Loss of Oncogenic H-ras-Induced Cell Cycle Arrest and p38 Mitogen-Activated Protein Kinase Activation by Disruption of Gadd45a (original) (raw)
Abstract
The activation of p53 is a guardian mechanism to protect primary cells from malignant transformation; however, the details of the activation of p53 by oncogenic stress are still incomplete. In this report we show that in _Gadd45a_−/− mouse embryo fibroblasts (MEF), overexpression of H-ras activates extracellular signal-regulated kinase (ERK) and c-Jun N-terminal kinase (JNK) but not p38 kinase, and this correlates with the loss of H-ras-induced cell cycle arrest (premature senescence). Inhibition of p38 mitogen-activated protein kinase (MAPK) activation correlated with the deregulation of p53 activation, and both a p38 MAPK chemical inhibitor and the expression of a dominant-negative p38α inhibited p53 activation in the presence of H-ras in wild-type MEF. p38, but not ERK or JNK, was found in a complex with Gadd45 proteins. The region of interaction was mapped to amino acids 71 to 96, and the central portion (amino acids 71 to 124) of Gadd45a was required for p38 MAPK activation in the presence of H-ras. Our results indicate that this Gadd45/p38 pathway plays an important role in preventing oncogene-induced growth at least in part by regulating the p53 tumor suppressor.
Regulation of genomic stability and protection from malignant transformation are complex processes involving a variety of different mechanisms, including permanent cell cycle arrest after expression of an activated oncogene (7). In normal primary cells, overexpression of an oncogene, such as activated Ha_-ras_, can trigger growth arrest with features of cell senescence, as well as apoptosis, in fibroblasts and epithelial cells (7, 9, 17, 35, 41). Such responses probably have a protective role in vivo by removing cycling cells when an oncogene has become active. The mechanism for premature senescence involves multiple pathways, including those involving p53 and Rb. To a large extent, Ras-induced cell cycle arrest is dependent on p53 signaling, except with certain human cells, such as IMR-90, where additional mechanisms are involved (41). In mouse cells, growth arrest after oncogenic stimulation is dependent on the p19/ARF pathway-mediated stabilization of p53 (17; S. Bates, A. C. Phillips, P. A. Clark, F. Stott, G. Peters, R. L. Ludwig, and K. H. Vousden, Letter, Nature **395:**124-125, 1998; I. Palmero, C. Pantoja, and M. Serrano, Letter, Nature **395:**125-126, 1998), which is not the case for some human cell lines in which p14/ARF is not induced (51). Although p53 accumulation is an important feature, full activation of p53 involves other events, including posttranslational modifications which involve a variety of regulatory kinases, including the mitogen-activated protein kinases (MAPK) (see reference 6 for more details).
In addition to the activation of the MEK1-extracellular signal-regulated kinase 1 and 2 (ERK1/2) pathway by oncogenic Ras, which can regulate p16/Ink4a levels (34, 63), there is increasing evidence that the other two major MAPK pathways, p38 MAPK (p38) and c-Jun N-terminal kinase (JNK), have important roles in the cellular response to oncogenic stress (40). In the case of Ha_-ras_ activation, all three (ERK, p38, and JNK) of the major branches of the MAPK pathway are stimulated. Sequential activation of the ERK pathway and then the p38 pathway has recently been reported to contribute to the induction of premature senescence by Ha-rasV12 (H-ras) (48). In this study, the argument was developed that H-ras activation of ERK and JNK can have growth-stimulating effects while premature senescence relies on the subsequent activation of p38. Growth arrest triggered by dominant-positive expression of MEK1, MKK3, or MAPK kinase 6 (MKK6) was blocked by inhibition of p38, which highlights the pivotal role of the p38 portion of the MAPK pathway as the cellular brake after oncogenic stress (3). In another recent study (40), pharmacologic inhibition of p38, together with Raf activation of ERK, was sufficient to mimic the morphological and growth transformations caused by oncogenic Ras. p38-mediated growth inhibition has been shown to involve stimulation of p53 (8) as well as inhibition of Cdc25B (10) and cyclin D1 (32). In human fibroblasts, overexpression of H-ras resulted in p53 posttranslation modifications at Ser33 and Ser46 (9), which are the same sites that exhibited p38-dependent phosphorylation after UV radiation (8). Growth inhibition of human fibroblasts by H-ras was prevented by pharmacologic inhibition of p38 (48). Studies with the Wip1 phosphatase, which can inactivate p38 (45), also support the contention that p38 is an important component in mediating stress-induced growth arrest. Wip1, which is p53 inducible, has been shown to mediate negative feedback regulation of p53 by inactivation of p38 after UV radiation (45). While single oncogenes such as those encoding H-ras, Myc, and Neu do not transform primary wild-type (wt) mouse embryo fibroblasts (MEF), coexpression of Wip1 did allow transformation of wt cells with these oncogenes (9). In the same study, frequent amplification of PPM1D, which encodes Wip1, was found in wt p53 human breast tumors, which further supports the hypothesis that the Wip1 gene can function as an oncogene in some circumstances while p38 has tumor suppressor properties.
Gadd45a (growth arrest and DNA damage inducible) has been associated with negative growth regulation since its isolation more than 12 years ago and is known to be regulated by a variety of proteins with tumor suppressor properties, such as p53 (recently reviewed in reference 21). Gadd45a is one member of a three-protein family of small acidic proteins that bind to several other proteins, including Cdc2, PCNA, p21 (Waf1/Cip1), core histones, and MTK1 (see reference 21 for more details). In the case of MTK1 (37, 47), a MAPK kinase kinase (MAPKKK), its murine equivalent, MEKK4, which is ubiquitously expressed, was originally reported to activate JNK but not p38 or ERK (18), but evidence exists that it can also activate p38. For example, overexpression of MTK1 in COS cells activated both p38 and JNK, and a dominant-negative form of MTK1 blocked p38 appreciably more than JNK (46). The Drosophila homolog of MTK1 has also been reported to activate p38 (25). All three Gadd45 proteins have been found to bind to and activate MTK1, leading to activation of both JNK and p38, and it has been proposed that induction of the Gadd45 protein is required for normal activation of JNK and p38 after stresses such as UV radiation (20, 37, 47).
The development of _Gadd45a_−/− mice has helped clarify the cellular roles of this stress protein (24). Interestingly, primary _Gadd45a_−/− MEF were able to undergo single oncogene transformation by H-ras. In the case of UV radiation, normal activation of the stress MAPK in _Gadd45a_−/− MEF was observed (49). A more recent observation supports some role(s) for the Gadd45 proteins in MAPK regulation. In T helper 1 cells from _Gadd45g_−/− mice, activation of p38 and JNK was found to be severely compromised in response to T-cell receptor signaling (36). Considering the central role of MAPK signaling and premature senescence in preventing H-ras transformation, we carried out an analysis of these events with _Gadd45a_−/− MEF. Oncogenic Ras triggered normal activation of ERK and JNK, but there was a nearly complete loss of p38 activation. Loss of p38 activation correlated with deficient p53 activation and loss of normal G1 and G2 checkpoint activation. Proficient JNK activation by H-ras indicated that proficient upstream signaling by MAPK kinase (MAPKK) and MAPKKK could occur and raised the possibility that Gadd45a may affect p38 directly. Gadd45a was found to interact with p38, and the central region of Gadd45a was required for p38 binding and H-ras activation of p38.
MATERIALS AND METHODS
Cell culture.
MEF were prepared from E13.5 embryos. Cells were maintained in Dulbecco modified Eagle medium with 10% fetal bovine serum. For introduction of an activated ras allele into MEF, Phoenix Eco packaging cells were transfected with pBabe-puro and pBabe-H-ras retroviral plasmids (provided by S. Lowe). pBabe-H-ras contains the H-rasV12 allele (41). Virus-containing medium was collected and was used for infection as previously described (41). Four days after selection with 2 μg of puromycin/ml, equal numbers of cells were seeded for experiments. In some cases, MEK1 inhibitor (50 μM PD98059) or p38 inhibitor (10 μM SB202190) was added immediately after selection and cells were cultivated in the presence of the inhibitor for 5 days. To analyze cells in S phase, pulse-labeling with 10 μM bromodeoxyuridine (BrdU) was done for 2 h as previously described (11). Senescence-associated-β-galactosidase-positive cells were stained as previously described (41). Cell line 33-F expressing Flag-tagged Gadd45a and the control line were obtained by stably transfecting human colorectal RKO cells with pFlag-Gadd45a and pcDNA3.1-Flag vectors, respectively (30).
Plasmids and transfection.
All Ras-related expression vectors have been described previously, and the chloramphenicol acetyltransferase (CAT) assay was carried out as previously described (8). Constructs for expression of hemagglutinin (HA)-tagged Gadd45a as well as Myc-tagged Gadd45a deletion mutant proteins in mammalian cells have been described previously (27, 30). Plasmids for expression of HA-tagged JNK1 and Flag-tagged p38α were kindly provided by M. Karin (University of California—San Diego) and J. Han (The Scripps Research Institute), respectively. Transfections were carried out with Effectene (Qiagen) or Lipofectamine 2000 (Gibco-BRL) reagent according to the manufacturer's protocol.
Immunoblot and in vitro kinase assays.
Cultures were washed twice in ice-cold phosphate-buffered saline, lysed in ice-cold radioimmunoprecipitation assay (RIPA) buffer (50 mM Tris-HCl [pH 8.0], 0.14 mM NaCl, 1% NP-40, 20 mM β-glycerophosphate, 10 μg of aprotinin/ml, 10 μg of leupeptin/ml, 1 mM phenylmethylsulfonyl fluoride), and centrifuged at 14,000 × g for 15 min. For immunoblot analyses, 50 to 100 μg of protein was applied to each gel lane. Analysis of protein levels was carried out by using polyclonal antibodies (Abs) against p16/ink4a (M-156; Santa Cruz) and p53 (AB7; Oncogene), monoclonal Abs against p21/Waf1 (AB4; Oncogene) and actin (AB1; Oncogene), anti-Flag monoclonal Ab M2 (Sigma) or anti-Flag monoclonal Ab M2 conjugated with horseradish peroxidase (Sigma), anti-HA polyclonal Ab (Covance Research Products), anti-Myc-tag monoclonal Ab 9E10 (Santa Cruz), anti-Gadd45a polyclonal Ab H-165 (Santa Cruz), anti-p38 polyclonal Ab C-20 (Santa Cruz), anti-JNK polyclonal Ab C-17 (Santa Cruz), and anti-ERK2 polyclonal Ab C-14 (Santa Cruz). Secondary Abs were sheep anti-mouse or anti-rabbit immunoglobulin G conjugated with horseradish peroxidase (Amersham Pharmacia). Endogenous ERK2 (MAPK2) or p38 was immunoprecipitated from MEF by incubation with the specific Ab. For immunoprecipitation, a sample of cleared total lysates (with total protein ranging from 0.5 to 10 mg) was incubated with affinity matrix-anti-Flag (M2) agarose (Sigma), anti-HA agarose (Covance Research Products), or anti-Myc agarose (Santa Cruz) for 2 h or overnight at 4°C. Isolated immunocomplexes were washed five times in RIPA buffer and subjected to immunoblot analysis as described above.
JNK kinase activity was analyzed after pull-down of protein extracts with 10 μg of glutathione _S_-transferase (GST)-Jun. Immunoprecipitates were washed twice with lysis buffer and then with kinase buffer (20 mM HEPES [pH 7.9], 20 mM MgCl2, 25 mM β-glycerophosphate). Kinase reactions were performed in 50 μl of kinase buffer containing 10% glycerol, 1 mM dithiothreitol, 50 μM unlabeled ATP, and 5 μCi of [γ-32P]ATP. For ERK2 and p38 analysis, 10 μg of myelin basic protein and 2 μg of GST-ATF2, respectively, were used as substrates. Reactions mixtures were incubated for 20 min at 30°C. Proteins were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis in 4 to 20% gradient gels, and the dried gels were exposed to X-ray film (Kodak).
In vitro association assays.
For in vitro association assays, the T7-based coupled transcription-translation system with rabbit reticulocytes (TNT; Promega) and the EasyTag-[35S] protein labeling mix (NEN Life Science Products) were used to produce 35S-labeled Gadd45a and p38α proteins. pCDNA-based HA-Gadd45a- (30) or Flag-p38α-expressing plasmid constructs were used in these reactions. In control reactions, only respective vectors were used. Transcription-translation-coupled reactions were carried out according to the manufacturer's protocol. Five microliters of reaction mixture (approximately one-tenth of the reaction mixture volume) was used for association assays. It was mixed with an aliquot of sample or control reaction mixture, diluted to 250 μl with ice-cold RIPA buffer supplemented with 100 μg of bovine serum albumin/ml, and incubated on ice for 30 min. These reaction mixtures were then precleared with nonrelated anti-Myc immunomatrix to minimize nonspecific precipitation. Supernatants of precleared reaction mixtures were immunoprecipitated with specific immunomatrix (either anti-HA or anti-Flag). In the case of the GST pull-down assay, 5 μl of 35S-labeled p38α reaction mixture was diluted to 250 μl with RIPA buffer and precleared with glutathione-agarose (Sigma). Precleared sample was incubated with GST-Gadd45-agarose or GST-agarose (control). Immunobeads were washed extensively with RIPA buffer and subjected to sodium dodecyl sulfate-12% polyacrylamide gel electrophoresis followed by protein electroblotting to a polyvinylidene difluoride membrane. Membranes were autoradiographed and/or immunoprobed with specific primary Abs.
RESULTS
_Gadd45a_−/− MEF do not undergo permanent cell cycle arrest in the presence of H-ras.
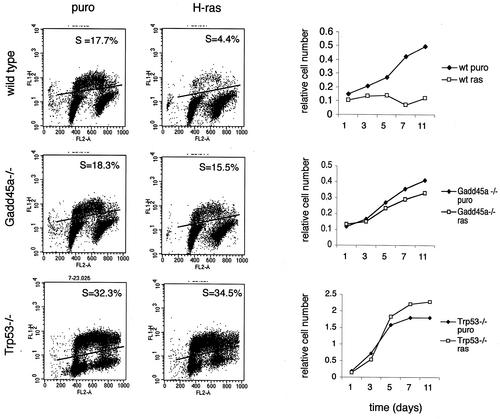
Since _Gadd45a_−/− MEF are able to undergo single oncogene transformation, unlike wt MEF (24), and since permanent cell cycle arrest (premature senescence) is believed to be a major protective response to an activated oncogene (41), growth inhibition by H-ras was studied in wt, _Gadd45a_−/−, and _Trp53_−/− MEF. Cells were infected with retrovirus containing either a puromycin resistance gene alone or the H-rasV12 gene and selected for 4 days in the presence of 2 μg of puromycin/ml as previously described (41). As reported previously (24), the _Gadd45a_−/− MEF used in this study did not undergo replicative senescence. Analysis of growth rate showed that H-ras overexpression blocked proliferation in wt MEF but not in _Gadd45a_−/− or _Trp53_−/− cells (Fig. 1, right panel). These data were further supported by analysis of BrdU-positive cells on day 5 after puromycin selection. Neither _Gadd45a_−/− nor _Trp53_−/− cell cultures had a decreased number of cells in S phase after H-ras overexpression. Moreover, analysis of senescence-associated-β-galactosidase-positive cells did not reveal any difference between pBabe-puro- and pBabe-H-ras-infected cells among either _Gadd45a_−/− or _Trp53_−/− MEF, while this senescence marker was increased in the wt cells (data not shown). These results demonstrate that, as is the case for _Trp53_−/− cells, the mechanism for Ras-induced long-term cell cycle arrest, premature senescence, is abolished after disruption of the Gadd45a gene.
FIG. 1.
H-ras-induced permanent cell cycle arrest is abolished in _Gadd45a_−/− MEF. wt, _Gadd45a_−/−, and _Trp53_−/− MEF were infected with retrovirus expressing oncogenic H-ras and selected for 4 days with 2 μg of puromycin (puro)/ml. The numbers of BrdU-positive cells (S-phase cells [S]) (left panel) and growth rates (right panel) after selection in puromycin are shown.
Deregulation of p38 and p53 activation after H-ras overexpression in _Gadd45a_−/− cells.
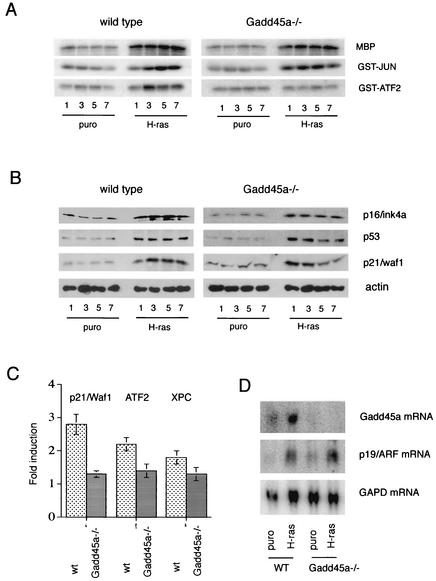
Since oncogenic H-ras can activate all three components of the MAPK pathway in wt cells (Fig. 2A, left panel) (19), we investigated the activation of these critical pathways in _Gadd45_−/− MEF. As shown in the right panel of Fig. 2A, there was normal activation of ERK and JNK, but p38 activation was completely abolished. Gadd45a can participate in the regulation of JNK and p38 kinase through positive regulation of an upstream kinase, MTK1, which presumably will lead to activation of both p38 and JNK (37, 47). Our results are consistent with this mechanism if a compensatory mechanism, such as one involving a MAPKKK other than MTK1, allows for the activation of JNK by Ras; e.g., in a previous study (46), a dominant-negative form of MTK1 primarily interfered with p38 stress activation. On the other hand, these results are also consistent with a defect in p38 signaling downstream of MTK1 at a point after the p38 and JNK pathways diverge.
FIG. 2.
Disruption of Gadd45a abolishes p38 activation after H-ras overexpression. (A) wt and _Gadd45a_−/− MEF were infected with H-ras-expressing retrovirus, and activities of ERK, JNK, and p38 were analyzed, with MBP, GST-Jun, and GST-ATF2 as substrates, respectively. puro, puromycin. (B) At the same time that the protein extracts were obtained, the levels of p16/Ink4a, p53, and p21/Waf1 were determined. (C) MEF were infected with either puromycin- or H-ras-expressing retroviruses, and 5 days after selection with puromycin, mRNA was purified and the levels of p53-inducible genes (those encoding p21/Waf1, XPC, and ATF2) were analyzed by using a quantitative filter hybridization procedure (29). The relative induction ratio was obtained after dividing the relative level of mRNA after infection with H-ras retrovirus by the level of mRNA after infection with puromycin vector alone. (D) The levels of Gadd45a and p19/ARF mRNA in wt and _Gadd45a_−/− MEF on day 5 after retroviral infection were measured by Northern blotting. GAPD was included as a loading control.
Other components of the MAPK pathways have been implicated in Ras-induced cell senescence; in particular, evidence exists that supports a role for the MEK/ERK pathway in the regulation of the p16/ink4a protein level as part of the mechanism to activate premature senescence in human and murine cells (34, 63). However, recent analysis of MEF from p16/Ink4a knockout mice showed that they remained susceptible to Ras-induced senescence (31, 42). As shown in Fig. 2B for wt and _Gadd45a_−/− MEF, there were no differences in the increases in p16/Ink4a protein levels after H-ras overexpression for the two genotypes, indicating a different mechanism for inhibition of H-ras-induced permanent cell cycle arrest in _Gadd45a_−/− MEF.
There is a major dependence on p53 for activated-oncogene-induced premature senescence in murine cells (41). Both MEKK1/JNK and p38 have been shown to efficiently induce p53-dependent transactivation (2, 8, 12). We next analyzed the levels of induction of p53 and p21/Waf1 proteins in wt and _Gadd45a_−/− MEF after infection with H-ras. H-ras induced the accumulation of both proteins in wt MEF (Fig. 2B, left panel). While the accumulation of p53 was not markedly affected in _Gadd45a_−/− MEF after infection with H-ras, the levels of p21/Waf1 showed some consistent differences. In contrast to wt cells, where p21/Waf1 levels remained elevated throughout the experiment, the initial induction of p21/Waf1 during the first 3 days was followed by a decrease at later times to levels approaching that of the control (Fig. 2B, right panel). Interestingly, growth rates of _Gadd45a_−/− MEF with or without H-ras were equivalent during the first 3 days after selection (Fig. 1) and diverged at later times, when p21 levels were reduced in the _Gadd45a_−/− cells. These results indicate that, even though accumulation of p53 by protein stabilization was not deregulated in _Gadd45a_−/− MEF, transactivation of p53 effectors, such as p21/Waf1, may be compromised. To confirm this prediction, we analyzed the levels of p21/Waf1 mRNA as well as those of two other p53-regulated genes, encoding ATF2 (4) and XPC (1, 5), by using a quantitative filter hybridization procedure. As shown in Fig. 2C, induction of all three genes was significantly reduced in _Gadd45a_−/− MEF compared to that in wt cells.
Since regulation of p53 stability after oncogene-induced stress is dependent on the p19_ARF_ gene (Bates et al., letter; Palmero et al., letter), the levels of p19_ARF_ mRNA in wt and Gadd45a_−/− MEF after infection with H-ras were determined. As shown in Fig. 2D, there was no appreciable difference in the p19_ARF levels for the genotypes after H-ras expression. This observation is consistent with our results for the p53 protein, of which comparable levels were seen for the two genotypes after H-ras overexpression (Fig. 2B). Taken together with earlier results for ERK and JNK, these results indicate that there is no generalized defect in H-ras signaling in _Gadd45a_−/− MEF other than that observed for p38.
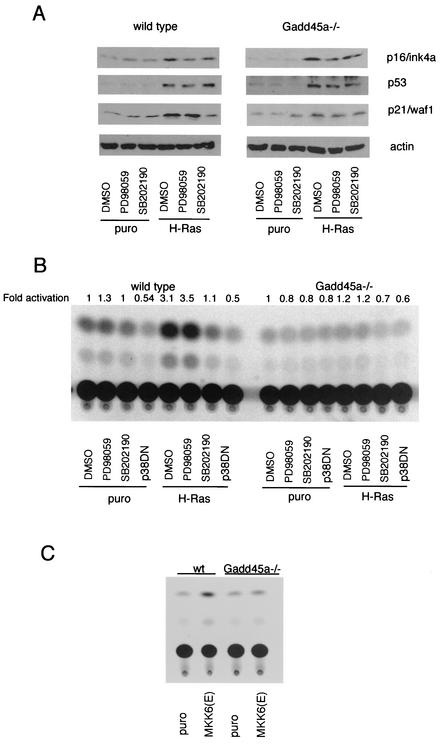
An important connection has already been established between p53 and p38 in the case of the cellular stress response to UV radiation. In particular, functional p38 is required for normal p53-dependent apoptosis and transactivation after UV radiation (8, 45). Since we found that disruption of the Gadd45a gene affects H-ras-induced p38 activation, we investigated whether inactivation of p38 alone is sufficient to attenuate p53 activation by H-ras. wt and _Gadd45a_−/− MEF were infected with either puromycin or H-ras, and specific inhibitors for MEK1 (PD98059) or p38 (SB202190) were added 4 days after the end of the selection in puromycin. Cells were grown in the presence of these inhibitors, and protein extracts were obtained 24 h later. Inactivation of MEK1 by PD98059 caused a reduction in p16/Ink4a accumulation but did not affect either the p53 or the p21/Waf1 protein levels in wt and _Gadd45a_−/− MEF (Fig. 3A). The p38 inhibitor had no effect on p16/Ink4a and p53 levels but did block the accumulation of p21/Waf1 after infection of wt MEF with H-ras, which is reminiscent of what was seen in _Gadd45a_−/− MEF without the inhibitor. In the case of _Gadd45a_−/− cells, H-ras had only a marginal effect on p21/Waf1 accumulation, which was reduced relative to that in the wt without the inhibitor, and the levels of p53 and p16/Ink4a proteins were comparable to those in wt cells.
FIG. 3.
Gadd45a and p38 are required for p53 activation after H-ras overexpression. (A) wt and _Gadd45a_−/− MEF were incubated in the presence of a MEK1 (50 μM PD98059) or p38 (10 μM SB202190) inhibitor, and protein extracts were obtained on day 5 after selection (see Materials and Methods). The levels of p16/Ink4a, p21/Waf1, and p53 proteins were analyzed. DMSO, dimethyl sulfoxide; puro, puromycin. (B) wt and _Gadd45a_−/− MEF were cotransfected with p53RE-CAT reporter plasmid and expression vectors containing either puromycin or H-ras. Some cells were additionally transfected with a dominant-negative p38α vector (p38DN). Four days later, cells were treated with either a MEK1 (PD90859) or p38 (SB202190) inhibitor, and CAT assays were carried out 12 h later. (C) wt and _Gadd45a_−/− MEF were cotransfected with p53RE-CAT reporter plasmid and expression vectors containing either puromycin or MKK6(E). Four days later, CAT activity was analyzed, and representative results are shown. Relative induction, as measured by increased CAT activity, was consistently twofold or greater in wt MEF compared to that in _Gadd45a_−/− MEF.
To rule out a p53-independent component in p21/Waf1 activation after H-ras overexpression, we analyzed p53 transactivation directly by using a reporter system containing a basal promoter with p53-binding sites (45, 60). Both wt and _Gadd45a_−/− cells were cotransfected with p53RE-CAT and either the puromycin or H-ras plasmid. For some samples, we included a dominant-negative p38α expression vector (p38DN). Three days later, cells were treated with either dimethyl sulfoxide, PD98059, or SB202190, and CAT activity was measured 24 h later. As shown in Fig. 3B, H-ras induced a substantial increase in p53-dependent transcriptional activity in wt MEF, while no appreciable increase occurred in _Gadd45a_−/− MEF (Fig. 3B). Inactivation of p38, but not the MEK1/ERK pathway, completely abolished p53 transactivation in wt MEF. To further demonstrate a role for Gadd45a in p38 signaling and to define at what points in the pathway this protein may be acting, p53 transcriptional activity was measured by the same approach as that used to obtain the results shown in Fig. 2B after introduction of a constitutively active form of the p38 upstream kinase MKK6, MKK6(E). wt and _Gadd45_−/− MEF were cotransfected with p53RE-CAT and the MKK6(E) expression vector, and 4 days later CAT activity was analyzed. As shown in Fig. 3C, the ability of MKK6(E) to activate p53 was reduced in _Gadd45a_−/− MEF. Taken together, our results show that either the disruption of the Gadd45a gene or the inactivation of p38 can attenuate p53 transcriptional activity after H-ras overexpression and that loss of Gadd45a results in a phenotype similar to that seen with direct inhibition of p38 by either a dominant-negative p38 expression vector or a specific chemical inhibitor.
The Gadd45a protein associates in vivo and in vitro with p38 but not with ERK or JNK.
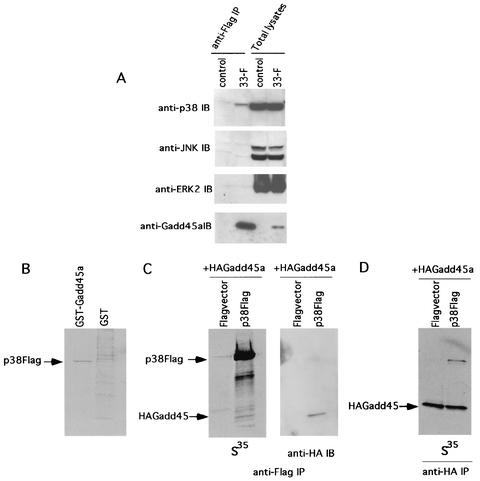
While there is clear evidence that the Gadd45 proteins can regulate upstream components of the p38/JNK portion of the MAPK pathway, such as MTK1, in some cell types (36, 37, 47), there is not convincing evidence with MEF, which may indicate a more downstream effect of Gadd45a in this cell type. As discussed earlier, _Gadd45a_−/− MEF showed normal UV activation of p38 and JNK (49) and no evidence was found for abnormal JNK regulation after H-ras overexpression (Fig. 2A). Moreover, MTK1 is far upstream of p38, and earlier studies have shown that compensation in the regulation of p38 could occur after disruption of intermediate kinases, such as MKK3, in this pathway (54). In addition, our results shown in Fig. 3C indicate that a signaling defect downstream of a MAPKK exists in _Gadd45a_−/− MEF and that activated MKK6 cannot rescue the phenotype. In light of these observations, a reasonable question to ask is whether Gadd45a has a more direct effect on p38 activation. As a first step, in vivo binding of p38 to Gadd45a was studied in a human RKO cell line, 33-F (30). This line stably expresses Flag-tagged Gadd45a at levels comparable to that of the endogenous protein in the parental RKO line after stress, when protein levels in this cell line can increase 10-fold (13, 30, 44). Anti-Flag affinity matrix immunoprecipitates from total lysates of 33-F and control cell lines were analyzed by immunoblotting with anti-p38, anti-JNK, or anti-ERK2 polyclonal Ab, with results shown in Fig. 4A. Only endogenous p38 was found by this analysis to coprecipitate with Gadd45a (Fig. 4A, two left lanes), suggesting a specific association of these proteins in vivo. The levels of all three MAPKs in 33-F and control cell lines were comparable, as demonstrated by immunoblot analysis (Fig. 4A, two right lanes). There was no significant coprecipitation of any endogenous MAPK from lysates of the control cells, which were stably transfected with Flag vector only (Fig. 4A, control).
FIG. 4.
Gadd45a associates with p38 both in vivo and in vitro. (A) Total lysates from 33-F cells stably expressing Flag-tagged Gadd45a and from control cell lines (approximately 10 mg of total protein) were immunoprecipitated (IP) with anti-Flag immunomatrix followed by immunoblot (IB) analysis of precipitates with anti-p38 (top panel), anti-JNK (second panel), anti-ERK2 (third panel), and anti-Gadd45a (bottom panel) polyclonal Abs. The lysates (approximately 100 μg per lane) were also analyzed directly by immunoblotting (two right lanes of each panel). (B) 35S-labeled Flag-p38α, obtained by coupled transcription-translation, was pulled down by GST-Gadd45a or GST beads and visualized by autoradiography. (C) 35S-labeled Flag-p38a and HA-Gadd45a obtained by coupled transcription-translation were mixed and precipitated with anti-Flag immunomatrix. Precipitates were analyzed either by using autoradiography (left panel) or by probing with anti-HA Ab (right panel). (D) Another experiment was carried out as described in the legend to Fig. 2C, left panel, except that anti-HA immunomatrix was used for precipitation. As controls for the experiments described in the legends to panels C and D, the 35S-labeled protein was mixed with in vitro transcription-translation labeling mixtures containing only vector.
To study this interaction in vitro, 35S-labeled HA-Gadd45a and Flag-p38α were produced by coupled transcription-translation and their interaction was analyzed in vitro by pull-down and coimmunoprecipitation assays (Fig. 4B to D). It was found that GST-Gadd45a beads can pull down 35S-labeled p38, thus supporting the contention for a direct interaction between these proteins (Fig. 4B). Further confirmation of this conclusion was obtained from coimmunoprecipitation experiments in which anti-Flag matrix was able to coprecipitate both Flag-p38α and HA-Gadd45a proteins (Fig. 4C) and, reciprocally, in which anti-HA immunomatrix coprecipitated HA-Gadd45a and Flag-p38α (Fig. 4D).
Mapping of Gadd45a interaction domain with p38 and its role in activation of p38 after H-ras overexpression.
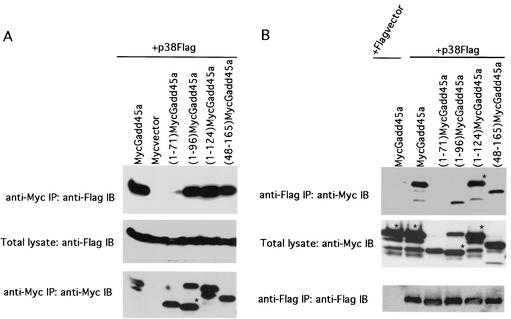
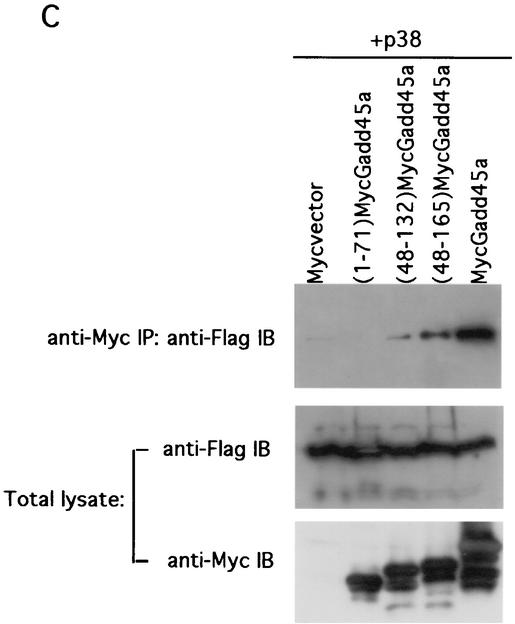
To define the Gadd45a region involved in the interaction with p38, a series of Myc-tagged Gadd45a deletion mutant proteins were generated. RKO cells were cotransfected with plasmids expressing Myc-tagged Gadd45a deletion mutant proteins and Flag-tagged p38α. The lysates from transfected cells were immunoprecipitated with either anti-Myc immunomatrix (to pull down Gadd45a) or anti-Flag immunomatrix (to pull down p38α), after which they were analyzed by immunoblotting with anti-Flag Abs (Fig. 5A and C, top panels) or anti-Myc Abs (Fig. 5B, top panel), respectively. The expression levels of Myc-tagged Gadd45a deletion mutant proteins as well as those of Flag-tagged p38α were analyzed in the total extracts and after immunoprecipitation (middle and bottom panels). The proteins were found to be abundant, and levels were comparable in all samples. Myc-tagged C-terminal deletion mutant proteins (1-96)Gadd45a and (1-124)Gadd45a as well as N-terminal deletion protein (48-165)Gadd45a were able to coprecipitate full-length Flag-tagged p38α, while C-terminal deletion protein (1-71)Gadd45a was deficient in coprecipitation (Fig. 5A, top panel). Consistently, full-length Flag-p38α was able to coprecipitate all of the above-mentioned Myc-tagged Gadd45a deletion mutant proteins except (1-71)Gadd45a (Fig. 5B, top panel), although the level of (1-96)Gadd45a was somewhat less than that of the other constructs containing additional carboxyl sequences. These results strongly suggest that the p38-interacting region is localized to the central part of Gadd45a protein, between amino acids 71 and 96. The ability of the Myc-tagged deletion mutant protein (48-132)Gadd45a, lacking both N-terminal and C-terminal parts, to still coprecipitate p38α (Fig. 5C, top panel) further supports this conclusion.
FIG. 5.
The central region of Gadd45a protein is involved in the interaction with p38. RKO cells were transiently transfected with Flag-p38α and Myc-tagged Gadd45a deletion proteins (1-71)Gadd45a, (1-96)Gadd45a, (1-124)Gadd45a, (48-132)Gadd45a, and (48-165)Gadd45a as well as full-size Myc-tagged Gadd45a. The total lysates of transfected cells were immunoprecipitated (IP) with either anti-Myc (A and C, top panels) or anti-Flag (B, top panel) immunomatrix, and the presence of deletion mutant proteins in precipitate was analyzed by immunoblotting (IB) with anti-Flag or anti-Myc Ab, respectively. The abundant expression of Flag-tagged p38α (A and C, middle panels) and Myc-tagged Gadd45a proteins (B, middle panel, and C, bottom panel) in total lysates was confirmed by immunoblotting with anti-Flag and anti-Myc Abs, respectively. The blots presented at the top of panels A and B were also reprobed with anti-Myc (bands that correspond to various forms of Gadd45a are marked with asterisks in lanes where there is more than one band) and anti-Flag Abs, respectively (A and B, bottom panels) to confirm comparable levels of protein in the primary immunoprecipitates. Cotransfection with Myc vector and Flag-p38α or Flag vector and full-size Myc-Gadd45a was used as for controls.
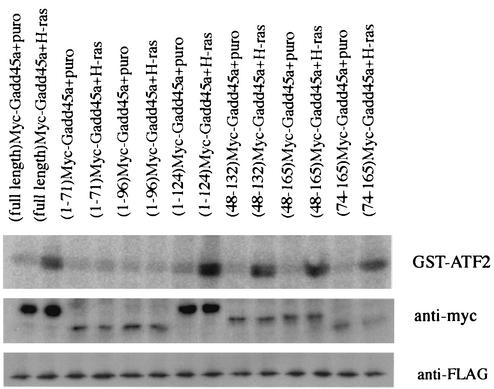
Next we analyzed which portion of Gadd45a is required for p38 activation after H-ras overexpression. _Gadd45a_−/− MEF were transiently transfected with Flag-tagged p38α, Myc-tagged deletion mutants of Gadd45a, and either the puromycin or H-ras expression vector. Two days later, p38 kinase was immunoprecipitated and p38 kinase activity was measured (Fig. 6). Transfection with the full-length Gadd45a expression vector did not induce appreciable activation of p38 in wt MEF (data not shown), nor did it activate p38 in _Gadd45a_−/− MEF (Fig. 6, first lane). However, simultaneous cotransfection with Gadd45a and H-ras resulted in the activation of p38 kinase relative to activity in the control cells transfected with the Gadd45a and control (puromycin) vectors (Fig. 6, first two lanes). These data suggest that Gadd45a is required but not sufficient for activation of p38 after oncogenic stress. Further analysis of p38 activation by H-ras and various Gadd45a deletion mutant proteins revealed that all the mutant proteins containing the central portion of Gadd45a were proficient in p38 activation. While the domain required for binding was mapped to amino acids 71 to 96, p38 activation mapped to amino acids 71 to 124, which again indicates a critical role for the central portion of this protein.
FIG. 6.
The central region of Gadd45a is required for p38 activation by H-ras. _Gadd45a_−/− MEF were cotransfected with p38α-Flag, different forms of Myc-Gadd45a, and plasmids with either puromycin (puro) or H-ras (1:3:9 ratio). Two days later, p38 activity was analyzed after immunoprecipitation with anti-Flag Ab in an in vitro kinase reaction with GST-ATF2 as the substrate.
DISCUSSION
Oncogene-induced cell senescence is an important protective mechanism in multicellular organisms to prevent aberrant growth and malignant transformation, and it has several critical regulatory components. These include a MEK/ERK signaling to p16/Ink4a, induction of p19/ARF, which stabilizes p53, and activation of p53 (7, 17, 63; Bates et al., letter; Palmero et al., letter). When H-ras was introduced into _Gadd45a_−/− MEF, p16 and p19 levels as well as p53 accumulation were equivalent to those in wt cells, while p53 activation, as measured by reduced p21/Waf1 induction and reduced p53 transcriptional activity, was markedly attenuated. Reduced p53 activation correlated with deficient p38 activation by H-ras. Deficient p38 signaling in Gadd45-deficient cells is not without precedent since reduced p38 signaling as well as reduced JNK signaling after activation of _Gadd45g_−/− T cells (36) and UV-irradiated _Gadd45a_−/− keratinocytes (20) has been reported. Our results further highlight the guardian role of p53 in preventing malignant transformation. Approximately half of all human tumors demonstrate mutations in TP53. Besides various mutations, several other mechanisms can inhibit p53 function, and these mechanisms may be important during the early stages of tumorigenesis. For example, methylation silencing of the p53 promoter and partial deletion of the human p14/ARF gene as well as amplification of Mdm2 are common mechanisms causing functional inactivation of p53 (16, 22, 39). As further discussed below, Wip1 amplification with resultant down-regulation of p38 activity can also compromise p53 function. Our results suggest that mutation of Gadd45a is another potential mechanism whereby p53 function can be compromised.
p38 signaling as a brake for cellular growth.
Our present findings provide new evidence for how p38 can be regulated by Gadd45a to prevent unregulated growth after oncogenic stimulation. In the case of human p53, we have found an important role for p38-dependent phosphorylation of key regulatory sites in p53 after UV irradiation (8). Moreover, recent findings on the interaction of p53 with Pin1 protein highlight the important role of p53 phosphorylation at certain sites in the regulation of the transcriptional activity of p53 (53, 58, 62). Intriguingly, mutation of p38 MAPK sites (serine 33 and serine 46) in human p53 was sufficient to nearly completely abolish p53 binding to Pin1 and thus indicates a critical role for p38 in the regulation of p53-Pin1 binding (53). The role of p38 in p53 activation is further supported by the finding that the Wip1 phosphatase, which inactivates p38, can block normal p53 activation (9, 45). Inhibition of p38 by either Wip1 or chemical inhibitors has been shown to prevent normal growth arrest after expression of H-ras (9, 40). In addition to its effects on p53, p38 can affect other growth-regulating mechanisms by means such as inhibiting Cdc25B phosphatase. p38 has been shown to directly phosphorylate inhibitory sites in Cdc25B phosphatase and to initiate a G2 checkpoint after UV irradiation (10) or osmotic stress (15). In the case of Ras-induced senescence in human fibroblasts, evidence has recently been reported that signaling by p38, unlike ERK and JNK, has a role in premature senescence (48). Considering that the Wip1 gene has oncogene-like properties (9, 33), the argument can be made that p38, which is inhibited by Wip1, has tumor suppressor-like properties. In support of this contention, we recently found that p38α-null MEF containing H-ras and E1A oncogenes developed substantially larger and more frequent tumors in nude mice compared to those observed with wt MEF (9).
The situation with stresses other than oncogenic H-ras and in different cell types is complicated for the Gadd45 proteins. While all three family members are ubiquitously expressed, Gadd45a was determined by real-time PCR to be the dominant isoform expressed in MEF, which may be why its disruption is not compensated for by the other two family members, which are expressed at lower levels and bind with lower affinity to p38 (data not shown). In contrast, Gadd45g is expressed at high levels during T-cell activation (36). In the case of UV radiation of _Gadd45a_−/− cells, T cells and keratinocytes appear to be unique in exhibiting reduced checkpoint activation, and this may indicate that compensatory pathways for p38 activation vary in different cell types. While it is clear that the Gadd45 proteins can bind to and contribute to the activation of MTK1, Gadd45a has lower affinity than Gadd45b and Gadd45g (37, 47), and thus, Gadd45a may have a more prominent effect downstream directly on p38 than upstream on MTK1 after expression of H-ras. The timing of the effect of Gadd45a on p53 and p38 is also complicated since some delay would be expected to be required to induce increased expression of Gadd45a. Gadd45a is known to be regulated by both p53 (28) and p53-independent signaling involving p38 (38), as well as by posttranscriptional transcript stability (26). Our results (Fig. 2 and 3) support such a role for Gadd45a in the sustained activation of both p38 and p53 after oncogenic stress.
Mechanisms for Gadd45a stimulation of p38.
There are two, not mutually exclusive, mechanisms whereby Gadd45a may contribute to the activation of p38. As discussed previously, there is evidence that Gadd45a can bind to and activate MTK1/MEKK4 (37, 47), which is upstream of p38 (46). This is supported by findings with _Gadd45g_−/− T cells (36) in which activation of JNK and p38 was deficient. The situation in _Gadd45a_−/− MEF and many other cell types is more complicated since defects in MAPK signaling or checkpoint control have not been observed previously, except with T cells (24, 50) and keratinocytes (20). In the case of UV radiation, normal JNK and p38 signaling was observed in _Gadd45a_−/− MEF (43, 49). Regardless of whether MTK1 activation by H-ras is deficient in _Gadd45a_−/− MEF, the argument could be made that the lack of any defect in JNK signaling indicates a more downstream effect of Gadd45a on the p38 pathway. There are multiple signaling components, such as the MAPKK, between upstream MTK1 and downstream p38, and it is possible that redundant compensatory mechanisms exist to counteract the loss of normal MTK1 activation. This possibility is supported by the observation that the disruption of the genes encoding MKK3, MKK4, and MEKK1 does not completely inhibit p38 or JNK stress activation (54, 56, 57). While the lack of a defect in JNK activation by H-ras in _Gadd45a_−/− MEF points to a primary role for Gadd45a downstream of MTK1, it does not directly preclude some contribution by MTK1 since compensatory mechanisms may differ for JNK and p38 signaling when MTK1 is not activated normally. For example, dominant-negative MTK1 was found to primarily affect p38 stress activation rather than JNK in overexpression studies with COS cells (46).
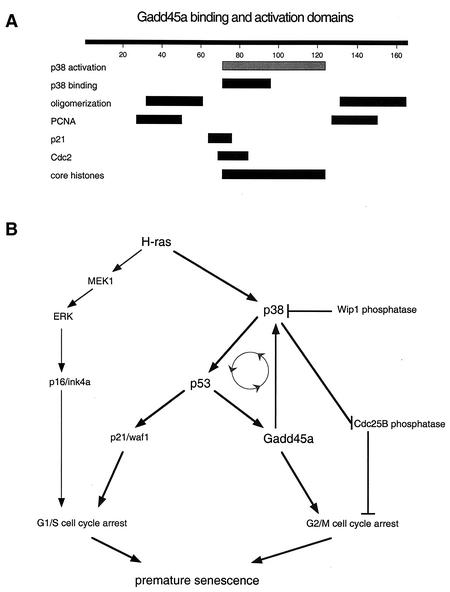
This work demonstrates that specific binding of Gadd45a to p38 but not to JNK or ERK is a potential mechanism by which Gadd45a has a direct role in p38 activation by H-ras. As summarized in Fig. 7A, the critical region in Gadd45a required for p38 binding was in the central portion of this small acidic protein. Expression of various Gadd45a deletion mutants along with H-ras demonstrated that the central region of Gadd45a was required, while N- and C-terminal regions were dispensable for both p38 binding and activation by H-ras. Interestingly, the domains for the binding of Gadd45 to MTK1 map to other regions in Gadd45 (D. Liebermann, personal communication), thus indicating that the primary effect of Gadd45a on p38 is direct and not via MTK1 in the presence of H-ras. The exact mechanism for p38 activation of Gadd45a remains to be determined since Gadd45a expression did not directly activate p38 in _Gadd45a_−/− MEF without H-ras (Fig. 6), thus indicating that Gadd45a is necessary but not sufficient for p38 activation. In addition, incubation of purified p38α protein with increasing concentrations of purified Gadd45a had no effect on p38 kinase activity in vitro (data not shown), and Gadd45a was a poor substrate for p38 kinase in vitro (data not shown). A reasonable explanation for these results is that Gadd45a functions as an adapter or scaffold protein in a complex with p38 and other proteins. Recent structural analysis of p38 complexes with binding proteins indicates a variety of overlapping and sometimes distinct binding sites (14). Association with various adapter proteins probably confers signaling specificity through p38 (52) and may account for the differences in p38 signaling that we have observed after oncogenic stress with H-ras and after genotoxic stress with UV radiation in _Gadd45a_−/− MEF, as well as differences between MEF and other cell types.
FIG. 7.
(A) Schematic diagram of regions involved in protein associations as determined by analysis of Gadd45a deletion proteins (see reference 21 for more detail). Results for p38 binding are taken from the experiment described in the legend to Fig. 5 and those for p38 activation by H-ras (gray bar) are from the experiment described in the legend to Fig. 6. (B) Model for growth-inhibitory responses triggered by oncogenic Ras and role of Gadd45/p38 pathway in activation of p53. The positive-feedback loop is designated by the gray circle with arrowheads (see text for further explanation). Some other relevant components, including Wip1, which is not induced by H-ras (9), are shown. To simplify the diagram, multiple components, such as MTK1, which also interacts with Gadd45 proteins (37, 47), have been omitted.
Defective signaling of p53 after oncogenic stress by H-ras in _Gadd45a_−/− MEF is remarkably similar to that seen when p38 activity is blocked. For example, Wip1 (9), a phosphatase that directly blocks p38, or chemical inhibition of p38 (8, 9) (Fig. 3A) blocked p53 transcriptional activity in a manner remarkably similar to that seen with _Gadd45a_−/− MEF (Fig. 2B and C and 3B). While the most straightforward explanation is that dampening of p38 activity is the mechanism for deficient p53 activation in _Gadd45a_−/− MEF after the expression of H-ras, an alternate explanation could be that Gadd45a has a role in a parallel signaling pathway that is also required for p53 activation after oncogenic stress. This latter possibility is unlikely based on our finding that p53 activation by constitutively active MKK6 [MKK6(E)], which is immediately upstream of p38, was reduced in _Gadd45a_−/− MEF (Fig. 3C). Another important conclusion from the MKK6(E) study is that a signaling defect exists in _Gadd45a_−/− MEF that is downstream of MAPKKK, such as MKT1, and the p38-specific MAPKK, MKK6. A role for Gadd45a in p38 signaling rather than in a parallel pathway is also supported by our finding that chemical inhibition of p38 resulted in no increase in transformation, as measured by colony growth in soft agar, in _Gadd45a_−/− MEF or in double-null MEF lacking Gadd45a as well as p21 or Trp53 (data not shown)
Evidence for a feedback loop involving p38, p53, and Gadd45a in H-ras-induced cellular senescence.
Aberrant oncogene expression triggers permanent growth arrest, so there must be one or more ongoing mechanisms to prevent escape from this senescent state. One component is the p53-regulated gene PPM1D, which encodes Wip1, whose induction after genotoxic stresses, such as UV radiation, can mediate negative feedback regulation of p53 by inactivation of p38 (45). While PPM1D induction occurs at later times after UV irradiation when human fibroblasts reenter the cell cycle, no induction was observed after the introduction of H-ras (9); as a result, p38 can remain active and positively regulates p53. Another required component for H-ras growth arrest is Gadd45a, which is known to be regulated by p53 (28). We now show that Gadd45a is required for normal H-ras activation of p38, and thus p38, p53, and Gadd45a form a positive-feedback loop during oncogene-induced senescence (Fig. 7B). There are several important implications of such a loop. Activation of p53 induces expression of genes involved in the regulation of the G1/S checkpoint and apoptosis. p53-dependent activation of 14-3-3σ and Gadd45a can also contribute to G2/M checkpoint control. Gadd45 can directly regulate Cdc2 activity by displacing cyclin B1 from the Cdc2 complex (59). Through its positive effect on p38 activity, Gadd45a can also be involved in inhibitory phosphorylation of Cdc25B phosphatase, which blocks progression into mitosis (10). Maintenance of this feedback loop should prevent escape from H-ras-induced growth arrest, leading to permanent growth arrest
The concept of a positive-feedback loop is particularly attractive since one prediction is that disruption of any component of this loop can facilitate cell transformation. While the connection with human carcinogenesis is established for p53 (7) and p38 (9, 33), the role of Gadd45a in human cancer is less certain in this regard than is the role of this protein in cancer in _Gadd45a_−/− mice. Such mice show genomic instability and increased carcinogenesis after exposure to ionizing radiation (24) or dimethylbenzanthracene (23), and _Gadd45a_−/− cells share some characteristics with _Trp53_−/− cells (24). Recently, a significant frequency of mutations in GADD45A in human pancreatic cancer specimens has been reported (55). In the case of GADD45G, gene silencing at the mRNA level in 83% of human pituitary adenomas was recently reported (61). Loss of expression of a Gadd45 family member may therefore contribute to tumor formation.
Acknowledgments
We thank D. Liebermann (Fels) for sharing unpublished data on the association between MTK1 and Gadd45 and M. Karin (UCSD) and J. Han (The Scripps Research Institute) for providing plasmid constructs.
REFERENCES
- 1.Adimoolam, S., and J. M. Ford. 2002. p53 and DNA damage-inducible expression of the xeroderma pigmentosum group C gene. Proc. Natl. Acad. Sci. USA 99**:**12985-12990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adler, V., M. R. Pincus, T. Minamoto, S. Y. Fuchs, M. J. Bluth, P. W. Brandt-Rauf, F. K. Friedman, R. C. Robinson, J. M. Chen, X. W. Wang, C. C. Harris, and Z. Ronai. 1997. Conformation-dependent phosphorylation of p53. Proc. Natl. Acad. Sci. USA 94**:**1686-1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ambrosino, C., and A. R. Nebreda. 2001. Cell cycle regulation by p38 MAP kinases. Biol. Cell 93**:**47-51. [DOI] [PubMed] [Google Scholar]
- 4.Amundson, S. A., M. Bittner, Y. D. Chen, J. Trent, P. Meltzer, and A. J. Fornace, Jr. 1999. cDNA microarray hybridization reveals complexity and heterogeneity of cellular genotoxic stress responses. Oncogene 18**:**3666-3672. [DOI] [PubMed] [Google Scholar]
- 5.Amundson, S. A., A. Patterson, K. T. Do, and A. J. J. Fornace. 2002. A nucleotide excision repair master-switch: p53 regulated coordinate induction of global genomic repair genes. Cancer Biol. Ther. 1**:**145-149. [DOI] [PubMed] [Google Scholar]
- 6.Appella, E., and C. W. Anderson. 2001. Post-translational modifications and activation of p53 by genotoxic stresses. Eur. J. Biochem. 268**:**2764-2772. [DOI] [PubMed] [Google Scholar]
- 7.Bringold, F., and M. Serrano. 2000. Tumor suppressors and oncogenes in cellular senescence. Exp. Gerontol. 35**:**317-329. [DOI] [PubMed] [Google Scholar]
- 8.Bulavin, D., S. I. Saito, M. C. Hollander, K. Sakaguchi, C. W. Anderson, E. Appella, and A. J. Fornace, Jr. 1999. Phosphorylation of human p53 by p38 kinase coordinates N-terminal phosphorylation and apoptosis in response to UV radiation. EMBO J. 18**:**6845-6854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bulavin, D. V., O. N. Demidov, S. Saito, P. Kauraniemi, C. Phillips, S. A. Amundson, C. Ambrosino, G. Sauter, A. R. Nebreda, C. W. Anderson, A. Kallioniemi, A. J. Fornace, Jr., and E. Appella. 2002. Amplification of PPM1D in human tumors abrogates p53 tumor-suppressor activity. Nat. Genet. 31**:**210-215. [DOI] [PubMed] [Google Scholar]
- 10.Bulavin, D. V., Y. Higashimoto, I. J. Popoff, W. A. Gaarde, V. Basrur, O. Potapova, E. Appella, and A. J. Fornace, Jr. 2001. Initiation of a G2/M checkpoint after ultraviolet radiation requires p38 kinase. Nature 411**:**102-107. [DOI] [PubMed] [Google Scholar]
- 11.Bulavin, D. V., N. D. Tararova, N. D. Aksenov, V. A. Pospelov, and T. V. Pospelova. 1999. Deregulation of p53/p21Cip1/Waf1 pathway contributes to polyploidy and apoptosis of E1A+cHa-ras transformed cells after gamma-irradiation. Oncogene 18**:**5611-5619. [DOI] [PubMed] [Google Scholar]
- 12.Buschmann, T., O. Potapova, A. Bar-Shira, V. N. Ivanov, S. Y. Fuchs, S. Henderson, V. A. Fried, T. Minamoto, D. Alarcon-Vargas, M. R. Pincus, W. A. Gaarde, N. J. Holbrook, Y. Shiloh, and Z. Ronai. 2001. Jun NH2-terminal kinase phosphorylation of p53 on Thr-81 is important for p53 stabilization and transcriptional activities in response to stress. Mol. Cell. Biol. 21**:**2743-2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carrier, F., M. L. Smith, I. Bae, K. E. Kilpatrick, C. Y. Chen, K. J. Johnson, W. D. Henner, T. Gilmer, M. B. Kastan, and A. J. Fornace, Jr. 1994. Characterization of human Gadd45, a p53-regulated protein. J. Biol. Chem. 269**:**32672-32677. [PubMed] [Google Scholar]
- 14.Chang, C. I., B. E. Xu, R. Akella, M. H. Cobb, and E. J. Goldsmith. 2002. Crystal structures of MAP kinase p38 complexed to the docking sites on its nuclear substrate MEF2A and activator MKK3b. Mol. Cell 9**:**1241-1249. [DOI] [PubMed] [Google Scholar]
- 15.Dmitrieva, N. I., D. V. Bulavin, A. J. Fornace, Jr., and M. B. Burg. 2002. Rapid activation of G2/M checkpoint after hypertonic stress in renal inner medullary epithelial (IME) cells is protective and requires p38 kinase. Proc. Natl. Acad. Sci. USA 99**:**184-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Esteller, M., S. Tortola, M. Toyota, G. Capella, M. A. Peinado, S. B. Baylin, and J. G. Herman. 2000. Hypermethylation-associated inactivation of p14(ARF) is independent of p16(INK4a) methylation and p53 mutational status. Cancer Res. 60**:**129-133. [PubMed] [Google Scholar]
- 17.Ferbeyre, G., E. de Stanchina, A. W. Lin, E. Querido, M. E. McCurrach, G. J. Hannon, and S. W. Lowe. 2002. Oncogenic ras and p53 cooperate to induce cellular senescence. Mol. Cell. Biol. 22**:**3497-3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gerwins, P., J. L. Blank, and G. L. Johnson. 1997. Cloning of a novel mitogen-activated protein kinase kinase kinase, MEKK4, that selectively regulates the c-Jun amino terminal kinase pathway. J. Biol. Chem. 272**:**8288-8295. [DOI] [PubMed] [Google Scholar]
- 19.Graham, S. M., S. M. Oldham, C. B. Martin, J. K. Drugan, I. E. Zohn, S. Campbell, and C. J. Der. 1999. TC21 and Ras share indistinguishable transforming and differentiating activities. Oncogene 18**:**2107-2116. [DOI] [PubMed] [Google Scholar]
- 20.Hildesheim, J., D. V. Bulavin, M. R. Anver, W. G. Alvord, M. C. Hollander, L. Vardanian, and A. Fornace, Jr. 2002. Gadd45a protects against UV irradiation-induced skin tumors, and promotes apoptosis and stress signaling via MAPK and p53. Cancer Res. 62**:**7305-7315. [PubMed] [Google Scholar]
- 21.Hildesheim, J., and A. J. Fornace, Jr. 2002. Gadd45a: an elusive yet attractive candidate gene in pancreatic cancer. Clin. Cancer Res. 8:2475-2479. [PubMed] [Google Scholar]
- 22.Ho, G. H., J. E. Calvano, M. Bisogna, Z. Abouezzi, P. I. Borgen, C. Cordon-Cardo, and K. J. van Zee. 2001. Genetic alterations of the p14ARF-hdm2-p53 regulatory pathway in breast carcinoma. Breast Cancer Res. Treat. 65**:**225-232. [DOI] [PubMed] [Google Scholar]
- 23.Hollander, M. C., O. Kovalsky, J. M. Salvador, K. E. Kim, A. D. Patterson, D. C. Hairnes, and A. J. Fornace, Jr. 2001. DMBA carcinogenesis in _Gadd45a_-null mice is associated with decreased DNA repair and increased mutation frequency. Cancer Res. 61**:**2487-2491. [PubMed] [Google Scholar]
- 24.Hollander, M. C., M. S. Sheikh, D. Bulavin, K. Lundren, L. Augeri-Henmueller, R. Shehee, T. Molinaro, K. Kim, E. Tolosa, J. D. Ashwell, M. D. Rosenberg, Q. Zhan, P. M. Fernández-Salguero, W. F. Morgan, C. X. Deng, and A. J. Fornace, Jr. 1999. Genomic instability in _Gadd45a_-deficient mice. Nat. Genet. 23**:**176-184. [DOI] [PubMed] [Google Scholar]
- 25.Inoue, H., M. Tateno, K. Fujimura-Kamada, G. Takaesu, T. Adachi-Yamada, J. Ninomiya-Tsuji, K. Irie, Y. Nishida, and K. Matsumoto. 2001. A Drosophila MAPKKK, D-MEKK1, mediates stress responses through activation of p38 MAPK. EMBO J. 20**:**5421-5430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jackman, J., I. Alamo, and A. J. Fornace, Jr. 1994. Genotoxic stress confers preferential and coordinate mRNA stability on the five gadd genes. Cancer Res. 54**:**5656-5662. [PubMed] [Google Scholar]
- 27.Jin, S., M. J. Antinore, F. D. Lung, X. Dong, H. Chao, F. Fan, A. B. Colchagie, P. Blanck, P. P. Roller, A. J. Fornace, Jr., and Q. Zhan. 2000. The GADD45 inhibition of Cdc2 kinase correlates with GADD45-mediated growth suppression. J. Biol. Chem. 275**:**16602-16608. [DOI] [PubMed] [Google Scholar]
- 28.Kastan, M. B., Q. Zhan, W. S. el-Deiry, F. Carrier, T. Jacks, W. V. Walsh, B. S. Plunkett, B. Vogelstein, and A. J. Fornace, Jr. 1992. A mammalian cell cycle checkpoint pathway utilizing p53 and GADD45 is defective in ataxia-telangiectasia. Cell 71**:**587-597. [DOI] [PubMed] [Google Scholar]
- 29.Koch-Paiz, C. A., R. Momenan, S. A. Amundson, E. Lamoreaux, and A. J. Fornace, Jr. 2000. Estimation of relative mRNA content by filter hybridization to a polyuridylic probe. BioTechniques 29**:**708-714. [DOI] [PubMed] [Google Scholar]
- 30.Kovalsky, O., F. D. Lung, P. P. Roller, and A. J. Fornace, Jr. 2001. Oligomerization of human gadd45a protein. J. Biol. Chem. 276**:**39330-39339. [DOI] [PubMed] [Google Scholar]
- 31.Krimpenfort, P., K. C. Quon, W. J. Mooi, A. Loonstra, and A. Berns. 2001. Loss of p16Ink4a confers susceptibility to metastatic melanoma in mice. Nature 413**:**83-86. [DOI] [PubMed] [Google Scholar]
- 32.Lavoie, J. N., G. L'Allemain, A. Brunet, R. Muller, and J. Pouyssegur. 1996. Cyclin D1 expression is regulated positively by the p42/p44MAPK and negatively by the p38/HOGMAPK pathway. J. Biol. Chem. 271**:**20608-20616. [DOI] [PubMed] [Google Scholar]
- 33.Li, J., Y. Yang, Y. Peng, R. J. Austin, W. G. van Eyndhoven, K. C. Nguyen, T. Gabriele, M. E. McCurrach, J. R. Marks, T. Hoey, S. W. Lowe, and S. Powers. 2002. Oncogenic properties of PPM1D located within a breast cancer amplification epicenter at 17q23. Nat. Genet. 31**:**133-134. [DOI] [PubMed] [Google Scholar]
- 34.Lin, A. W., M. Barradas, J. C. Stone, L. van Aelst, M. Serrano, and S. W. Lowe. 1998. Premature senescence involving p53 and p16 is activated in response to constitutive MEK/MAPK mitogenic signaling. Genes Dev. 12**:**3008-3019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin, A. W., and S. W. Lowe. 2001. Oncogenic ras activates the ARF-p53 pathway to suppress epithelial cell transformation. Proc. Natl. Acad. Sci. USA 98**:**5025-5030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lu, B., H. Yu, C. Chow, B. Li, W. Zheng, R. J. Davis, and R. A. Flavell. 2001. GADD45gamma mediates the activation of the p38 and JNK MAP kinase pathways and cytokine production in effector T(H)1 cells. Immunity 14**:**583-590. [DOI] [PubMed] [Google Scholar]
- 37.Mita, H., J. Tsutsui, M. Takekawa, E. A. Witten, and H. Saito. 2002. Regulation of MTK1/MEKK4 kinase activity by its N-terminal autoinhibitory domain and GADD45 binding. Mol. Cell. Biol. 22**:**4544-4555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oh-Hashi, K., W. Maruyama, and K. Isobe. 2001. Peroxynitrite induces GADD34, 45, and 153 via p38 MAPK in human neuroblastoma, SH-SY5Y cells. Free Radic. Biol. Med. 30**:**213-221. [DOI] [PubMed] [Google Scholar]
- 39.Oliner, J. D., K. W. Kinzler, P. S. Meltzer, D. L. George, and B. Vogelstein. 1992. Amplification of a gene encoding a p53-associated protein in human sarcomas. Nature 358**:**80-83. [DOI] [PubMed] [Google Scholar]
- 40.Pruitt, K., W. M. Pruitt, G. K. Bilter, J. K. Westwick, and C. J. Der. 2002. Raf-independent deregulation of p38 and JNK kinases are critical for Ras transformation. J. Biol. Chem. 277**:**31808-31817. [DOI] [PubMed] [Google Scholar]
- 41.Serrano, M., A. W. Lin, M. E. McCurrach, D. Beach, and S. W. Lowe. 1997. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell 88**:**593-602. [DOI] [PubMed] [Google Scholar]
- 42.Sharpless, N. E., N. Bardeesy, K. H. Lee, D. Carrasco, D. H. Castrillon, A. J. Aguirre, E. A. Wu, J. W. Horner, and R. A. DePinho. 2001. Loss of p16Ink4a with retention of p19Arf predisposes mice to tumorigenesis. Nature 413**:**86-91. [DOI] [PubMed] [Google Scholar]
- 43.Shaulian, E., and M. Karin. 1999. Stress-induced JNK activation is independent of Gadd45 induction. J. Biol. Chem. 274**:**29595-29598. [DOI] [PubMed] [Google Scholar]
- 44.Smith, M. L., I. T. Chen, Q. Zhan, P. M. O'Connor, and A. J. Fornace, Jr. 1995. Involvement of the p53 tumor suppressor in repair of UV-type DNA damage. Oncogene 10**:**1053-1059. [PubMed] [Google Scholar]
- 45.Takekawa, M., M. Adachi, A. Nakahata, I. Nakayama, F. Itoh, H. Tsukuda, Y. Taya, and K. Imai. 2000. p53-inducible wip1 phosphatase mediates a negative feedback regulation of p38 MAPK-p53 signaling in response to UV radiation. EMBO J. 19**:**6517-6626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Takekawa, M., F. Posas, and H. Saito. 1997. A human homolog of the yeast Ssk2/Ssk22 MAP kinase kinase kinases, MTK1, mediates stress-induced activation of the p38 and JNK pathways. EMBO J. 16**:**4973-4982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Takekawa, M., and H. Saito. 1998. A family of stress-inducible GADD45-like proteins mediate activation of the stress-responsive MTK1/MEKK4 MAPKKK. Cell 95**:**521-530. [DOI] [PubMed] [Google Scholar]
- 48.Wang, W., J. X. Chen, R. Liao, Q. Deng, J. J. Zhou, S. Huang, and P. Sun. 2002. Sequential activation of the MEK-extracellular signal-regulated kinase and MKK3/6-p38 mitogen-activated protein kinase pathways mediates oncogenic _ras_-induced premature senescence. Mol. Cell. Biol. 22**:**3389-3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang, X., M. Gorospe, and N. J. Holbrook. 1999. gadd45 is not required for activation of c-jun N-terminal kinase or p38 during acute stress. J. Biol. Chem. 274**:**29599-29602. [DOI] [PubMed] [Google Scholar]
- 50.Wang, X. W., Q. Zhan, J. D. Coursen, M. Khan, H. U. Kontny, L. Liu, M. C. Hollander, P. M. O'Connor, A. J. Fornace, Jr., and C. C. Harris. 1999. Gadd45 induction of a G2-M cell cycle checkpoint. Proc. Natl. Acad. Sci. USA 96**:**3706-3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wei, W., R. M. Hemmer, and J. M. Sedivy. 2001. Role of p14ARF in replicative and induced senescence of human fibroblasts. Mol. Cell. Biol. 21**:**6748-6757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weston, C. R., D. G. Lambright, and R. J. Davis. 2002. Signal transduction. MAP kinase signaling specificity. Science 296**:**2345-2347. [DOI] [PubMed] [Google Scholar]
- 53.Wulf, G. M., Y. C. Liou, A. Ryo, S. W. Lee, and K. P. Lu. 2002. Role of Pin1 in the regulation of p53 stability and p21 transactivation, and cell cycle checkpoints in response to DNA damage. J. Biol. Chem. 277**:**47976-47979. [DOI] [PubMed] [Google Scholar]
- 54.Wysk, M., D. D. Yang, H. T. Lu, R. A. Flavell, and R. J. Davis. 1999. Requirement of mitogen-activated protein kinase kinase 3 (MKK3) for tumor necrosis factor-induced cytokine expression. Proc. Natl. Acad. Sci. USA 96**:**3763-3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yamasawa, K., Y. Nio, M. Dong, K. Yamaguchi, and M. Itakura. 2002. Clinicopathological significance of abnormalities in Gadd45 expression and its relationship to p53 in human pancreatic cancer. Clin. Cancer Res. 8**:**2563-2569. [PubMed] [Google Scholar]
- 56.Yang, D., C. Tournier, M. Wysk, H. T. Lu, J. Xu, R. J. Davis, and R. A. Flavell. 1997. Targeted disruption of the MKK4 gene causes embryonic death, inhibition of c-Jun NH2-terminal kinase activation, and defects in AP-1 transcriptional activity. Proc. Natl. Acad. Sci. USA 94**:**3004-3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yujiri, T., S. Sather, G. R. Fanger, and G. L. Johnson. 1998. Role of MEKK1 in cell survival and activation of JNK and ERK pathways defined by targeted gene disruption. Science 282**:**1911-1914. [DOI] [PubMed] [Google Scholar]
- 58.Zacchi, P., M. Gostissa, T. Uchida, C. Salvagno, F. Avolio, S. Volinia, Z. Ronai, G. Blandino, C. Schneider, and S. G. Del. 2002. The prolyl isomerase Pin1 reveals a mechanism to control p53 functions after genotoxic insults. Nature 419**:**853-857. [DOI] [PubMed] [Google Scholar]
- 59.Zhan, Q., M. J. Antinore, X. W. Wang, F. Carrier, M. L. Smith, C. C. Harris, and A. J. Fornace, Jr. 1999. Association with Cdc2 and inhibition of Cdc2/cyclin B1 kinase activity by the p53-regulated protein Gadd45. Oncogene 18**:**2892-2900. [DOI] [PubMed] [Google Scholar]
- 60.Zhan, Q., F. Carrier, and A. J. Fornace, Jr. 1993. Induction of cellular p53 activity by DNA-damaging agents and growth arrest. Mol. Cell. Biol. 13**:**4242-4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang, X., H. Sun, D. C. Danila, S. R. Johnson, Y. Zhou, B. Swearingen, and A. Klibanski. 2002. Loss of expression of GADD45 gamma, a growth inhibitory gene, in human pituitary adenomas: implications for tumorigenesis. J. Clin. Endocrinol. Metab. 87**:**1262-1267. [DOI] [PubMed] [Google Scholar]
- 62.Zheng, H., H. You, X. Z. Zhou, S. A. Murray, T. Uchida, G. Wulf, L. Gu, X. Tang, K. P. Lu, and Z. X. Xiao. 2002. The prolyl isomerase Pin1 is a regulator of p53 in genotoxic response. Nature 419**:**849-853. [DOI] [PubMed] [Google Scholar]
- 63.Zhu, J., D. Woods, M. McMahon, and J. M. Bishop. 1998. Senescence of human fibroblasts induced by oncogenic Raf. Genes Dev. 12**:**2997-3007. [DOI] [PMC free article] [PubMed] [Google Scholar]