A Novel Antiviral Intervention Results in More Accurate Assessment of Human Immunodeficiency Virus Type 1 Replication Dynamics and T-Cell Decay In Vivo (original) (raw)
Abstract
Mathematical models provide an understanding of in vivo replication kinetics of human immunodeficiency virus type 1 (HIV-1). With a novel intervention designed for increased potency, we have more accurately deduced the half-lives of virus-producing CD4+ T cells, 0.7 day, and the generation time of HIV-1 in vivo, approximately 2 days, confirming the dynamic nature of HIV-1 replication.
In 1996, we developed a mathematical model to analyze a set of plasma human immunodeficiency virus type 1 (HIV-1) RNA data from five chronically infected individuals treated with ritonavir monotherapy (5). On the basis of these results, we estimated the half-life (_t_1/2) of free virions to be 0.24 day and the _t_1/2 of productively infected CD4+ T cells to be 1.6 days. These turnover rates were considered minimum estimates because the analysis assumed that viral replication was completely suppressed by the therapy. From these data and measured baseline viral loads, we calculated minimal daily virion production to be ∼1010 particles in a typical patient. Moreover, the dynamic turnover of HIV-1 and productively infected cells formed the scientific rationale for combination antiretroviral therapy (1, 5), which has led to significant reductions in HIV-1-related morbidity and mortality (3).
We have subsequently performed two additional experiments to better define c, the clearance rate constant of plasma virions. The results of these studies (6) allowed us to adjust the previous estimate for virion t_1/2 to a new value of ∼30 min. Similarly, we have also adjusted the estimate for δ,_ the rate of loss of productively infected CD4+ T cells, in a follow-up study (4). The new estimate of the _t_1/2 of virus-producing cells was ∼1.1 days, again assuming that the combination antiretroviral therapy used was completely suppressive.
Advances in the development of antiretroviral agents have yielded a number of drugs that are more effective in blocking HIV-1 replication in vivo (7). Thus, we have better tools in hand to readdress the issue of the relative potency of our therapeutic regimens and, hence, to better estimate δ. As we have previously reported, the slope of the initial phase of plasma HIV-1 RNA decline depends on the degree of suppression of viral replication, as well as on δ (1, 4, 5). As the potency of combination therapy approaches 100%, the determination of δ becomes more precise. We now report findings from a Rockefeller University Institutional Review Board-approved novel interventional trial to reassess δ, as well as the relative potency of our current antiretroviral therapies.
Nine chronically HIV-1-infected individuals (Table 1) were treated with lopinavir-ritonavir (1,066 and 266 mg/day, respectively), efavirenz (600 mg/day), lamivudine (300 mg/day), and tenofovir DF (300 mg/day). Study subjects were hospitalized for the first 72 h of therapy, when plasma viral loads were measured by reverse transcription-PCR (Roche Amplicor Ultrasensitive Cobas 1.5; detection limit of 50 copies/ml) at 6-h intervals. Thereafter, measurements were taken daily when the patients were outpatients until day 10. The plasma viral load in each patient began to decline after an average lag of 0.76 ± 0.71 day (data not shown). This lag was followed by an exponential decline in viremia that was substantially faster than those previously observed during standard combination retroviral therapy. The first phase of HIV-1 RNA decay in this trial ended after 2.5 to 7.0 days (mean of 4.1 days), compared to 7 to 10 days in previous studies (1, 5).
TABLE 1.
Summary of pretreatment status and rate of loss of productively infected CD4+ T cells in nine study subjects
| Patient | Pretreatment | Rate of loss of productively infected CD4+ T cells | ||||
|---|---|---|---|---|---|---|
| HIV-1 RNA Log no. of copies/ml of plasma | No. of CD4 cells/mm3 | δ (day−1) | _t_1/2 (days) | 95% Confidence limit | ||
| Lower | Upper | |||||
| 202 | 4.7 | 224 | 0.6 | 1.1 | 0.5 | 1.9 |
| 204 | 4.4 | 365 | 1.2 | 0.6 | 1.0 | 1.5 |
| 205 | 5.9 | 167 | 1.4 | 0.5 | 0.9 | 1.9 |
| 206 | 5.8 | 339 | 1.3 | 0.5 | 0.7 | 2.8 |
| 207 | 4.6 | 289 | 1.3 | 0.6 | 0.8 | 2.4 |
| 209 | 4.6 | 280 | 0.8 | 0.9 | 0.6 | 1.7 |
| 210 | 5.2 | 372 | 0.9 | 0.8 | 0.6 | 1.6 |
| 211 | 4.5 | 377 | 1.0 | 0.7 | 0.7 | 1.6 |
| 212 | 4.3 | 700 | 0.9 | 0.8 | 0.6 | 1.3 |
| Mean ± SD | 4.9 ± 0.6 | 346 ± 151 | 1.0 ± 0.3 | 0.7 ± 0.2 |
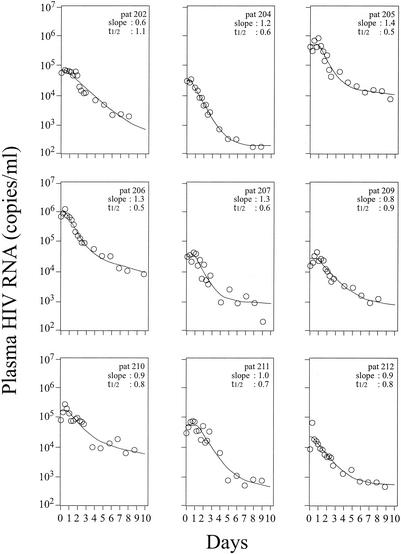
By using nonlinear regression analysis, we estimated δ for each patient by fitting plasma viral load data to our published mathematical model (4, 5), using a value for c of 23 day−1 (6) (Fig. 1). The best-fit values of δ ranged from 0.6 to 1.4 day−1, with a mean of 1.0 day−1. The latter value translates into a mean _t_1/2 of 0.7 day (Table 1), which is significantly shorter than our last estimate of 1.1 days (4). Although the cohort was small, the rate of clearance of virus-producing cells, δ, was not correlated to the baseline CD4+ T-cell count or baseline levels of plasma viremia. It is important to note that this decay rate for virus-producing cells is indicative of the time from initial virion production to cessation of particle production, presumably because of cell death. This underscores the relatively short period of time for which an infected cell is an available target for HIV-1-specific cytotoxic T cells.
FIG. 1.
HIV-1 RNA decay in the plasma of nine patients (pat) after initiation of therapy with lopinavir-ritonavir, tenofovir DF, lamivudine, and efavirenz. The best fit of measured HIV-1 RNA levels in plasma (circles) to a multicomponent model of HIV-1 RNA decay (4) (solid lines) is shown.
In this study, by using both reverse transcriptase and protease inhibitors, we observed a mean lag of ∼0.76 day before plasma viremia began to decline in patients. It is reassuring that the delay here is shorter than the 1.0 day observed for protease inhibitor therapy (5) because the present lag reflects the time from reverse transcription to particle release whereas the previous lag reflects the time from virus binding to particle release. Thus, by inference, the average time required to go from virus binding to reverse transcription is ∼0.24 day in vivo, which is consistent with experimental results in vitro.
By mathematically analyzing the sharper decline of plasma viremia seen with the current antiretroviral regimen, we have also determined δ, the rate of loss of productively infected CD4+ T cells, with greater precision. The new value of δ is ∼0.7 day−1, which is statistically significantly faster (P = 0.018, Mann-Whitney test) than our last minimal estimate of ∼1.0 day−1 (4). The faster rate of loss of virus-producing cells has two major implications. First, it shows that the generation time for HIV-1 in vivo is correspondingly shorter, ∼2.0 days, which is obtained by summing up 1/c, 1/δ, and the eclipse time of ∼1.0 day (5). This value indicates that HIV-1 typically undergoes 180 generations per year in an infected person. Coupled with the high mutation rate of reverse transcription, it is therefore not surprising that HIV-1 infection is a prime example of Darwinian evolution fast forward. Second, by using the faster decay rate for productively infected cells to reanalyze published data on the decline of plasma viremia in prior treatment trials, we come to the inescapable conclusion that combination antiretroviral regimens in common use today have relative potencies substantially lower than that observed in the present study, which, in turn, may be well short of ideal (2).
Acknowledgments
This study was supported by grants from the National Institutes of Health (A141387, AIY47033, AI28433, RR06555, MO1-RR00102, and A142848), Abbott Laboratories, Gilead Sciences, and Bristol-Myers Squibb. Portions of this work were performed under the auspices of the U.S. Department of Energy.
REFERENCES
- 1.Ho, D. D., A. U. Neumann, A. S. Perelson, W. Chen, J. M. Leonard, and M. Markowitz. 1995. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature 373**:**123-126. [DOI] [PubMed] [Google Scholar]
- 2.Louie, M., C. Hogan, M. Di Mascio, A. Hurley, V. Simon, J. Rooney, N. Ruiz, S. Brun, E. Sun, A. S. Perelson, D. D. Ho, and M. Markowitz. Determining the relative efficacy of highly active antiretroviral therapy. J. Infect. Dis., in press. [DOI] [PubMed]
- 3.Palella, F. J., Jr., K. M. Delaney, A. C. Moorman, M. O. Loveless, J. Fuhrer, G. A. Satten, D. J. Aschman, S. D. Holmberg, et al. 1998. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N. Engl. J. Med. 338**:**853-860. [DOI] [PubMed] [Google Scholar]
- 4.Perelson, A. S., P. Essunger, Y. Cao, M. Vesanen, A. Hurley, K. Saksela, M. Markowitz, and D. D. Ho. 1997. Decay characteristics of HIV-1-infected compartments during combination therapy. Nature 387**:**188-191. [DOI] [PubMed] [Google Scholar]
- 5.Perelson, A. S., A. U. Neumann, M. Markowitz, J. M. Leonard, and D. D. Ho. 1996. HIV-1 dynamics in vivo: virion clearance rate, infected cell life-span, and viral generation time. Science 271**:**1582-1586. [DOI] [PubMed] [Google Scholar]
- 6.Ramratnam, B., S. Bonhoeffer, J. Binley, A. Hurley, L. Zhang, J. E. Mittler, M. Markowitz, J. P. Moore, A. S. Perelson, and D. D. Ho. 1999. Rapid production and clearance of HIV-1 and hepatitis C virus assessed by large volume plasma apheresis. Lancet 354**:**1782-1785. [DOI] [PubMed] [Google Scholar]
- 7.Yeni, P. G., S. M. Hammer, C. C. Carpenter, D. A. Cooper, M. A. Fischl, J. M. Gatell, B. G. Gazzard, M. S. Hirsch, D. M. Jacobsen, D. A. Katzenstein, J. S. Montaner, D. D. Richman, M. S. Saag, M. Schechter, R. T. Schooley, M. A. Thompson, S. Vella, and P. A. Volberding. 2002. Antiretroviral treatment for adult HIV infection in 2002: updated recommendations of the International AIDS Society—USA Panel. JAMA 288**:**222-235. [DOI] [PubMed] [Google Scholar]