Analysis and accurate quantification of CpG methylation by MALDI mass spectrometry (original) (raw)
Abstract
As the DNA sequence of the human genome is now nearly finished, the main task of genome research is to elucidate gene function and regulation. DNA methylation is of particular importance for gene regulation and is strongly implicated in the development of cancer. Even minor changes in the degree of methylation can have severe consequences. An accurate quantification of the methylation status at any given position of the genome is a powerful diagnostic indicator. Here we present the first assay for the analysis and precise quantification of methylation on CpG positions in simplex and multiplex reactions based on matrix-assisted laser desorption/ ionisation mass spectrometry detection. Calibration curves for CpGs in two genes were established and an algorithm was developed to account for systematic fluctuations. Regression analysis gave R2 ≥ 0.99 and standard deviation around 2% for the different positions. The limit of detection was ∼5% for the minor isomer. Calibrations showed no significant differences when carried out as simplex or multiplex analyses. All variable parameters were thoroughly investigated, several paraffin-embedded tissue biopsies were analysed and results were verified by established methods like analysis of cloned material. Mass spectrometric results were also compared to chip hybridisation.
INTRODUCTION
With the availability of the draft sequence of the human genome, research is now turning towards the elucidation of gene function and regulation. Epigenetics, which can be defined as the study of heritable changes in gene expression without alteration of the DNA sequence itself (1), will be one of the key areas. DNA methylation occurs in mammals almost exclusively on the 5 position of cytosine in the context of a CpG dinucleotide (2,3). This dinucleotide is under-represented in the genome, probably because it is a mutation hotspot, and is generally methylated. CpG-rich clusters (CpG islands) are often found in the promoter region and first exons of genes, and are mostly not methylated. DNA methylation is associated with X chromosome inactivation (4), genomic imprinting (5), maintenance of genome integrity by transcriptional silencing of repetitive DNA sequences (6) and control of gene expression (7). Aberrant methylation patterns have been reported in various diseases (7), especially cancer (8,9). In tumour cells global hypomethylation of the genome as well as hypermethylation of CpG islands is observed (8,9). Methylation patterns can be shared by different types of tumours as well as being tumour-type specific (10,11). For the analysis of DNA methylation, sensitive and quantitative techniques are needed to detect even subtle changes in the degree of methylation as biological samples often represent a heterogeneous mixture of different cells, especially tumour and non-tumour cells from tissue biopsies. A variety of methods for the analysis of CpGs using either digestion of DNA with methylation-sensitive restriction enzymes or bisulphite conversion of DNA have been presented in the last year (12). Restriction enzymes have been widely used for techniques such as restriction landmark genomic scanning (10), methylation-sensitive arbitrarily primed PCR (13), methylated CpG island amplification (14) and differential methylation hybridisation (15). However, due to their dependence on restriction sites accessible to methylation-sensitive restriction enzymes, these methods are limited in their applicability. Bisulphite treatment of genomic DNA converts all non-methylated cytosines to uracils, whereas methylated cytosines are not changed under the reaction conditions. The methylation status can then be determined among other techniques by either direct sequencing or sequencing of subclones (16,17), methylation-sensitive PCR (MSP) (18), fluorescent real-time PCR (MethyLight; 19) (ConLight-MSP; 20), combined bisulphite restriction analysis (COBRA) (21), single-strand conformation polymorphism (SSCP) (22), melting curves (23,24) or denaturing gel electrophoresis (25). Methylation-sensitive single nucleotide primer extension (Ms-SNuPE) has been combined with radioactive (26) or ion-pair reverse phase HPLC (SNuPE IP RP HPLC) detection (27). Genome-wide analysis of specific methylation positions has been performed using microarray-based methods (28–30). While mass spectrometry is one of the key detection platforms for the analysis of genetic variations (31), it has only been applied to the assessment of global DNA methylation levels of digested DNA samples by HPLC–electrospray ionisation mass spectrometry (32,33).
For the analysis of genetic variations such as single nucle otide polymorphisms (SNPs), MALDI mass spectrometry (34) combined with primer extension for allele discrimination has been shown to be an accurate and quantitative tool for the determination of allele frequencies in pools of genomic DNA (35–38). Dilution of a known quantity of a heterozygous sample into a homozygous sample at different concentrations yielded highly linear calibration curves with correlation factors between 0.979 and 0.994 (35). When assays are performed in several replicates, accuracy (deviation from the expected allele frequency) is around 2–3% with a limit of quantification of 5–10% and the limit of detection is around 5% for the minor allele. The accuracy is independent of pool size (37) and when compared to different techniques for quantification, MALDI mass spectrometric analysis performed substantially better; it resulted in the lowest standard error of the mean and required the fewest replicates per pool (39). One of the advantages of mass spectrometers is their multichannel analysis capability; no significant differences between results obtained in simplex or multiplex (quadruplex) assay formats were found (38). This quality in combination with the number of methylation variable positions (MVPs) of a CpG island that should be tested simultaneously make mass spectrometry and DNA methylation analysis a good match.
Here we present the first assay for the analysis and accurate quantification of MVPs based on mass spectrometric detection. This epigenotyping assay combines bisulphite treatment of genomic DNA with the GOOD assay (40–42) that has been adapted to the special requirements of the low sequence diversity of bisulphite-treated DNA. The GOOD assay is a purification-free sample preparation method for high throughput, low cost and reliable SNP genotyping and molecular haplotyping (43) that uses primer extension for allele discrimination and MALDI mass spectrometry for detection. All possible variable parameters of the molecular biology steps as well as of the detection were thoroughly investigated. Calibration curves are recorded to compensate for preferential allele amplification and different annealing behaviour of extension primers. Statistical methods for accumulation and analysis of the mass spectra are developed to yield highly accurate and quantitative information about the methylation degree of CpG positions in simplex and multiplex reactions. This assay performed well in the analysis of even low quality DNA from paraffin-embedded tissue biopsies. As one model system for isolated intragenic CpG positions, we chose exon 14 of the coagulation factor VIII (FVIII) (44). Two studies reported heterogeneous methylation levels (40–80%) on CpGs in exon 14, making it an ideal target for our quantification assay (45,46). To prove the applicability of the GOOD assay for epigenotyping for CpG islands the gene segment encompassing the transcription start of glutathione _S_-transferase π (GSTP1) was analysed. This gene is well described and known to be hypermethylated in various cancers, particularly prostate cancer, whereas it is unmethylated in normal tissue (47). Efforts have been made at prostate cancer diagnosis by analysis of the methylation status of this gene from body fluids (48).
MATERIALS AND METHODS
Primers for PCR amplification and microarray experiments were purchased from MWG (Ebersberg, Germany), primers for primer extension from Biotez (Berlin, Germany), Platinum™ Taq High Fidelity DNA polymerase from Invitrogen Life Technologies (Carlsbad, CA), shrimp alkaline phosphatase (SAP) from Amersham (Little Calfont, UK), TMA31FS DNA polymerase from Roche Molecular Systems (Alameda, CA), phosphodiesterase II (from calf spleen) from Worthington Biochemical Corp. (Lakewood, NJ), dNTPs from Hybaid (Ashford, UK), α-S-ddNTPs from Biolog (Bremen, Germany), _Mss_I from MBI Fermentas (St Leon-Rot, Germany), _Sss_I from New England Biolabs (Beverly, MA), proteinase K from Roth (Buchenau, Germany), SeaPlaque™ low melting point agarose from FMC (Philadelphia, PA) and mixed human DNA from Promega (Madison, WI). PCR products of FVIII and GSTP1 derived from clones of bisulphite-treated completely methylated and non-methylated human genomic DNA, respectively, were provided by Epigenomics AG (Berlin, Germany). PCR products were purified with the QIAquick PCR purification kit (Qiagen, Hilden, Germany) or Exo-SAP-IT™ from USB Corp. (Cleveland, OH). DNA quantification was performed using the PicoGreen™ double-stranded (ds)DNA quantification kit (Molecular Probes, Eugene, OR) on a SpectraMax Gemini UV spectrometer (Molecular Devices, Sunnyvale, CA). DNA extracted from tissues was purified either with Wizard™ Genomic DNA Purification kits from Promega or QIAamp DNA Mini kits (Qiagen). Cloning was performed using the TOP10 TA Cloning™ kit with a pCR™ 2.1 vector from Invitrogen Life Technologies. Standard reagents were bought from either Aldrich (Milwaukee, WI) or Merck (Darmstadt, Germany) and were of analytical grade, if not otherwise stated. Thermocycling procedures were carried out in an Eppendorf 96 Gradient Mastercycler (Hamburg, Germany). Bisulphite sequencing was performed with the BigDye Terminator Cycle Sequencing kit (ABI, Foster City, CA) and analysed on an ABI 310 sequencer. Mass spectrometric analyses were performed using a Bruker Reflex III time-of-flight mass spectrometer (Bruker Daltonik, Bremen, Germany) equipped with a Scout MTP™ ion source with delayed extraction.
Extraction of DNA
Samples were provided by Charité (Berlin, Germany). DNA from fresh colon cancer tissues and matched normal controls was extracted with the Wizard™ Genomic DNA Purification kit. Punched needle biopsies were taken from different positions of paraffin-embedded tissue from an advanced prostate tumour. Approximately 12 mg of tissue from each specimen was used. The samples were overlaid with 180 µl of lysis buffer (50 mM Tris–HCl, pH 8.4, 0.5% SDS, 1 mM EDTA) and boiled for 10 min. An aliquot of 20 µl of proteinase K (20 mg/ml) was added to each sample and incubated for 4 h at 55°C. DNA was further purified using the QIAamp DNA Mini kit.
Preparation of _Sss_I-treated DNA
Aliquots of 7.5 µl of NE buffer 2, 10 nmol _S_-adenosylmethionine (SAM) and 6 U _Sss_I were added to 4.5 µg of human genomic DNA in a final volume of 67.5 µl. The solution was incubated at 37°C in a water bath. After 3 h and again after an additional 2 h, 10 nmol SAM and 6 U _Sss_I were added and the reaction was incubated overnight at 37°C. The enzyme was inactivated at 95°C for 5 min and the solution was directly used for bisulphite treatment.
Bisulphite conversion of genomic DNA
The sodium bisulphite treatment was performed according to the procedure described by Olek et al. (49) with minor modifications. Samples of 40 µg of isolated chromosomal DNA were digested with 25 U of _Mss_I at 37°C for 16 h. The enzyme was heat inactivated at 90°C for 10 min and the DNA stored at 4°C for further use. An aliquot of 700 ng of genomic DNA was diluted to 21 µl and denatured at 99°C for 10 min. The DNA was briefly centrifuged, 4 µl of 2 M NaOH was added and the solution was mixed with 2 vol of 2% low melting point agarose. Agarose beads were formed by pipetting 10 µl aliquots onto cooled parafilm. Samples of 55 mg hydroquinone and 1.9 g sodium bisulphite were dissolved in a mixture containing 2.5 ml H2O and 720 µl 2 M NaOH. Aliquots of 700–800 µl were cooled to 0°C and the beads were added. After 10 min at 0°C, the beads were incubated for 4 h at 55°C with exclusion of light. The treatment was terminated by removal of the bisulphite solution and equilibration against 1 ml of 1× TE (4 × 15 min) and 1 ml H2O (1 × 15 min). Desulphonation was achieved by incubation (37°C) with 0.3 M NaOH (2 × 15 min) followed by washing steps with 1 ml of 1× TE (2 × 15 min) and 1 ml H2O (2 × 15 min). The beads were melted and used within 2 weeks.
Bisulphite conversion control
The success of the bisulphite treatment was tested using direct bisulphite sequencing of selected PCR products. Bisulphite sequencing was performed with the forward primers used for PCR amplification. PCR amplicons were purified using Exo-SAP-IT™ and subsequently a cycle sequencing reaction was performed using the BigDye Terminator Sequencing kit. Analysis was performed on an ABI 310 sequencer.
Preparation of PCR product mixtures
PCR products derived from clones from bisulphite-treated completely methylated and non-methylated genomic DNA were purified with the QIAquick PCR purification kit. DNA concentrations were adjusted after measurement of the concentration using the PicoGreen™ dsDNA quantification kit. For calibration mixtures in 10% increments were prepared.
Cloning
The PCR product of the GSTP1 fragment amplified from bisulphite-treated DNA of one prostate sample was cloned using the TOP10 TA Cloning™ kit with a pCR™ 2.1 vector according to the manufacturer’s recommendations. The GSTP1 fragment was amplified from 192 clones under the conditions described in the GOOD assay for epigenotyping. The ratio of the number of the clones containing a C or T at the MVP analysed was used to determine the degree of methylation of the sample.
‘GOOD assay’ protocol for epigenotyping
PCR. PCR primers were generally designed to be fully complementary to the deaminated strand and did not contain any possible methylation positions.
For amplification of exon 14 of the FVIII gene, 5 pmol each of forward (5′-AGGGAGTTTTTTTTAGGGAATAGAGGGA) and reverse (5′-TAATCCCAAAACCTCTCCACTACAACAA) primer and 5 ng of bisulphite-converted DNA or 5 fmol of purified PCR product, respectively, as template was used to yield a 561 bp product. The reaction conditions were 60 mM Tris–SO4 (pH 8.9), 18 mM (NH4)2SO4, 1 mM MgSO4, 50 µM dNTPs and 1.0 U Platinum Taq HiFi polymerase in a 10 µl volume. Mixtures were denatured for 4 min at 95°C, then thermocycled for 30 s at 95°C, 45 s at 61.5°C and 60 s at 72°C, repeating the cycle 30 times. A final extension step at 72°C for 4 min terminated the programme.
For amplification of the gene segment spanning the transcription start of GSTP1, 5 pmol each of forward (5′-GTGATTTAGTATTGG) and reverse (5′-AACTCTAAACCCCATC) primer and 5 ng of bisulphite-converted DNA or 5 fmol of purified PCR product, respectively, as template was used to yield a 144 bp product. The reaction conditions were the same as described above with an annealing temperature of 50°C and extension time of 20 s.
SAP digest. An aliquot of 0.5 U of SAP was added to the PCR and the solution was incubated for 1 h at 37°C, followed by heat inactivation at 90°C for 10 min.
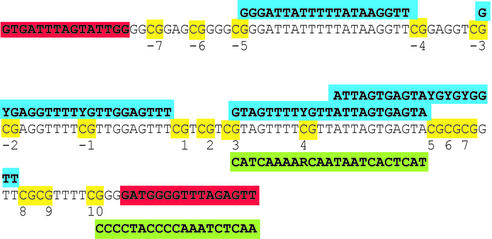
Primer extension reaction. All primers for the single base primer extension were designed either as forward or as reverse primers depending on the sequence context and had calculated melting temperatures of 58°C. Extension primers that spanned other CpGs were synthesized with C/T (Y) or A/G (R) wild-cards at the corresponding MVPs. Aliquots of 15 pmol of extension primer and 1.0 U TMA31FS were added. MgCl2 was adjusted to a final concentration of 4.3 mM, Tris–SO4 to 40 mM, (NH4)2SO4 to 17 mM and KCl to 12 mM (pH ∼8.2). The respective α-S-ddNTPs were used at a concentration of 50 µM in a reaction volume of 20 µl. An initial denaturing step of 2 min at 95°C was used followed by 40 cycles of 3 s at 95°C and 5 s at 53°C. The sequences for the extension primers are shown in Table 1. In the case of FVIII exon 14 the nomenclature refers to the number of the CpG position in the fragment, in the case of GSTP1 (see Fig. 4) numbering refers to the transcription start.
Table 1. Sequences of the extension primers used for analysis of CpG positions in the FVIII and GSTP1 fragments.
| Gene and CpG | Direction | Sequence of the extension primer |
|---|---|---|
| FVIII M1 | Reverse | 5′-CTATTTACTTCATTCCACTTAAptTCTptC |
| FVIII M2 | Forward | 5′-AAAGGATATTATTTTGTTTTTGptACTptA |
| FVIII M4 | Forward | 5′-CTTTTTAAAATTTATTAGTTTTGAptACTptA |
| FVIII M6 | Reverse | 5′-TTAATCTAACTAAAAAATAATptACTptC |
| FVIII M7 | Forward | 5′-TGAGGATGAAAATTAGAGTTptTCTptT |
| GSTP1 M–4 | Forward | 5′-GGGATTATTTTTATAAGGptTCTptT |
| GSTP1 M1 | Forward | 5′-GYGAGGTTTTYGTTGGAGTptTCTptT |
| GSTP1 M3 | Reverse | 5′-TACTCACTAATAACRAAAACTptACTptC |
| GSTP1 M5 | Forward | 5′-GTAGTTTTYGTTATTAGTGAGptTCTptA |
| GSTP1 M8 | Forward | 5′-ATTAGTGAGTAYGYGYGGptTCTptT |
| GSTP1 M10 | Reverse | 5′-AACTCTAAACCCCATCptCptCCTptC |
Figure 4.
Sequence of the GSTP1 amplicon. CpG positions are highlighted in yellow and the extension primers with which they are analysed are shown (blue for forward primers, green for reverse primers). Extension primers were checked for self-extension in a simplex format and cross-talk in multiplex assays. For multiplex analysis only non-overlapping extension primers were combined, as otherwise competition would occur between the primers querying different MVPs.
All primers were checked for self-extension in the absence of template. Extension primers carry amino-modified bases at the second base from the 3′-end. This base is bracketed by two phosphorothioate bridges. The amino function is used to attach charge tags (CT). Charge tagging of the amino-modified oligonucleotides was done according to the procedure previously described (40) with 6-trimethylammoniumhexyryl-_N_-hydroxysuccinimidyl ester or 6-trimethyl ammoniumbutyryl-_N_-hydroxysuccinimidyl ester. Quality of oligonucleotides was checked by MALDI mass spectrometry before and after charge tagging.
PDE digestion. Aliquots of 1 µl of 0.5 M acetic acid and 3 µl of phosphodiesterase II (3.4 × 10–3 U/µl) were added to the reaction followed by incubation for 60 min at 37°C.
Alkylation reaction. A mixture of 37 µl acetonitrile, 1.4 µl 2 M triethylammonium hydrogen carbonate buffer (pH 7.5), 1.4 µl 2 M Tris buffer (pH 7.5) and 16 µl iodomethane was added and incubated at 40°C for 25 min. Upon cooling a biphasic system was obtained. Water was added (20 µl) and the mixture was allowed to stand for 5 min at room temperature. A 20 µl sample of the upper layer was taken off and diluted with 45 µl of 40% acetonitrile.
Sample preparation. A 1.5% solution of α-cyano-4-hydroxycinnamic acid methyl ester in acetone was used as the matrix for MALDI analysis. The matrix was spotted onto the target using a BasePlate robot (The Automation Partnership, Royston, UK). The analyte (0.5 µl) was manually pipetted on top of the dry matrix.
MALDI analysis. All mass spectra were measured automatically using the AutoXecute software (Bruker Daltonik). Spectra were recorded in positive ion linear time-of-flight mode. The typical acceleration potential was –18 kV. Ion extraction was delayed by 200 ns. The Genotools SNP manager (50) was used to automatically analyse the spectra and yield the relative intensities of the peaks. For methylation quantification at MVPs the assay was carried out four times for each DNA, and each probe was prepared on six positions of the MALDI target, giving overall 24 preparations per DNA. A total of 20 × 10 shots were recorded on each preparation, while no more than 10 shots were allowed on the same spot of the preparation. The resolution (m/Δ_m_) had to be >400 and the signal-to-noise ratio >15. If more than 10 consequent spots did not fulfil the requirements, the entire preparation was rejected. The laser attenuation was adjusted for each target (varying between 45 and 50) and was fixed for the entire measurement.
Chip hybridisation
A recently published method using oligonucleotide microarrays was used as a reference method to verify the methylation data obtained (28). Oligonucleotides with a C6 amino modification at the 5′-end were spotted with a 4-fold redundancy on activated glass slides. In contrast to the published procedure triplet clusters of CpG positions were analysed. For each analysed CpG triplet site two oligonucleotides N1CGN2CGN3CGN4 and N1TGN2TGN3TGN4 (index 1–4 = 1–8 bases), reflecting the methylated and non-methylated status of the analysed DNA region, were spotted and immobilised on the glass slide. The oligonucleotide microarray representing three different CpG cluster sites of the GSTP1 gene was hybridised with Cy5-labelled PCR fragments, produced with 5′-Cy5-labelled PCR primers. For the direct comparison of chip hybridisation and MALDI mass spectrometry PCR products were split and analysed on both platforms. Subsequently, the fluorescent image of the hybridised slides was recorded with a GenePix 4000 microarray scanner (Axon Instruments Inc., Union City, CA). Hybridisation experiments were repeated five times. The ratio of signal CG/(CG + TG) was used for quantification of the methylation at individual CpG triplets.
RESULTS
Assays for the analysis of SNPs using primer extension reactions for allele discrimination and MALDI mass spectrometric detection of small DNA molecules have been demonstrated to allow quantification. As the quantification of bisulphite-treated DNA for the analysis of DNA methylation patterns using primer extension and mass spectrometric detection was not previously reported we considered it necessary to thoroughly investigate all parameters before applying the assay to the analysis and quantification of CpG positions. The quality and performance of our epigenotyping procedure is demonstrated in a series of experiments: (i) evaluation of factors influencing the performance of the PCR and primer extension reactions; (ii) investigation of the mass spectrometry parameters; (iii) calibration of isolated CpG positions by mixing PCR products obtained from bisulphite-treated methylated and unmethylated DNA templates; (iv) calibration of isolated CpG positions with PCR amplification from mixtures of methylated and non-methylated DNA templates; (v) extension to the analysis of CpG positions in CpG islands using degenerate extension primers; (vi) analysis of cancerous samples from paraffin-embedded tissue biopsies; and (vii) comparison of the results obtained with established techniques for the analysis of DNA methylation including cloning.
Evaluation of molecular biology and instrument parameters
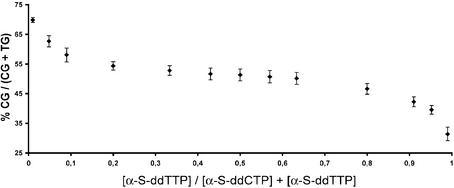
Several factors influencing the accurate quantification have to be considered when combining primer extension reactions for allele discrimination and MALDI mass spectrometry for detection: (i) preferential amplification of one allele in the PCR (PCR bias); (ii) preferential incorporation of a terminating ddNTP in the primer extension reaction; (iii) an instrumental and detection bias towards one of the allele-specific products; and (iv) an error in the mixes for standardisation. A single base extension protocol for allele discrimination is applied. Thus products differ by only 15 (T/C) or 16 Da (A/G) in their masses and their base composition is very similar so that ionisation efficiency (due to the use of charge tags) as well as a detection bias towards smaller molecules are of limited influence. For the primer extension reaction a simplified thermocycling profile was applied, which consists only of a denaturing and an annealing step. An extension step was omitted, as primers would not stay annealed to the template DNA at the preferred extension temperature of the DNA polymerase due to the high A/T content of bisulphite-treated DNA. Variation of parameters in the primer extension reaction such as annealing temperature, magnesium concentration and the use of different polymerases such as ThermoSequenase and TMA31FS showed little effect on the relative results of quantification. These parameters did have an effect on overall signal intensity. Assay conditions were optimised with the aim of universal applicability. Optimised parameters consist of TMA31FS as DNA polymerase, a buffer with a magnesium concentration between 4 and 4.5 mM and pH ∼8.3 and a temperature profile alternating between 53 and 95°C. The respective terminating α-S-ddNTPs were titrated against each other to investigate any preferential incorporation and no significant difference in incorporation rate up to a 10-fold excess of one of the two α-S-ddNTPs was found (Fig. 1). In consideration of a potential PCR bias, mixtures of clones prepared from methylated and unmethylated DNA were analysed by the GOOD assay with and without prior amplification. Results of these experiments are described in detail in the paragraphs detailing the methylation analysis of the respective genes.
Figure 1.
Evaluation of incorporation rates of terminating α-S-ddNTPs in the primer extension reaction using a primer in the forward direction. Results are similar for the primers in the reverse direction using α-S-ddATP/α-S-ddGTP for termination.
Low reproducibility of the crystallisation, differing ionisation efficiencies and potential signal-to-signal interdependencies could complicate quantitative analyses by MALDI mass spectrometry. The distribution of the peak intensity ratios of the mass spectra for an assigned probe was determined as a Gaussian distribution by a χ2 test. To accurately determine the mean value with a probability of 99.7% (± 0.02) approximately 200 shots per preparation on a MALDI spot are necessary. To account for differences in the performance of the enzymatic steps of the assay each analyte was processed four times.
Analysis and quantification of isolated CpG positions
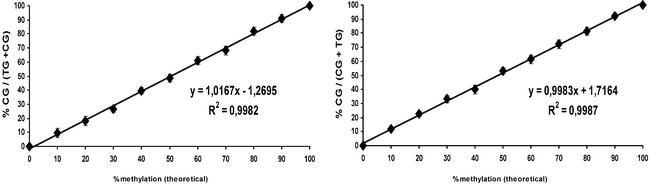
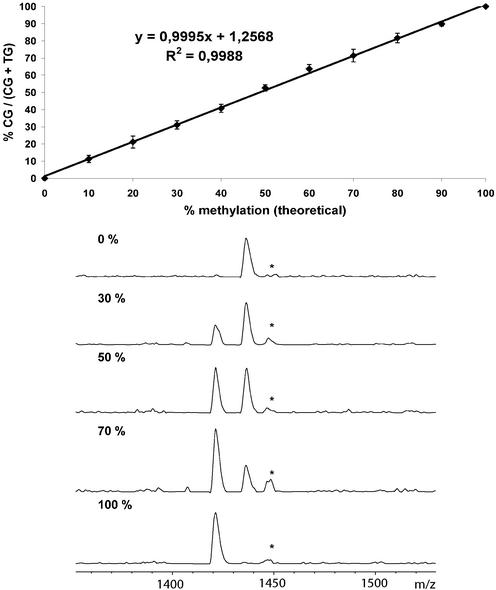
For our first experiments we amplified the region of exon 14 of the FVIII gene from clones prepared from bisulphite-treated _Sss_I-methylated and unmethylated mixed human DNA (Promega), respectively, by PCR. Eleven mixtures of the two PCR products representing methylation states ranging from 0 to 100% (in 10% increments) were analysed at five of the eight CpG positions contained in the fragment. Extension primers were designed either in forward or reverse direction. For all positions linear calibration curves with a correlation factor ≥0.99 and a slope of ∼1 were obtained. The standard deviation was ∼2% or lower for all calibration points. The limit of detection was around 2% while a limit of accurate quantification of 5% for the minor allele was determined. No significant difference was observed when the calibration was performed in either simplex or duplex (using two non-overlapping extension primers) (Fig. 2). Methylation analysis of the identical five CpG positions was then performed by mixing the two cloned DNA templates (11 mixtures as described above for PCR mixes) prior to PCR amplification (Fig. 3). The calibration curves were completely reproducible within the range of the standard deviation of the mixtures. This demonstrates that in the absence of a PCR bias as in the present case, the GOOD assay for epigenotyping can be used for accurate quantification without the need for calibration curves, thus decreasing the amount of time and labour. Minor deviations from the ideal calibration curve can be attributed to uncertainties in the determination of the DNA concentration and pipetting errors in adjusting the mixtures. To show the applicability of our FVIII assay to the analysis of bisulphite-treated genomic DNA extracted from tissue samples, we screened five samples derived from colon cancer tissues and two normal controls. All samples could be successfully analysed, however, all were completely methylated in the cancer and normal tissue.
Figure 2.
Comparison of mixtures of PCR products derived from cloned bisulphite-treated methylated and non-methylated DNA in a simplex (left) and duplex (right) experiment. The position analysed is CpG 6 in the FVIII gene. The duplex experiment was performed by simultaneous analysis of CPG 6 and 1.
Figure 3.
Calibration curve for CpG position 4 in the FVIII gene. Mixtures of clones from bisulphite-treated completely methylated and non-methylated DNA were amplified by PCR. The correlation coefficient is given on the curve. Standard deviation is indicated by vertical bars and was calculated from 24 measurements of each mixture. The MALDI spectra represent a selection of the calibration measurements. Product masses of the alleles are: d(AptACTptAptdC), 1422 Da; d(AptACTptAptdT), 1436 Da. The extension primer was charge tagged with 6-trimethylammoniumbutyryl-_N_-hydroxysuccinimidyl ester. The peak marked with an asterix corresponds to an incomplete primer digestion.
Analysis and quantification of CpG positions in CpG islands
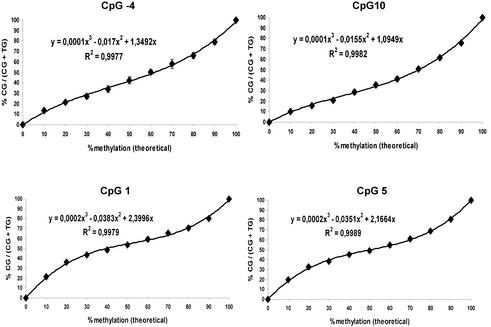
As a second model system, we chose a fragment of the well-described GSTP1 gene spanning the transcription start. With this system we expanded the applicability of our assay to the analysis of MVPs in CpG islands. The selected fragment of 140 bp contains 17 CpG positions (Fig. 4). This results in a sequence difference of >12% between completely methylated and non-methylated template after bisulphite treatment. This difference strongly influences the melting and annealing properties during PCR amplification (potential for PCR bias). In addition CpG positions are so closely spaced to each other that primers used in the primer extension reaction have to contain degenerate bases with potentially different annealing behaviour. Calibration curves were recorded for six of the CpG positions in the fragment by amplifying mixtures of two cloned DNA templates prepared from bisulphite-treated _Sss_I-methylated and unmethylated mixed human DNA (Promega), respectively, by PCR (Fig. 5). In contrast to FVIII, the calibration did not show a linear trend. A polynomial fit of third order gave correlation coefficients >0.99 and standard deviations were again ∼2% for each sample and each position. Several factors could cause the sigmoid shape of the calibration curves. One is a certain PCR bias towards the unmethylated allele. A second potential reason is the sequence-specific annealing behaviour of the extension primers that contain between none and three degenerate bases, resulting in possible mismatches. The sigmoid trend of the calibrations was intensified with increasing number of degenerate bases in the extension primer. Calibration curves were recorded for simplex, duplex and triplex analysis. For multiplex analysis only extension primers were chosen that did not overlap in their sequence. Here two major advantages of the GOOD assay become obvious. (i) The use of different charge tags attached to the extension primers allows the separation of the mass spectrometric signals corresponding to different MVPs in the same PCR fragment in a multiplex reaction. Due to the low sequence diversity of the bisulphite-treated DNA the maximum number of simultaneously analysed positions was four. (ii) The use of degenerate bases within the extension primers does not cause spectral complexity as the parts of the primers containing these bases are removed by the 5′-phosphodiesterase digestion following the primer extension reaction.
Figure 5.
Calibration curves for four CpG positions in the GSTP1 fragment (Fig. 4). The correlation coefficient is given in each curve. Standard deviation is indicated by vertical bars and was calculated from 24 measurements of each calibration mixture. Primers for positions –4 and 10 contain no wild-cards; those for positions 5 and 1 contain one and two degenerate bases, respectively.
Analysis of the methylation status in prostate biopsy samples
We analysed 10 samples taken from different regions of paraffin-embedded cancerous prostate tissue biopsies (Table 2). The results of the analysis demonstrate a high degree of co-methylation between the different positions analysed. As the fragment of interest of the GSTP1 gene is known to be fully methylated in prostate cancer tissues and non-methylated in normal prostates (47), the percentage of methylation found in the samples reflects the percentage of cancerous cells in the sample. The degree of methylation of a certain sample therefore correlates with the position of sampling and its distance to the centre of the tumour. Analysis was carried out in simplex or triplex format (Fig. 6). To verify our assay we decided to clone the amplified GSTP1 fragment of the bisulphite-treated DNA of one of the prostate samples. Analysing a large number of clones has to be considered the gold standard for the quantification of DNA methylation. 192 clones were picked of which 146 were unambiguously analysed using our assay with the GSTP1 M1 extension primer. The methylation status of the studied sample was determined to be 20.6% by counting the clones derived from methylated or non-methylated DNA, respectively. The proportion of methylated cells obtained with quantitative mass spectrometric analysis was 20.7 ± 2.1%. For further verification the results for three prostate probes were compared to those obtained by quantitative chip hybridisation, a method which has been used for the high-throughput quantification of the methylation states of many genes in parallel (28) (Table 3). PCR products were split and analysed both by chip hybridisation and MALDI mass spectrometry. No significant differences between the results obtained by both methods were found. These results underline the applicability of our assay even to fragments difficult to interrogate due to a high density of MVPs and the accurate quantification of methylation even in the presence of perturbing factors such as a possible PCR bias.
Table 2. MALDI analysis of 6 CpG positions within the GSTP1 amplicon in paraffin-embedded cancerous prostate samples.
| Sample | Methylation (%) By mass spectrometry | |||||
|---|---|---|---|---|---|---|
| CpG –4 | CpG 1 | CpG 3 | CpG 5 | CpG 8 | CpG 10 | |
| 1 | 79.8 ± 1.9 | 81.2 ± 2.2 | 81.4 ± 1.5 | 70.5 ± 2.5 | 74.5 ± 2.1 | 60.2 ± 1.9 |
| 2 | 82.5 ± 1.8 | 85.6 ± 2.1 | 84.9 ± 2.4 | 75.3 ± 2.1 | 80.1 ± 2.6 | 66.1 ± 1.8 |
| 3 | 56.8 ± 2.0 | 58.4 ± 1.9 | 54.9 ± 2.0 | 51.7 ± 1.4 | 47.5 ± 1.9 | 51.7 ± 1.7 |
| 4 | 23.2 ± 1.8 | 20.7 ± 2.1 | 22.0 ± 2.1 | 16.9 ± 1.9 | 24.7 ± 1.2 | 19.4 ± 2.4 |
| 5 | 78.6 ± 2.5 | 79.9 ± 2.5 | 80.0 ± 2.7 | 79.4 ± 2.2 | 77.5 ± 2.1 | 78.2 ± 1.6 |
| 6 | 76.9 ± 1.7 | 75.4 ± 2.2 | 79.9 ± 2.4 | 81.0 ± 1.5 | 79.2 ± 1.2 | 64.5 ± 2.9 |
| 7 | 29.0 ± 2.1 | 29.6 ± 2.4 | 25.7 ± 1.5 | 26.0 ± 2.2 | 28.5 ± 1.9 | 24.6 ± 1.7 |
| 8 | 27.7 ± 2.2 | 32.6 ± 2.1 | 35.2 ± 1.8 | 33.1 ± 1.5 | 31.3 ± 2.2 | 29.9 ± 2.2 |
| 9 | 48.3 ± 1.9 | 39.6 ± 2.5 | 41.0 ± 1.8 | 44.0 ± 1.6 | 42.5 ± 2.1 | 44.7 ± 1.6 |
| 10 | 27.0 ± 1.9 | 33.8 ± 2.2 | 31.5 ± 2.4 | 29.2 ± 1.6 | 31.9 ± 2.1 | 34.5 ± 2.6 |
Figure 6.
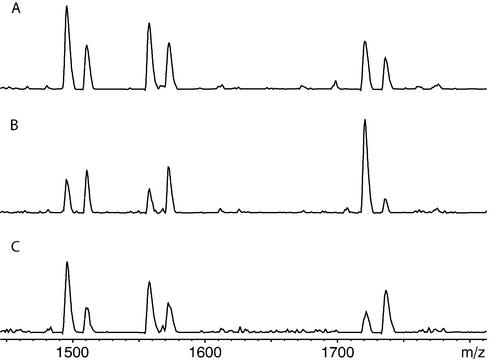
Simultaneous analysis of three MVPs (M1, M5 and M10) in three different prostate samples. Relative peak heights of the sum spectra were measured and results were obtained after calibration. Results deviate at most by 3% from the values given for simplex analysis (Table 2), which is in the range of standard deviation. (A) Sample 1, (B) sample 4 and (C) sample 5. Product masses of the alleles in the MALDI spectra are: d(TptTCTptTptdC), 1497 Da and d(TptTCTptTptdT), 1512 Da for M1; d(GptTCTptAptdC), 1559 Da and d(GptTCTptAptdT), 1574 Da for M5; d(CptCptCCTptCptdA), 1693 Da and d(CptCptCCTptCptdG), 1709 Da for M10. To ensure unambiguous calculation the masses of the alleles were shifted by the use of different charge tags (CT). The extension primer querying position M1 was charge tagged with 6-trimethylammoniumbutyryl-_N_-hydroxysuccinimidyl ester, while the other two were charge tagged with 6-trimethylammoniumhexyryl-_N_-hydroxysuccinimidyl ester.
Table 3. Comparison of the methylation quantification results of three prostate samples by MALDI mass spectrometry with the results obtained by quantitative chip hybridisation.
| Sample | Methylation (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| By mass spectrometry | By chip hybridisation | ||||||||
| CpG –4 | CpG 1 | CpG 3 | CpG 5 | CpG 8 | CpG 10 | CpG –4, –3, –2 | CpG 1, 2, 3 | CpG 8, 9, 10 | |
| 1 | 79.8 ± 1.9 | 81.2 ± 2.2 | 81.4 ± 1.5 | 70.5 ± 2.5 | 74.5 ± 2.1 | 60.2 ± 1.9 | 80.7 | 78.8 | 62.3 |
| 2 | 82.5 ± 1.8 | 85.6 ± 2.1 | 84.9 ± 2.4 | 75.3 ± 2.1 | 80.1 ± 2.6 | 66.1 ± 1.8 | 87.8 | 84.5 | 74.4 |
| 3 | 56.8 ± 2.0 | 58.4 ± 1.9 | 54.9 ± 2.0 | 51.7 ± 1.4 | 47.5 ± 1.9 | 51.7 ± 1.7 | 58.0 | 60.4 | 46.7 |
DISCUSSION
In this study we successfully demonstrate the first method for the analysis and accurate quantification of the methylation status at CpG positions by MALDI mass spectrometry. MALDI mass spectrometry provides a higher degree of multiplexing compared to most other detection techniques as many data points can be recorded in a single experiment. We showed a multiplexed methylation analysis at three MVPs. Restrictions are mainly caused by the spectral overlap of peaks due to the low sequence diversity of bisulphite-treated DNA. Another advantage of mass spectrometry is the speed of data accumulation. With modern mass spectrometers, capable of automatic target loading, several targets can be measured unattended. Thereby, the accumulation of 200 laser shots per spot does not prohibit high-throughput studies for epigenotyping. Furthermore, mass spectrometry relies on the direct determination of products rather than on indirect analysis of tags. Assay formats using primer extension combined with radioactive labelling have been presented for quantification (26); however, no limits of detection and quantification were described. In addition, radioactive labelling is expensive and of limited acceptance. Recently, two hybridisation-based approaches using microarrays and fluorescently labelled PCR products for the genome-wide assessment of the methylation status in many genes were demonstrated (28– 30). These approaches allow the establishment of molecular fingerprints of cancer tissues. However, microarray-based approaches lack flexibility.
Primer extension assays with mass spectrometric detection have been widely applied for the determination of allele frequencies of SNPs and other polymorphisms in pooled genomic DNAs (36–38). The incorporation of a complementary nucleotide by a DNA polymerase distinguishes more accurately between the two alleles than the different thermal stabilities of hybridising allele-specific probes (51). The GOOD assay for epigenotyping applies single base primer extension for allele discrimination. This is in contrast to other assays with mass spectrometric detection, in which one allele is extended by one base while the other is extended by at least two bases, resulting in allele-specific products differing by at least 300 Da in their masses, as otherwise resolution may not be sufficient in the range of detection for quantification. Products of the protocols are therefore more prone to be confused due to instrumental bias. Since for most traditional applications differences in the allele frequencies between cases and controls in association studies were analysed, no absolute quantification was necessary. In some cases results were corrected with a factor estimated from defined heterozygous samples, thus taking preferential amplification, incorporation of terminating ddNTPs and different ionisation efficiency into account. However, in the case of DNA methylation analysis positions of interest can comprise all possible degrees of methylation, ranging from 0 to 100%. Therefore, a comparable one-point calibration is not feasible for this application. In addition, the use of extension primers containing degenerate positions would cause spectral complexity, as each different allele would result in a different signal. In the range of detection of these assays the different signals could not be separated, making accurate quantification of the two alleles impossible. The phosphodiesterase used to digest the extension primer in the GOOD assay to a core sequence consisting of four to five bases removes the bases at the degenerate positions. Accordingly, the mass spectrometric signatures remain simple and allow multiplexing. As MVPs in CpG islands are in close proximity the design of extension primers without degenerate bases is often impossible.
PCR bias, as for the GSTP1 fragment, is sometimes observed in quantitative methylation analysis (52). Methyl ated and non-methylated DNA result in different sequences as a consequence of bisulphite treatment reflected in a different C/G content. This may lead to a preferential amplification of one set of bisulphite-treated DNA molecules. As PCR bias is both sequence and strand specific, it is almost impossible to predict. Through appropriate quality control of the bisulphite conversion, like direct bisulphite sequencing, it can be ruled out that quantification results are confounded by incomplete bisulphite conversion. In any case, our approach of recording calibration curves for the PCR fragments of interest allows for accurate quantification. The calibration effort is easily outweighed by throughput and accuracy of the resulting assay. However, extreme PCR biases resulting in curves with a low slope will complicate the accurate quantification of the affected methylation states.
Analysis of DNA methylation is a rapidly advancing field of research. The biological mechanism by which DNA methylation is established and maintained is not yet fully understood. Aberrant methylation patterns have an influence on the progress of diseases, but the genome also seems to be tolerant towards these variations within certain limits (53). In the field of cancer it is widely debated if DNA methylation is the reason for or the consequence of aberrant tumour suppressor gene silencing (54). The presented GOOD assay for epigenotyping is a fast, reliable and high-throughput assay for the accurate analysis and quantification of DNA methylation.
Acknowledgments
ACKNOWLEDGEMENTS
We thank Sandra Nicodeme (CNG) for help with the cloning and Anne Hentschel and Erik Leu (Epigenomics) for help with the chip hybridisation experiments. The colon cancer samples and the prostate tissue sample were kindly provided by Dr Glen Kristiansen (Charité, Berlin, Germany). This work was in part supported by a grant from the European Union (QLRT-1999-30417).
REFERENCES
- 1.Wu C.-T. and Morris,J.R. (2001) Genes, genetics and epigenetics: a correspondence. Science, 293, 1103–1104. [DOI] [PubMed]
- 2.Bird A. (2002) DNA methylation patterns and epigenetic memory. Genes Dev., 16, 6–21. [DOI] [PubMed]
- 3.Novik K.L., Nimmrich,I., Genc,B., Maier,S., Piepenbrock,C., Olek,A. and Beck,S. (2002) Epigenomics: genome-wide study of methylation phenomena. Curr. Issues Mol. Biol., 4, 111–128. [PubMed]
- 4.Avner P. and Heard,E. (2001) X-chromosome inactivation: counting, choice and initiation. Nature Rev. Genet., 2, 59–67. [DOI] [PubMed]
- 5.Reik W. and Walter,J. (2001) Genomic imprinting: parental influence on the genome. Nature Rev. Genet., 2, 21–32. [DOI] [PubMed]
- 6.Yoder J.A., Walsh,C.P. and Bestor,T.H. (1997) Cytosine methylation and the ecology of intragenomic parasites. Trends Genet., 13, 335–340. [DOI] [PubMed]
- 7.Robertson K.D. and Wolffe,A.P. (2000) DNA methylation in health and disease. Nature Rev. Genet., 1, 11–19. [DOI] [PubMed]
- 8.Jones P.A. and Baylin,S.B. (2002) The fundamental role of epigenetic events in cancer. Nature Rev. Genet., 3, 415–428. [DOI] [PubMed]
- 9.Plass C. (2002) Cancer epigenomics. Hum. Mol. Genet., 11, 2479–2488. [DOI] [PubMed]
- 10.Costello J.F., Frühwald,M.C., Smiraglia,D.J., Rush,L.J., Robertson,G.P., Gao,X., Wright,F.A., Feramisco,J.D., Peltomäki,P., Lang,J.C. et al. (2000) Aberrant CpG-island methylation has non-random and tumour-type-specific patterns. Nature Genet., 24, 132–138. [DOI] [PubMed]
- 11.Esteller M., Corn,P.G., Baylin,S.B. and Herman,J.G. (2001) A gene hypermethylation profile of human cancer. Cancer Res., 61, 3225–3229. [PubMed]
- 12.Fraga M.F. and Esteller,M. (2002) DNA methylation: a profile of methods and applications. Biotechniques, 33, 632, 634, 636–649. [DOI] [PubMed]
- 13.Gonzalgo M.L., Liang,G., Spruck,C.H.,III, Zingg,J.M., Rideout,W.M.,III and Jones,P.A. (1997) Identification and characterization of differentially methylated regions of genomic DNA by methylation-sensitive arbitrarily primed PCR. Cancer Res., 57, 594–599. [PubMed]
- 14.Toyota M., Ho,C., Ahuja,N., Jair,K.W., Li,Q., Ohe-Toyota,M., Baylin,S.B. and Issa,J.P. (1999) Identification of differentially methylated sequences in colorectal cancer by methylated CpG island amplification. Cancer Res., 59, 2307–2312. [PubMed]
- 15.Huang T.H., Perry,M.R. and Laux,D.E. (1999) Methylation profiling of CpG islands in human breast cancer cells. Hum. Mol. Genet., 8, 459–470. [DOI] [PubMed]
- 16.Frommer M., McDonald,L.E., Millar,D.S., Collis,C.M., Watt,F., Grigg,G.W., Molloy,P.L. and Paul,C.L. (1992) A genomic sequencing protocol that yields a positive display of 5-methylcytosine residues in individual DNA strands. Proc. Natl Acad. Sci. USA, 89, 1827–1831. [DOI] [PMC free article] [PubMed]
- 17.Clark S.J., Harrison,J., Paul,C.L. and Frommer,M. (1994) High sensitivity mapping of methylated cytosines. Nucleic Acids Res., 22, 2990–2997. [DOI] [PMC free article] [PubMed]
- 18.Herman J.G., Graff,J.R., Myohanen,S., Nelkin,B.D. and Baylin,S.B. (1996) Methylation-specific PCR: a novel PCR assay for methylation status of CpG islands. Proc. Natl Acad. Sci. USA, 93, 9821–9826. [DOI] [PMC free article] [PubMed]
- 19.Eads C.A., Danenberg,K.D., Kawakami,K., Saltz,L.B., Blake,C., Shibata,D., Danenberg,P.V. and Laird,P.W. (2000) MethyLight: a high-throughput assay to measure DNA methylation. Nucleic Acids Res., 28, e32. [DOI] [PMC free article] [PubMed]
- 20.Rand K., Qu,W., Ho,T., Clark,S.J. and Molloy,P. (2002) Conversion-specific detection of DNA methylation using real-time polymerase chain reaction (ConLight-MSP) to avoid false positives. Methods, 27, 114–120. [DOI] [PubMed]
- 21.Xiong Z. and Laird,P.W. (1997) COBRA: a sensitive and quantitative DNA methylation assay. Nucleic Acids Res., 25, 2532–2534. [DOI] [PMC free article] [PubMed]
- 22.Burri N. and Chaubert,P. (1999) Complex methylation patterns analyzed by single-strand conformation polymorphism. Biotechniques, 26, 232–234. [DOI] [PubMed]
- 23.Worm J., Aggerholm,A. and Guldberg,P. (2001) In-tube DNA methylation profiling by fluorescence melting curve analysis. Clin. Chem., 47, 1183–1189. [PubMed]
- 24.Akey D.T., Akey,J.M., Zhang,K. and Jin,L. (2002) Assaying DNA methylation based on high-throughput melting curve approaches. Genomics, 80, 376–384. [DOI] [PubMed]
- 25.Uhlmann K., Marczinek,K., Hampe,J., Thiel,G. and Nürnberg,P. (1999) Changes in methylation patterns identified by two-dimensional DNA fingerprinting. Electrophoresis, 20, 1748–1755. [DOI] [PubMed]
- 26.Gonzalgo M.L. and Jones,P.A. (1997) Rapid quantification of methylation differences at specific sites using methylation-sensitive single nucleotide primer extension (Ms-SNuPE). Nucleic Acids Res., 25, 2529–2531. [DOI] [PMC free article] [PubMed]
- 27.El-Maarri O., Herbiniaux,U., Walter,J. and Oldenburg,J. (2002) A rapid, quantitative, non-radioactive bisulfite-SNuPE-IP RP HPLC assay for methylation analysis at specific CpG sites. Nucleic Acids Res., 30, e25. [DOI] [PMC free article] [PubMed]
- 28.Adorjan P., Distler,J., Lipscher,E., Model,F., Müller,J., Pelet,C., Braun,A., Florl,A.R., Gütig,D., Grabs,G. et al. (2002) Tumour class prediction and discovery by microarray-based DNA methylation analysis. Nucleic Acids Res., 30, e21. [DOI] [PMC free article] [PubMed]
- 29.Gitan R.S., Shi,H., Chen,C.M., Yan,P.S. and Huang,T.H. (2002) Methylation-specific oligonucleotide microarray: a new potential for high-throughput methylation analysis. Genome Res., 12, 158–164. [DOI] [PMC free article] [PubMed]
- 30.Shi H., Maier,S., Nimmrich,I., Yan,P.S., Caldwell,C.W., Olek,A. and Huang,T.H. (2003) Oligonucleotide-based microarray for DNA methylation analysis: principles and applications. J. Cell. Biochem., 88, 138–143. [DOI] [PubMed]
- 31.Tost J. and Gut,I.G. (2003) Genotyping single nucleotide polymorphisms by mass spectrometry. Mass Spectrom. Rev., in press. [DOI] [PubMed]
- 32.Babinger P., Kobl,I., Mages,W. and Schmitt,R. (2001) A link between DNA methylation and epigenetic silencing in transgenic Volvox carteri. Nucleic Acids Res., 29, 1261–1271. [DOI] [PMC free article] [PubMed]
- 33.Friso S., Choi,S.W., Dolnikowski,G.G. and Selhub,J. (2002) A method to assess genomic DNA methylation using high-performance liquid chromatography/electrospray ionization mass spectrometry. Anal. Chem., 74, 4526–4531. [DOI] [PubMed]
- 34.Karas M. and Hillenkamp,F. (1988) Laser desorption ionization of proteins with molecular masses exceeding 10000 daltons. Anal. Chem., 60, 2299–2301. [DOI] [PubMed]
- 35.Ross P., Hall,L. and Haff,L.A. (2000) Quantitative approach to single-nucleotide polymorphism analysis using MALDI-TOF mass spectrometry. Biotechniques, 29, 620–626, 628–629. [DOI] [PubMed]
- 36.Buetow K.H., Edmonson,M., MacDonald,R., Clifford,R., Yip,P., Kelley,J., Little,D.P., Strausberg,R., Köster,H., Cantor,C.R. et al. (2001) High-throughput development and characterization of a genomewide collection of gene-based single nucleotide polymorphism markers by chip-based matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Proc. Natl Acad. Sci. USA, 98, 581–584. [DOI] [PMC free article] [PubMed]
- 37.Werner M., Sych,M., Herbon,N., Illig,T., König,I.R. and Wjst,M. (2002) Large-scale determination of SNP allele frequencies in DNA pools using MALDI-TOF mass spectrometry. Hum. Mutat., 20, 57–64. [DOI] [PubMed]
- 38.Rodi C.P., Darnhofer-Patel,B., Stanssens,P., Zabeau,M. and van den Boom,D. (2002) A strategy for the rapid discovery of disease markers using the MassARRAY system. Biotechniques, suppl., 62–66, 68–69. [PubMed]
- 39.Le Hellard S., Ballereau,S.J., Visscher,P.M., Torrance,H.S., Pinson,J., Morris,S.W., Thomson,M.L., Semple,C.A., Muir,W.J., Blackwood,D.H. et al. (2002) SNP genotyping on pooled DNAs: comparison of genotyping technologies and a semi-automated method for data storage and analysis. Nucleic Acids Res., 30, e74. [DOI] [PMC free article] [PubMed]
- 40.Sauer S., Lechner,D., Berlin,K., Lehrach,H., Escary,J.L., Fox,N. and Gut,I.G. (2000) A novel procedure for efficient genotyping of single nucleotide polymorphisms. Nucleic Acids Res., 28, e13. [DOI] [PMC free article] [PubMed]
- 41.Sauer S., Lechner,D., Berlin,K., Plancon,C., Heuermann,A., Lehrach,H. and Gut,I.G. (2000) Full flexibility genotyping of single nucleotide polymorphisms by the GOOD assay. Nucleic Acids Res., 28, e100. [DOI] [PMC free article] [PubMed]
- 42.Sauer S., Lechner,D. and Gut,I.G. (2001) The GOOD assay. In Housby,N. (ed.), Mass Spectrometry and Genomic Analysis. Kluwer Aceademic Publishers, Dordrecht, The Netherlands, pp. 54–65.
- 43.Tost J., Brandt,O., Boussicault,F., Derbala,D., Caloustian,C., Lechner,D. and Gut,I.G. (2002) Molecular haplotyping at high throughput. Nucleic Acids Res., 30, e96. [DOI] [PMC free article] [PubMed]
- 44.Lenting P.J., van Mourik,J.A. and Mertens,K. (1998) The life cycle of coagulation factor VIII in view of its structure and function. Blood, 92, 3983–3996. [PubMed]
- 45.Millar D.S., Krawczak,M. and Cooper,D.N. (1998) Variation of site-specific methylation patterns in the factor VIII (F8C) gene in human sperm DNA. Hum. Genet., 103, 228–233. [DOI] [PubMed]
- 46.El-Maarri O., Olek,A., Balaban,B., Montag,M., van der Ven,H., Urman,B., Olek,K., Caglayan,S.H., Walter,J. and Oldenburg,J. (1998) Methylation levels at selected CpG sites in the factor VIII and FGFR3 genes, in mature female and male germ cells: implications for male-driven evolution. Am. J. Hum. Genet., 63, 1001–1008. [DOI] [PMC free article] [PubMed]
- 47.Millar D.S., Ow,K.K., Paul,C.L., Russell,P.J., Molloy,P.L. and Clark,S.J. (1999) Detailed methylation analysis of the glutathione S-transferase pi (GSTP1) gene in prostate cancer. Oncogene, 18, 1313–1324. [DOI] [PubMed]
- 48.Cairns P., Esteller,M., Herman,J.G., Schoenberg,M., Jeronimo,C., Sanchez-Cespedes,M., Chow,N.H., Grasso,M., Wu,L., Westra,W.B. et al. (2001) Molecular detection of prostate cancer in urine by GSTP1 hypermethylation. Clin. Cancer Res., 7, 2727–2730. [PubMed]
- 49.Olek A., Oswald,J. and Walter,J. (1996) A modified and improved method for bisulphite based cytosine methylation analysis. Nucleic Acids Res., 24, 5064–5066. [DOI] [PMC free article] [PubMed]
- 50.Pusch W., Kräuter,K.O., Fröhlich,T., Stalgies,Y. and Kostrzewa,M. (2001) Genotools SNP manager: a new software for automated high-throughput MALDI-TOF mass spectrometry SNP genotyping. Biotechniques, 30, 210–215. [DOI] [PubMed]
- 51.Pastinen T., Kurg,A., Metspalu,A., Peltonen,L. and Syvänen,A.C. (1997) Minisequencing: a specific tool for DNA analysis and diagnostics on oligonucleotide arrays. Genome Res., 7, 606–614. [DOI] [PubMed]
- 52.Warnecke P.M., Stirzaker,C., Melki,J.R., Millar,D.S., Paul,C.L. and Clark,S.J. (1997) Detection and measurement of PCR bias in quantitative methylation analysis of bisulphite-treated DNA. Nucleic Acids Res., 25, 4422–4426. [DOI] [PMC free article] [PubMed]
- 53.Humpherys D., Eggan,K., Akutsu,H., Hochedlinger,K., Rideout,W.M.,III, Biniszkiewicz,D., Yanagimachi,R. and Jaenisch,R. (2001) Epigenetic instability in ES cells and cloned mice. Science, 293, 95–97. [DOI] [PubMed]
- 54.Baylin S. and Bestor,T.H. (2002) Altered methylation patterns in cancer cell genomes: cause or consequence? Cancer Cell, 1, 299–305. [DOI] [PubMed]