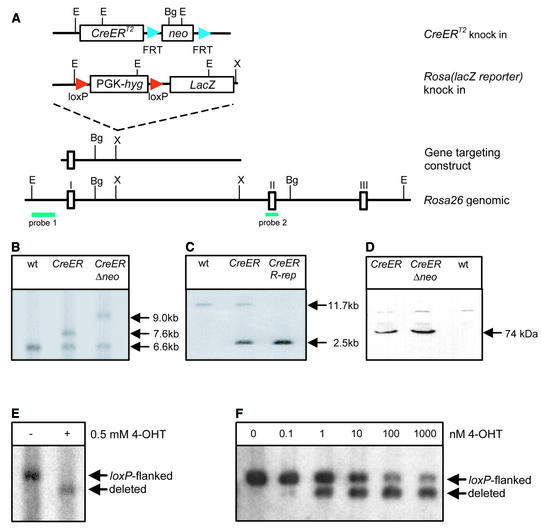
To derive an optimized system for inducible gene targeting in mice, we generated a fusion protein consisting of Cre recombinase and the mutated ligand-binding domain of the estrogen receptor ERT2 (17). The ERT2 domain is composed of amino acids 282–595 of the human estrogen receptor and carries three mutations (G400V/M543A/L544A) that confer a 10× enhanced sensitivity to the synthetic ligand 4-hydroxytamoxifen in mice as compared to the previously used ERT (G521R) domain (18). The CreER T2 coding region was inserted into the ubiquitously expressed Rosa26 locus through homologous recombination to obtain a configuration that allows the inducible inactivation of genes in all organs (Fig. 1). The Rosa26 targeting vector containing the CreER T2 gene is depicted in Figure 1A. We used the hybrid ES cell lines V6.5 [(C57BL/6 × 129S4Sv/Jae) F1] and ART4.12 [(C57BL/6 × 129S6/SvEvTac) F1] for homologous recombination, since these lines are capable of producing completely ES cell-derived mice (ES mice) through tetraploid blastocyst complementation with high efficiency (16). Independent recombinant ES cell clones were obtained at a frequency of 2% as verified by Southern blot analysis (Fig. 1B). Deletion of the neo gene did not affect the level of CreER T2 expression as determined by western blot analysis (Fig. 1D). A _loxP_-flanked hygromycin resistance gene was introduced into the second allele of Rosa26 (Fig. 1A and C) to provide a similar test substrate for Cre as the widely used Rosa26 reporter (19,20). In ES cells carrying both the rosa(CreER T2) and the rosa(lacZ reporter) alleles, complete recombination of the _loxP_-flanked segment was achieved upon treatment with 500 nM 4-hydroxytamoxifen for 4.5 days (Fig. 1E). Half-maximal activation of CreERT2 occurred at a concentration of 10 nM 4-hydroxytamoxifen (Fig. 1F) as compared to 100 nM for the CreERT fusion protein (12).
Figure 1.
Generation of targeted ES cells. (A) Scheme of the gene targeting strategy. Both constructs were sequentially inserted into the Rosa26 locus by homologous recombination as depicted. E, _Eco_RV; X, _Xba_I; Bg, _Bgl_I. (B) Southern blot analysis of genomic DNA from ES cells containing the targeted insertion of CreER T2 before and after FLP-mediated deletion of the neomycin resistance gene. The DNA of ES cells was digested with Bgl_I and hybridized with probe 2. The sizes of the wild-type, targeted and Δ_neo alleles are 6.6, 7.6 and 9.0 kb, respectively. (C) Southern blot analysis of genomic DNA extracted from double-targeted ES cells carrying the rosa(CreER T2) and rosa(lacZ reporter) alleles. Homologous recombination at the Rosa26 locus is detectable using _Eco_RV-digested genomic DNA and probe 1, resulting in an 11.7 kb band for the wild-type and a 2.5 kb band for the targeted allele. (D) Western blot analysis of CreERT2 expression in targeted ES cells before and after neo deletion. The western blot was probed with a Cre antiserum as described in Materials and Methods. (E) Southern blot analysis of inducible recombination of the rosa(lacZ reporter) allele. Cells were treated for 4.5 days with 500 nM 4-hydroxytamoxifen (4-OHT). Southern blot analysis of _Eco_RV-digested DNA was performed using the _rosa26_-specific probe 1 depicted in (A). (F) Titration of 4-OHT inducible recombination. Cells were treated for 3 days with increasing concentrations of 4-OHT as indicated. Southern blot analysis of _Eco_RV-digested DNA was performed using the _rosa26_-specific probe 1.
Generation of ES cell-derived mice
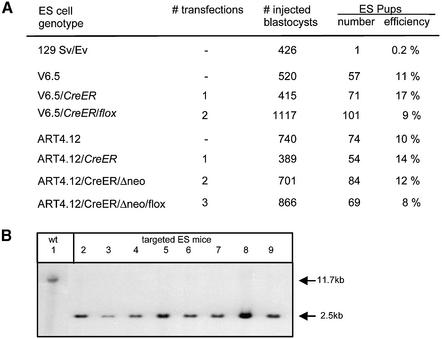
The three types of recombinant and wild-type V6.5 and ART4.12 ES cells were injected into tetraploid blastocysts and ES mice were obtained upon transfer of these blastocysts into pseudopregnant females. The proportion of transferred blastocysts that further developed into live born pups was in the range 8–17% and did not significantly decrease when ES cells were used that had undergone up to three sequential rounds of transfection (Fig. 2A). In contrast, the production of ES mice using inbred ES cells was inefficient (0.2%), confirming the ‘hybrid vigor’ effect described in Eggan et al. (16) (Fig. 2A). Southern blot analysis of genomic DNA using a _Rosa26_-specific probe showed that the V6.5/CreER/flox ES mice were indeed solely derived from the recombinant ES cells and did not contain any detectable tetraploid cells from the injected blastocysts (Fig. 2B). These results demonstrate that mice can be produced by tetraploid blastocyst complementation using cells that have undergone three consecutive rounds of transfection, including two drug selection cycles.
Figure 2.
Production efficiency of completely ES cell-derived mice. (A) ES cells with the indicated genotype were injected into tetraploid blastocysts. The percentages of blastocysts that developed into live born ES pups are depicted. (B) Southern blot analysis of DNA extracted from liver of ES mice (lanes 2–9). Genomic tail DNA from a wild-type ES mouse is used as a control (lane 1). DNA was digested with _Eco_RV and probed with the _Rosa26_-specific probe 1 as depicted in Figure 1A.
Ubiquitously inducible gene targeting in mice
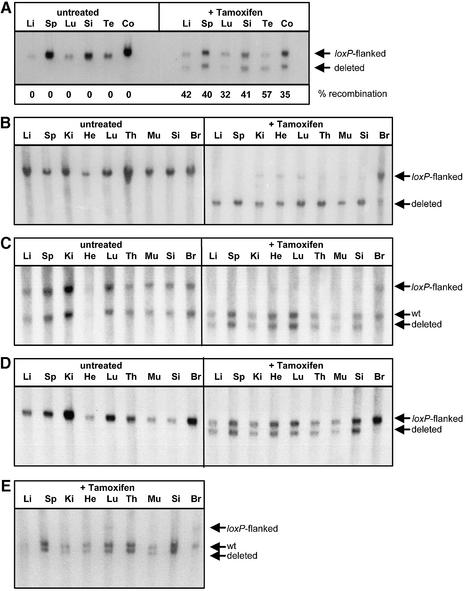
For the induction of CreERT2 in mice, we applied 1–5 mg tamoxifen orally for 5 days in the following experiments. We preferred to use this protocol, as the LD50 of tamoxifen in mice is 15-fold higher via the oral route than by i.p. injection (200 mg versus 5 g/kg) (21). It has been recently demonstrated that the oral application of tamoxifen is as efficient as i.p. injection of 4-hydroxytamoxifen for the activation of CreER (22). Rosa(CreER T2/lacZ reporter) mice were fed with daily 5 mg tamoxifen for 5 days and recombination of the lacZ reporter was analyzed 3 days after the last administration. Southern analysis of genomic DNA from different organs showed up to 50% recombination, without detectable background activity in untreated animals (Fig. 3A). This is a significantly higher degree of inducible recombination as compared to the results obtained with the Rosa26-CreER T strain (11), indicating that the ERT2 domain is indeed more sensitive to 4-hydroxytamoxifen. Upon recombination, the lacZ gene turned out to be inserted non-functionally, presumably due to a second start codon upstream of the coding region.
Figure 3.
Inducible recombination in mice. Mice carrying different _loxP_-flanked alleles were fed daily with 5 mg tamoxifen (A–D) or 1 mg tamoxifen (E) for 5 days. Cre-mediated deletion of the _loxP_-flanked region was detected by Southern analysis 3 days after the treatment. (A) Rosa(CreER T2/lacZ reporter) mice. Genomic DNA from various organs was digested with _Eco_RV and hybridized with a _lacZ_-specific probe. The percentages of deletion of the _loxP_-flanked allele, as quantified with a Bio-Imaging Analyzer, are indicated. (B) pol_β_flox/rosa(CreER T2) mice. Cre-mediated excision of the 1.5 kb _loxP_-flanked region was detected by Southern analysis. Genomic DNA from various organs was digested with _Bam_HI and hybridized with a probe specific for the polymerase β gene (4). (C) ect2 flox/rosa(CreER T2) mice. The loxP sites are 2.5 kb apart from each other flanking a _FRT_-flanked neomycin selection marker and exon 6 of the ect2 gene (described in Materials and Methods). Genomic DNA was digested with _Hpa_I and hybridized with an _ect2_-specific probe hybridizing upstream of the _loxP_-flanked region. (D) ect2 flox_Δ_neo/rosa(CreER T2) mice. The ect2 flox_Δ_neo allele was generated through FLP-mediated deletion of the neomycin selection marker from the ect2 flox allele resulting in a _loxP_-flanked region of 0.5 kb containing exon 6 of the ect2 gene (described in Materials and Methods). Genomic DNA was digested with _Hpa_I and hybridized with an _ect2_-specific probe. (E) ect2 flox/rosa(CreER T2) mice treated with 1 mg tamoxifen for 5 days. Genomic DNA was digested with _Hpa_I and hybridized with an _ect2_-specific probe hybridizing upstream of the _loxP_-flanked region. Li, liver; Sp, spleen; Ki, kidney; He, heart; Lu, lung; Th, thymus; Mu, muscle; Si, small intestine; Br, brain; Te, testis; Co, colon.
Since the Rosa26 locus has a limited accessibility for Cre (11), we were interested to test the rosa(CreER T2) configuration in the context of other _loxP_-flanked loci. As the second substrate, we used the _loxP_-flanked DNA polymerase β gene segment (pol_β_flox) (4). This allele had been used to characterize a number of tissue-specific (4,12,23–26) as well as inducible Cre strains (6,12), and shows an intermediate accessibility for Cre (F. Schwenk and J. Seibler, unpublished observations). The pol_β_flox/rosa(CreER T2) mice were fed with 5 mg tamoxifen/day for 5 days and analyzed 3 days later. Southern blot analysis revealed that the _loxP_-flanked polymerase β gene segment was excised in more than 90% of cells in all organs except brain, whereas no background recombination was detected in untreated mice (Fig. 3B). As a third substrate, we used a 2.5 kb _loxP_-flanked segment of the ect2 gene. Again, up to 100% recombination in most tissues was achieved upon tamoxifen treatment of ect2 flox/rosa(CreER T2) mice, without detectable background recombination in uninduced controls (Fig. 3C). A similar degree of recombination was achieved when a shorter (0.5 kb) _loxP_-flanked segment in the same position of the ect2 gene was used (Fig. 3D). ‘Low dose application’ of 5 × 1 mg tamoxifen was sufficient to achieve complete recombination of the ect2 flox allele in most organs, further demonstrating the improved sensitivity of the ERT2 domain in vivo.
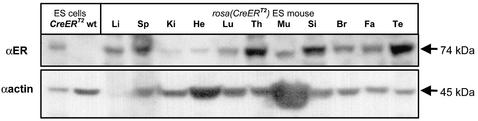
To investigate whether the low recombination efficiency in brain was due to low CreER T2 expression we performed western analysis using antibodies specific for the human estrogen receptor. The 74 kDa band corresponding to the CreERT2 fusion protein was detectable in all organs including brain, with the highest levels in thymus, small intestine and testis (Fig. 4). The limited degree of recombination in brain may thus reflect a lower local concentration of 4-hydroxytamoxifen rather than a reduced expression level of CreERT2.
Figure 4.
Western blot analysis of CreER T2 expression in ES mice. Proteins were extracted from rosa(CreER T2) targeted ES mice and analyzed as described in Materials and Methods. Protein extracts from wild-type and rosa(CreER T2) targeted ES cells are used as controls. The positions of bands representing CreERT2 and actin are indicated. Li, liver; SP, spleen; Ki, kidney; He, heart; Lu, lung; Th, thymus; Mu, muscle; Si, small intestine; Br, brain; Fa, fat tissue; Te, testis.
DISCUSSION
We describe an optimized system that allows ubiquitously inducible gene targeting in mice. This system is based on a fusion protein consisting of Cre recombinase and a mutated ligand-binding domain of the estrogen receptor that is expressed under the control of the ubiquitously active Rosa26 promoter (19). In contrast to the Rosa26-CreER T mouse strain published by Vooijs et al. (11), we used the ERT2 domain that responds at an ∼10-fold lower dose of the synthetic inducer 4-hydroxytamoxifen as compared to the ERT domain (17,18). In mice, the rosa(CreER T2) configuration achieved nearly complete inducible excision of multiple _loxP_-flanked gene segments in all organs except brain, without background recombination in the absence of inducer (Fig. 3). Thus, our system permits the tight temporal control of ubiquitous gene inactivation, allowing precise gene function analysis in animals that have undergone normal embryonic development. The lower degree of deletion observed with the Rosa26 allele (lacZ reporter) confirms the position dependency of Cre-mediated recombination described by Vooijs et al. (11). Since all other targets tested so far achieved near complete excision with the rosa(CreER T2) configuration (Fig. 2 and unpublished observations), a limited accessibility may be restricted to only a minority of _loxP_-flanked loci. As the rosa(CreER T2) allele permits inducible gene inactivation in all organs, it should find broad application as a research tool in reverse mouse genetics.
We have employed the tetraploid blastocyst complementation approach for in vivo analysis of the rosa(CreER T2) configuration, allowing the generation of ES cell-derived mice (ES mice) in a single step without the requirement of time-consuming breeding. Tetraploid blastocysts are not capable of completing normal development, but, when complemented by the introduction of diploid ES cells, support the development of solely ES cell-derived mice. The efficient generation of such ES mice using non-transgenic hybrid ES cells has been recently demonstrated (16). In this study, the authors also succeeded in generating a single ES mouse through tetraploid blastocyst complementation using transfected cells that had undergone two consecutive rounds of selection. However, it remained unclear whether ES cells maintain their potency to complement tetraploid embryos with sufficient efficiency and reproducibility even after additional genetic modifications, which is a prerequisite for the strategy depicted in Figure 5B. We introduced both the CreER T2 coding region and a lacZ reporter construct into hybrid ES cells by targeted insertion into the Rosa26 locus. The targeting strategy involved three consecutive rounds of transfection and selection with the antibiotics G418 and hygromycin B. We found that all targeted ES cell clones produced ES-derived mice with high efficiency. The proportion of transferred blastocysts that developed into live born pups was in the range 8–17% and did not significantly decrease using ES cells that had undergone three consecutive transfections (Fig. 2A). Thus, we demonstrate that hybrid ES cells can be exposed to at least three transfection cycles without affecting the potency to complement tetraploid embryos, allowing the analysis of complex genetic alterations in ES cell-derived mice within the time of a single mouse generation.
Our findings provide the proof of principle for a new concept allowing the rapid production of inducible knockout mice using tetraploid blastocyst complementation. So far, the derivation of conditional mouse mutants had been a time-consuming undertaking since three breeding steps (3 months each) are usually required to obtain mice combining two copies of the _loxP_-flanked allele and the recombinase transgene (Fig. 5A). To address this limitation, we propose a new strategy that involves the tetraploid blastocyst complementation approach for the one step generation of conditional mouse mutants from genetically engineered ES cells. The generation of ES cells from Cre transgenic mice is feasible using standard protocols (15), as demonstrated by O’Gorman and colleagues (27). Three transfection cycles are sufficient to introduce loxP sites into both alleles of the target gene in ES cells, following the strategy depicted in Figure 5B. Thus, by using Cre transgenic ES cell lines for the generation of the _loxP_-flanked alleles, it will be possible to produce conditional mouse mutants directly from the targeted ES cells through tetraploid blastocyst complementation. Using this scheme, adult (2 months old) inducible mouse mutants available for phenotypic analyses can be obtained within 6 months, required for three gene targeting steps (1 month each) and one mouse generation time (3 months) (Fig. 5B). This saves more than 50% of time as compared to the current conditional gene targeting protocol, which requires 14 months including two targeting steps and four mouse generations (Fig. 5A). Since the described approach is simple and technically not more demanding than the current knockout protocol, we expect that this strategy will become a widely used tool in reverse mouse genetics.
Acknowledgments
ACKNOWLEDGEMENTS
We thank Francis Stewart (MPI for Cell Biology and Genetics, Dresden), Frank Edenhofer, Thomas Wunderlich (Institute for Genetics, University of Cologne) and Sandra Niehaves (Artemis Pharmaceuticals, Cologne) for scientific discussions and technical advice. We are grateful to Gordon Stott and Jens Tampe (Artemis Pharmaceuticals, Cologne) for critically reading the manuscript. This work was supported by the German Ministry for Education and Science (BMBF) grant no. 0311956.
REFERENCES
- 1.Rajewsky K., Gu,H., Kühn,R., Betz,U.A., Muller,W., Roes,J. and Schwenk,F. (1996) Conditional gene targeting. J. Clin. Invest., 98, 600–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lewandoski M. (2001) Conditional control of gene expression in the mouse. Nature Rev. Genet., 2, 743–755. [DOI] [PubMed] [Google Scholar]
- 3.Kwan K.M. (2002) Conditional alleles in mice: practical considerations for tissue-specific knockouts. Genesis, 32, 49–62. [DOI] [PubMed] [Google Scholar]
- 4.Gu H., Marth,J.D., Orban,P.C., Mossmann,H. and Rajewsky,K. (1994) Deletion of a DNA polymerase beta gene segment in T cells using cell type specific gene targeting. Science, 265, 103–106. [DOI] [PubMed] [Google Scholar]
- 5.Kahn C.R., Bruning,J.C., Michael,M.D. and Kulkarni,R.N. (2000) Knockout mice challenge our concepts of glucose homeostasis and the pathogenesis of diabetes mellitus. J. Pediat. Endocrinol. Metab., 13, 1377–1383. [DOI] [PubMed] [Google Scholar]
- 6.Kühn R., Schwenk,F., Aguet,M. and Rajewsky,K. (1995) Inducible gene targeting in mice. Science, 269, 1427–1429. [DOI] [PubMed] [Google Scholar]
- 7.Feil R., Brocard,J., Mascrez,B., LeMeur,M., Metzger,D. and Chambon,P. (1996) Ligand-activated site-specific recombination in mice. Proc. Natl Acad. Sci. USA, 93, 10887–10890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holzenberger M., Zaoui,R., Leneuve,P., Hamard,G. and Le Bouc,Y. (2000) Ubiquitous postnatal LoxP recombination using a doxycycline auto-inducible Cre transgene (DAI-Cre). Genesis, 26, 157–159. [PubMed] [Google Scholar]
- 9.Dietrich P., Dragatsis,I., Xuan,S., Zeitlin,S. and Efstratiadis,A. (2000) Conditional mutagenesis in mice with heat shock promoter-driven cre transgenes. Mamm. Genome, 11, 196–205. [DOI] [PubMed] [Google Scholar]
- 10.Guo C., Yang,W. and Lobe,C.G. (2002) A Cre recombinase transgene with mosaic, widespread tamoxifen-inducible action. Genesis, 32, 8–18. [DOI] [PubMed] [Google Scholar]
- 11.Vooijs M., Jonkers,J. and Berns,A. (2001) A highly efficient ligand-regulated Cre recombinase mouse line shows that LoxP recombination is position dependent. EMBO Rep., 2, 292–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schwenk F., Kühn,R., Angrand,P.O., Rajewsky,K. and Stewart,A.F. (1998) Temporally and spatially regulated somatic mutagenesis in mice. Nucleic Acids Res., 26, 1427–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Friedrich G. and Soriano,P. (1991) Promoter traps in embryonic stem cells: a genetic screen to identify and mutate developmental genes in mice. Genes Dev., 9, 1513–1523. [DOI] [PubMed] [Google Scholar]
- 14.Buchholz F., Angrand,P.O. and Stewart,A.F. (1996) A simple assay to determine the functionality of Cre or FLP recombination targets in genomic manipulation constructs. Nucleic Acids Res., 24, 3118–3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hogan B., Beddington,R., Costantini,F. and Lacy,E. (1994) Manipulating the Mouse Embryo: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, pp. 253–289.
- 16.Eggan K., Akutsu,H., Loring,J., Jackson-Grusby,L., Klemm,M., Rideout,W.M.,III, Yanagimachi,R. and Jaenisch,R. (2001) Hybrid vigor, fetal overgrowth and viability of mice derived by nuclear cloning and tetraploid embryo complementation. Proc. Natl Acad. Sci. USA, 98, 6209–6214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feil R., Wagner,J., Metzger,D. and Chambon,P. (1997) Regulation of Cre recombinase activity by mutated estrogen receptor ligand-binding domains. Biochem. Biophys. Res. Commun., 237, 752–757. [DOI] [PubMed] [Google Scholar]
- 18.Indra A.K., Warot,X., Brocard,J., Bornert,J.M., Xiao,J.H., Chambon,P. and Metzger,D. (1999) Temporally-controlled site-specific mutagenesis in the basal layer of the epidermis: comparison of the recombinase activity of the tamoxifen-inducible Cre-ER(T) and Cre-ER(T2) recombinases. Nucleic Acids Res., 27, 4324–4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Soriano P. (1999) Generalized lacZ expression with the ROSA26 Cre reporter strain. Nature Genet., 21, 70–71. [DOI] [PubMed] [Google Scholar]
- 20.Mao X., Fujiwara,Y. and Orkin,S.H. (1999) Improved reporter strain for monitoring Cre recombinase-mediated DNA excisions in mice. Proc. Natl Acad. Sci. USA, 96, 5037–5042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Furr B.J.A. and Jordan,V.C. (1984) The pharmacology and clinical uses of tamoxifen. Pharmacol. Ther., 25, 127–205. [DOI] [PubMed] [Google Scholar]
- 22.Kuhbandner S., Brummer,S., Metzger,D., Chambon,P., Hofmann,F. and Feil,R. (2000) Temporally controlled somatic mutagenesis in smooth muscle. Genesis, 28, 15–22. [DOI] [PubMed] [Google Scholar]
- 23.Schwenk F., Baron,U. and Rajewsky,K. (1995) A cre-transgenic mouse strain for the ubiquitous deletion of loxP-flanked gene segments including deletion in germ cells. Nucleic Acids Res., 23, 5080–5081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Betz U.A.K., Voßhenrich,C.A.J., Rajewsky,K. and Müller,W. (1996) Bypass of lethality with mosaic mice generated by Cre-loxP mediated recombination. Curr. Biol., 6, 1307–1316. [DOI] [PubMed] [Google Scholar]
- 25.Rickert R.C., Roes,J. and Rajewsky,K. (1997) B lymphocyte-specific, Cre-mediated mutagenesis in mice. Nucleic Acids Res., 25, 1317–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clausen B.E., Burkhardt,C., Reith,W., Renkawitz,R. and Forster,I. (1999) Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res., 8, 265–277. [DOI] [PubMed] [Google Scholar]
- 27.O’Gorman S., Dagenais,N.A., Qian,M. and Marchuk,Y. (1997) Protamine-Cre recombinase transgenes efficiently recombine target sequences in the male germ line of mice, but not in embryonic stem cells. Proc. Natl Acad. Sci. USA, 94, 14602–14607. [DOI] [PMC free article] [PubMed] [Google Scholar]