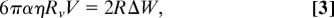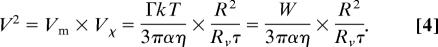Vesicles surfing on a lipid bilayer: Self-induced haptotactic motion (original) (raw)
Abstract
Haptotaxis is a mechanism proposed at the end of the 1960s to explain cell motility. It describes cell movement induced by an adhesion gradient. In this work, we present evidence for self-induced haptotaxis using negatively charged giant vesicles interacting with positively charged supported lipid bilayers, which has not been previously described. Depending on the charge of the vesicle, we observed different behaviors. At low charge, no adhesion occurs. At high charge, the vesicle adheres but does not move. In a restricted range of intermediate charge densities, we found that the vesicle moves spontaneously with velocities of the order of a few micrometers per second over distances of >100 μm. We show that a local lipid transfer between the giant vesicle and the supported lipid bilayer takes place during the adhesion, breaking the symmetry and inducing a lateral charge gradient. This charge gradient polarizes the giant vesicle and induces its motion. To explain our observations, we propose a scaling model that relates the adhesion energy to the velocity of vesicle motion and to the characteristic lipid transfer time. Our measurements indicate that the effective adhesion energy is strongly reduced by counterions, which are dynamically trapped between the vesicle and the supported bilayer.
Keywords: adhesion, electrostatic interaction, giant unilamellar vesicles, haptotaxis, lipid membrane
Cellular adhesion is currently studied very actively both from biological and physical perspectives. At the level of the cell membrane, cell–cell or cell–extracellular matrix adhesion is mediated by specific ligand–receptor pairs, such as cadherin–cadherin or integrin–fibronectin (1). Many efforts have been devoted to decipher cell adhesion on different types of substrates (2–6) and to analyze the intracellular signaling pathway leading to the formation of complex adhesion structures (7, 8). In parallel, physicists have used simple model systems without cytoskeleton to understand the static and dynamic behavior of membranes adhering on solid substrates. The spreading of giant unilamellar vesicles (GUVs) containing specific ligands on surface coated with the corresponding receptors has been described in detail (9, 10). A relatively slow spreading has been reported leading to the formation of a stable and homogeneous adhesion zone. Alternatively, nonspecific interactions between GUVs and different substrates also have been studied, in particular electrostatic. Contact between charged vesicles and a polylysine-coated surface with a very high charge density leads to immediate lysis and destruction of GUVs (11). However, if a charged GUV is brought onto an oppositely charged lipid monolayer with moderate charge density, the vesicle spreads gently with the final contact zone presenting a heterogeneous pattern of counterion-rich blisters (12). Note that in this reference, the GUV membrane had a much lower fluidity, because it consisted of an equimolar mixture of cholesterol and 1,2-dimyristoyl-_sn_-glycero-3-phosphocholine (DMPC) (13). Interesting lipid patterning has also been observed when red blood cells are brought onto a polylysine-coated surface (14).
Nonspecific electrostatic adhesion of a giant vesicle on a fluid substrate has not been investigated yet. In this work, we study the dynamic of adhesion of a negatively charged giant vesicle on a positively charged supported bilayer by using optical interferometry. Reflection interference contrast microscopy (RICM) is a quantitative interference technique, widely used to determine the optical thickness and separation distances of thin films (13). It is therefore well adapted to investigate adhesion phenomena for GUVs or cells. Contact between the two fluid membranes leads to a symmetry breaking in the charge distribution in the contact zone that induces the spontaneous motion of the vesicle. The “surfing vesicle” moves over hundreds of micrometers before stopping and completing its adhesion. This process is formally similar to haptotaxis. This phenomenon was proposed 40 years ago to explain cell migration and to describe the displacement of cells due to the presence of adhesion gradient associated with a concentration gradient of adhesion molecules (15). Although cell motility is more complex and requires energy, the haptotaxis mechanism can describe our observations. Moreover, exact calculations have been performed recently to determine the velocity of a vesicle on a surface prepared with a constant adhesion gradient (16) and the force applied to the vesicle (17).
In the present system, the adhesion gradient is not externally prepared but results from internal redistribution of charges. To account for this effect, we developed a scaling model based on the “free-running droplet,” where an adhesion gradient is created by the local modification of the wettability of the substrate at the droplet position by chemical reactions (18, 19), transfer of surfactants (20), or externally light-activating rotaxane molecules assembled on a substrate and, thus, switching its polarizability (21).
Results
Characterization of the Supported Lipid Bilayer (SLB).
Positively charged SLBs are formed by the method of small vesicle spreading (see Fig. 5 and Supporting Text, which are published as supporting information on the PNAS web site, for details on the preparation). The small unilamellar vesicles (SUVs) contain a mixture of zwitterionic 1,2-dioleoyl-_sn_-glycero-3-phosphocholine (DOPC) and cationic 1,2-dioleoyl-3-trimethylammonium-propane (DOTAP) (9:1 mol/mol), supplemented by a small fraction (1%) of fluorescently labeled lipids. The SLBs are characterized by using RICM, confocal microscopy, atomic force microscopy, and fluorescence recovery after photobleaching (FRAP).
The major part of the surface appeared smooth as expected for an SLB (22). A small number of protrusions were although found (see bright spots in Fig. 6, which is published as supporting information on the PNAS web site), that covered <1% of the surface. Their typical height (5–10 nm) may indicate that some nonfused SUVs remained on the bilayer or, alternatively, may correspond to some DOTAP impurities (23). These defects, as we will show, may be responsible for the attachment of lipid tubes, which slow down the vesicles motion.
To investigate the lipid mobility in the SLBs, we performed FRAP experiments, which showed the following: (i) most lipids are mobile (mobile fraction ≈ 90%), and (ii) their diffusion constant D = (0.80 ± 0.31) μm2/s (see Supporting Text and also Fig. 7, which is published as supporting information on the PNAS web site) is smaller than for suspended bilayers, 13.4 ± 0.7 μm2/s (24), because the bottom leaflet interacts with the substrate (25). The small immobile fraction is probably associated with the defects described previously.
Adhesion and Motion of a Negatively Charged Giant Vesicle on a Positively Charged SLB.
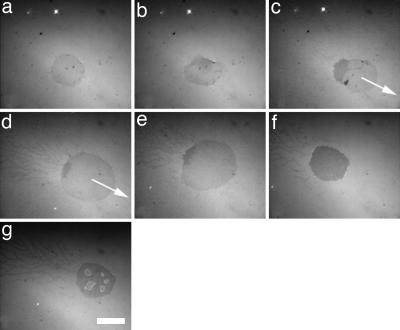
We used RICM to investigate the interaction between GUVs, made from a mixture of negatively charged 1,2-dioleoyl-_sn_-glycero-3-phosphatidylserine (DOPS) and zwitterionic DOPC, and positively charged SLBs. RICM is a classical optical method to characterize the contact zone between a vesicle and a substrate (26), which is also well suited for a GUV and an SLB. Fig. 1 and Movie 1, which is published as supporting information on the PNAS web site, show a representative example of the adhesion dynamics of a GUV made from a lipid mixture containing 10% DOPS on a positively charged SLB (10% DOTAP) without any added salt. After injecting the GUVs in the chamber coated with the SLB, the GUV gradually sediments onto the supported bilayer up to be in contact after a few minutes. Upon contact, the vesicle spreads quickly (within <1 s) and forms a contact zone, apparent as a darker circular area of homogeneous brightness, typically 100 μm2 in size (Fig. 1_a_). Similar adhesion patches have already been observed with biotinylated vesicles spreading on streptavidin-functionalized solid substrates (10) and with positively charged vesicles adhering on a negatively charged lipid monolayer (12). A few seconds after spreading (typically 4–5 s), the contact zone is no longer laterally homogeneous and symmetric. On one side, the adhesion zone becomes darker, and on the opposite side, the contact zone spreads, forming a sort of lamellipodia (Fig. 1_b_). The darker area indicates that the distance between the vesicle and the supported lipid membrane is smaller in this zone. In all cases, the vesicle begins to move linearly in the direction opposite to the location of the dark area (Fig. 1_c_) over a few hundred micrometers with a velocity of a few micrometers per second. During this motion, the dark area remains at the rear of the adhesion zone, and the total size of the adhesion patch increases by >2-fold. Moreover, a remarkable network of tethers forms behind the moving vesicle (Fig. 1 d and e), resulting in a particular adhesion pattern (“comet shape”) (Fig. 2aii). We note that neither the vesicle movement nor the unusual shape of the adhesion pattern has been observed previously on solid substrates or on lipid monolayers in the conditions of ref. 12. Typically, 30 s after the beginning of the motion, the vesicle stops. Two apparently contradictory phenomena are then observed within the following 30 s (Fig. 1 e and f): (i) the total size of the adhesion patch decreases, and (ii) the size of the darker zone increases, indicating a tighter adhesion between the membranes, until it covers the entire adhesion area (Fig. 1f). Eventually, light patches grow inside the dark adhesion zone over a time of typically 5 min. These patches, termed “blisters,” have been reported by Nardi et al. (12). Adhesion patterns with an isotropic distribution of tethers (“sun shape”) are occasionally observed (Fig. 2ai). Interestingly, in some cases we could observe vesicle division induced by the motion of two parts of the vesicle in opposite directions (see Movie 2, which is published as supporting information on the PNAS web site).
Fig. 1.
RICM observation of the adhesion dynamics of a negatively charged vesicle (10% DOPS, wt/wt) on a positively charged bilayer (10% DOTAP, wt/wt). (a) t = 0. The vesicle adheres and spreads in <1 s. (b) t = 5 s. The contact zone becomes inhomogeneous (a darker zone appears) and deformed. (c) t = 10 s. The vesicle begins to move straight in the opposite direction of the darker zone. (d) t = 20 s. The contact zone grows during vesicle motion (from c) to d, and many tethers are left behind the vesicle. (e) t = 30 s. After moving over a few hundred micrometers, the vesicle stops. (f) t = 40 s. The contact zone becomes homogeneously dark and decreases in area. (g) t = 300 s. Counterion blisters appear in the contact zone. (Scale bar: 10 μm.)
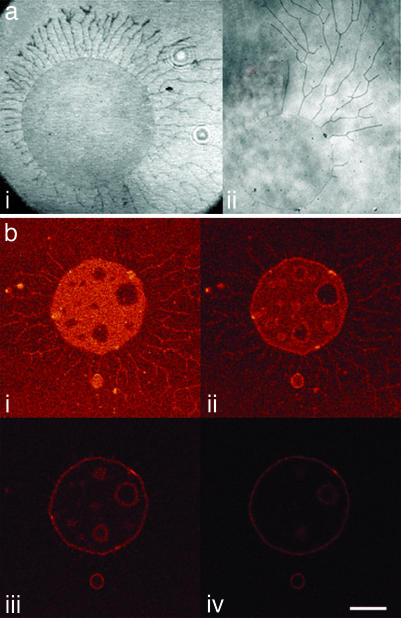
Fig. 2.
Long-time adhesion patterns of GUV. (a) Typical adhesion patterns observed by RICM. (i) Sun-shape pattern with tethers distributed isotropically around the contact zone of the vesicle. (ii) Comet-shape pattern with anisotropically distributed tethers. (b) Confocal microscopy images of an initially non fluorescent negatively charged vesicle (10% DOPS) adhering on a fluorescent {1% 2-(12-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino)dodecanoyl-1-hexadecanoyl-_sn_-glycero-3-phosphocholine (_C_12-NBD-PC)} positively charged SLB (10% DOTAP). The image’s sections are oriented parallel to the SLB with the focus being 0, 200, 400, and 600 nm above the SLB. (i) The contact zone is more fluorescent than the SLB, indicating a lipid transfer between the SLB and the GUV. We can also observe the presence of many tethers on the bilayer and of inhomogeneities in the contact zone corresponding to ion blisters. (ii_–_iv) The vesicle is completely fluorescent. (Scale bar: 10 μm.)
The above-described adhesion dynamics were found to be very sensitive to the charge density in both membranes. When the DOTAP fraction in the supported bilayer is <10%, giant vesicles containing the same fraction of negatively charged lipids do not adhere. This effect is probably due to a dissymmetry in the positive charge distribution in the two leaflets of the supported bilayer induced by the negative charges of the glass coverslip (27), leading to a depletion of positive charges in the upper leaflet and then to a strong reduction or suppression of the attractive force between the GUV and the SLB. In contrast, if the DOTAP concentration in the bilayer is >15%, no motion is observed, but the vesicle adheres strongly, as revealed by the presence of a dark adhesion zone and blisters appearing after a few minutes (see Movie 3, which is published as supporting information on the PNAS web site). As described, when the concentrations of negative and positive lipids are ≈10% in the GUV and SLB, respectively, adhesion and motion can be observed.
Lipid Transfer Between the Two Bilayers.
By using confocal microscopy, we have recorded the fluorescence in different sections parallel to the substrate, as illustrated in Fig. 2b. An originally nonfluorescent vesicle was brought in contact with a fluorescently labeled SLB and imaged 15 min after spreading. Fig. 2bi corresponds to a section at the level of the bilayer; the other images represent sections separated by 200 nm each and located above the first one. The presence of fluorescence in the vesicle membrane and in the surrounding tethers provides evidence for a significant exchange of fluorescent lipids between the vesicle and the bilayer. A quantitative fluorescence intensity analysis in Fig. 2bi yields a ratio of 1.6 ± 0.2 between the fluorescence intensity at the contact zone and the fluorescence intensity of the bilayer. This result suggests that the lipid transfer occurs only between the bilayer and the outer leaflet of the vesicle membrane, because the translocation of fluorescent lipids into the inner leaflet of the vesicle would result in a ratio of 2. This finding also eliminates the possibility of a hemifusion process with a stable circular diaphragm consisting of two monolayers in the contact zone, because this scenario would result in a fluorescence ratio of 0.5 or 1 (28). The asymmetric fluorescence distribution cannot be relaxed over the short time of the experiments, because flip-flop is a very slow process for phospholipids (29). A similar lipid transfer has already been reported between negatively charged vesicles and a positively charged monolayer (30), as well as between SLBs and oppositely charged SLB-coated beads (31). We can also notice the presence of blisters in the contact zone, as observed by RICM after a similar period. The observation of the lateral distribution of the fluorescence on a giant vesicle originally nonfluorescent during its motion indicates that the lipid transfer is not homogeneous on the whole contact zone (see Movie 4, which is published as supporting information on the PNAS web site). In this movie, the rear of the vesicle appears more fluorescent than the front, indicating that the transfer occurs mainly at the rear of the vesicle, i.e., at the zones of tight contact as revealed by the dark areas in the RICM images.
Discussion
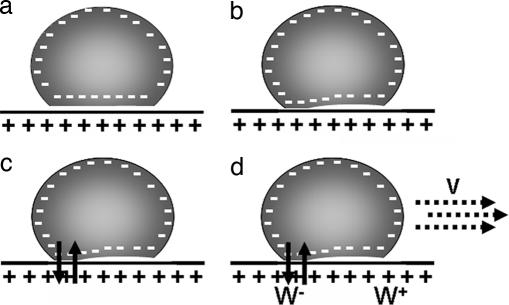
Previous studies have shown that the spontaneous motion of a liquid droplet can be induced when a chemical reaction at the interface between the droplet and the substrate modifies the surface energy of the solid substrate locally, thereby creating a hydrophobicity gradient. It was the case for the “running droplet” observed by Dos Santos and Ondarçuhu (19). A silane-containing alkane droplet was pushed on an initially hydrophilic substrate, inducing a symmetry breaking (32). A self-sustained movement was then induced by the grafting of the hydrophobic silane moieties to the substrate. Alternatively, a haptotactic mechanism due to an adhesion gradient can also produce movement (15–17). We propose that the spontaneous motion of a charged giant vesicle on an oppositely charged supported bilayer described in this work is a combination of these two mechanisms. The adhesion gradient is in this case self-generated by a local transfer of charged lipids between the vesicle and the SLB, which modulates the electrostatic interaction, and thus the adhesion, and leads to vesicle motion as described for haptotaxis. The details of the proposed mechanism are shown in Fig. 3. First, the vesicle adheres on the bilayer (Fig. 3a), and the distance between the two membranes is reduced. Thermal fluctuations may establish a locally restricted zone of close contact between the two bilayers (Fig. 3b), allowing the transfer of lipids, including charged species (Fig. 3c), and thereby locally reducing the membrane charge. This process leads to a local decrease of the adhesion energy and to the apparition of an adhesion gradient between this part of the vesicle (rear) and the opposite part (front), which initiates the motion (Fig. 3d) in the direction of the front part. As the vesicle moves, its front is exposed to a high adhesion energy, whereas the energy at the rear is continuously reduced by the transfer of charges. This scenario is fully consistent with the fluorescence experiments described in Materials and Methods.
Fig. 3.
Mechanism proposed to explain the vesicle motility. (a) The vesicle first adheres on the positively charged bilayer. (b) Thermal fluctuations bring the two membranes locally into close contact. (c) Lipid transfer (represented by arrows) and a partial neutralization of the vesicle charge follows. (d) This local neutralization induces a charge gradient between the rear and the back of the vesicle and an adhesion gradient W+ − _W_−, which induces a motion at a velocity V in the direction of the gradient.
We develop a simple scaling model to describe this self-induced vesicle motion. The problem we describe here differs from haptotaxis, because the adhesion gradient is self-created by the vesicle and not fixed externally.
The adhesion energy of two oppositely charged surfaces in the absence of salt is generally given by
where Γ represents the charge density of the less-charged surface (33). This adhesion energy originates from the entropy gain due to the liberation of counterions originally trapped between the two surfaces. The charge density is directly correlated to the number of charged lipids in the membrane. The lipid transfer therefore diminishes the charge density Γ during the neutralization process. Because the exchange rate between the two surfaces is most likely proportional to the charge density, the variation of charge density in the vesicle bilayer is given by
where τ is the characteristic exchange time between the two membranes.
The velocity V of the vesicle on the surface depends on the difference in adhesion energy Δ_W_ = W+ − _W_− between the front and the rear of the vesicle (Fig. 3d). In a stationary regime, an equilibrium between the force due to the adhesion gradient and the friction force should be reached. Assuming that the vesicle is rolling, the balance of forces can be written as
where Rv is the vesicle radius, and R is the contact zone radius (see Fig. 8, which is published as supporting information on the PNAS web site). α is a numerical coefficient (α ≈ 10) including the friction on the liquid wedge at the contact line (34) and the friction between the rolling vesicle and the surrounding fluid (35). Δ_W_ is directly related to the charge density difference ΔΓ = Γ+ − Γ− between the front and the rear, Δ_W_ = ΔΓ_kT_. To estimate ΔΓ, we write the simple rate Eq. 2. If the transit time t = R/V is much smaller than τ, Eq. 2 leads to ΔΓ = (Γ/τ)·(R/V). Finally, from the last equations, we deduce the vesicle velocity V, which can be written as the product of a mechanical component related to the adhesion, Vm = (Γ_kT_)/(3παη), and a chemical component reflecting the lipid transfer, _V_χ = _R_2/_Rv_τ
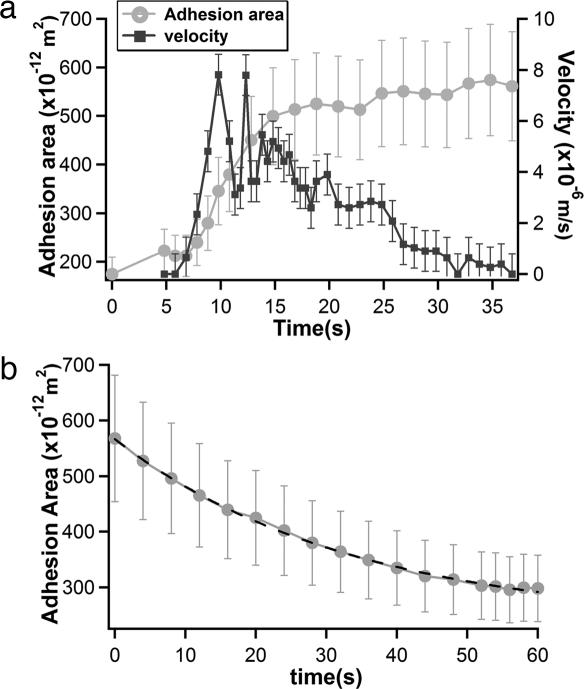
Therefore, the vesicle velocity depends directly on the adhesion energy and the characteristic time τ of the exchange of lipids. We have measured the evolution of the velocity of the vesicle and of the area of the contact zone during the motion (Fig. 4a). When the vesicle starts moving, we observe a strong increase in the velocity up to 5 μm/s in ≈5 s, concomitant with an increase of the contact zone area corresponding to its spreading. The velocity saturates during the following 10 s and then decreases, apparently linearly, until a final stop after 30 s. During the phase of decreasing velocity, the size of the contact zone is nearly constant. Once the vesicle is not moving anymore, we observe a continuous decrease of the contact zone (Fig. 4b). We expect from Eq. 2 that the adhesion energy decreases exponentially with time during the neutralization process; a similar behavior is expected for the contact area A. From the fit in Fig. 4b, we estimate the characteristic time τ for the lipid transfer to be of the order of 30 s. Note that the mean velocity of vesicles during the motion period ranges between 1 and 5 μm/s (see Fig. 9, which is published as supporting information on the PNAS web site); this experiment is thus representative of series of experiments.
Fig. 4.
Time variation of the contact area and the GUV velocity. (a) Evolution of velocity and adhesion area of the giant vesicle during its motion. (b) Decrease of the adhesion area after arrest of the vesicle (the new origin of time corresponds to t = 35 s in a). An exponential decay with a characteristic time τ = 29 s is measured using A(t) = _A_exp[−(t/τ)] + _A_0, with A = 330.3 × 10−12 m−2 and _A_0 = 236.3 × 10−12 m−2 (dotted line).
Taking a vesicle velocity of 5 μm/s and a transfer rate of τ = 30s, Eq. 4 provides an estimate of the adhesion energy of W ≈ 10−5 J/m2; this value corresponds to a driving force (Eq. 3) of ≈300 pN, interestingly in the same order of magnitude range as the force generated by actin network polymerization (36). Note that this value is relatively high, particularly as compared with the adhesion energy of surfaces coated with complementary ligand–receptor pairs, typically ranging between 10−7 and 10−6 J/m2 (37). This adhesion energy W would correspond to a charge density of Γ = 2 × 1017 m−2, thus an effective charged lipid fraction of the order of 0.1%. This value is 100-fold lower than the actual density of charged lipids in our membranes. This very large discrepancy arises because all counterions from the contact zone are actually not released. Indeed, we observe the apparition of characteristic blisters between the two membranes a few minutes after arrest of the vesicle’s motion (Fig. 1g), in good agreement with a reduced adhesion energy and the hypothesis of trapped counterions during the motion. The absence of tight adhesion between the two membranes can be seen in Fig. 1 during vesicle motion, because the adhesion zone is not dark at the front of the vesicle. During motion, the redistribution of ions is hindered; they remain confined between the membranes, and the vesicle is literally “surfing” on a counterion layer. The kinetic trapping of counterions is favored by the planar geometry of the supported bilayer and the large size of the GUV. It would not be relevant for a GUV interacting with small particles or small vesicles of opposite charge, because counterions could escape more easily from the contact zone in these cases. A similar important lowering of the adhesion energy due to counterion blisters in the adhesion zone has already been shown by Nardi et al. (12) in experiments described above. Unfortunately, no direct measurement of the adhesion energy between two planar surfaces with trapped counterions, by using surface force apparatus for instance, is available so far.
The different adhesion patterns that we observe could be related to variations in the position of the nucleation site for lipid transfer. When the transfer is initiated close to the center of the contact zone, the charge gradient is isotropic, the vesicle remains immobile, and the tethers are isotropically pulled during the retraction period associated with charge neutralization (sun shape). However, when the nucleation occurs near the edge, the symmetry is broken, and the vesicle is polarized, which in turn gives rise to a net translation (comet shape). In particular for larger GUVs, >100 μm in diameter, several nucleation sites are formed simultaneously, leading to opposite motion of two parts of the vesicle and vesicle division (Movie 2).
Even if the mechanism of vesicle motion that we suggest here is very different from cell motility on a stiff substrate, some similarities exist, such as the increase of the contact zone during motion as well as the formation of multiple tethers pulled behind. Such trails have been observed behind many motile cells, including neutrophils, or during cell division (38). For cells, the trails are not only composed of membrane but also contain some actin (39). For neutrophils, these tethers even have antibacterial properties (40). Tether formation from cells or vesicles has already been widely investigated, both experimentally and theoretically (see, for instance, refs. 41–43). In most cases, a mechanical point force is applied to a membrane, and a tether is pulled out. If the membrane is attached to a surface by a small adhesion patch, tube extrusion can be obtained when a flow is applied to the system (44). The diameter of the zone over which the force is applied is a crucial parameter, because it determines directly the force overshoot necessary to form a tube (45). If the adhesion patch is too large, the pulling system may not be able to extract a tube, although the force to extend the tube, once formed, is perfectly accessible. In the present case, the tether extrusion force is provided by the vesicle itself: The membrane is locally pinned on the surface defects (bumps or holes), and the driving force acting on the vesicle induces the formation of tubes. Each tube exerts a resistance force of the order 10–30 pN (43). Moreover, the presence of either a few large defects or many small defects should explain the absence of vesicle motion observed in some cases. Our observations are in qualitative agreement with the present understanding of membrane tubes. Unfortunately, the system appears too complex, and the relevant parameters (membrane tension and density of membrane defects, for instance) not well controlled enough to perform a complete analysis. Nevertheless, we can notice that the reduction of vesicle velocity correlates with an increased number of extracted tubes. We can estimate that typically ≈10 tubes are sufficient to stop the motion. It also may explain why no motion is observed in the strong adhesion regime corresponding to charge densities >15%, which leads to high membrane tension and thus larger forces for tube extrusion.
In summary, we have shown that a simple nonspecific adhesion of oppositely charged membranes can induce a new form of motion of a giant vesicle. This system presents a spontaneous symmetry breaking due to a local lipid transfer between the two membranes. This lipid transfer induces a charge gradient at the level of the contact zone, which is at the origin of the motion. Interestingly, no external energy is required, because the system self-induces the movement. With a scaling model, we have succeeded in directly relating the velocity of the vesicle to the characteristic time for intermembrane lipid transfer. Our measurements lead to an adhesion energy of order 10−5 J/m2, which is 2 orders of magnitude smaller than an estimation considering that all charged lipids contribute to the adhesion. The difference is accounted for by kinetic trapping of counterions during motion, as also observed by Nardi et al. (12). The moving vesicle deposits behind a trail of lipid nanotubes. They are responsible of the stop of the motion of the running vesicles after few hundred micrometers.
Materials and Methods
Reagents.
DOPC, DOPS, and DOTAP were purchased from Avanti Polar Lipids (Alabaster, AL), and 2-(12-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino)dodecanoyl-1-hexadecanoyl-_sn_-glycero-3-phosphocholine (C12-HPC-NBD) was purchased from Molecular Probes (Eugene, OR). All chemicals were purchased from Sigma-Aldrich (St. Louis, MO).
Coverslips used were from Erie Scientific Co. (Portsmouth, NH; No. 1 quality, 0.13–0.16 mm, 40 × 60 mm) and Fisher Scientific International (Hampton, NH; No. 1 quality, 0.13–0.17 mm, 25 × 25 mm).
GUVs.
GUVs were grown in a 170-mOsM sucrose solution by using the electroformation technique (46). They were prepared by using different molar fractions of DOPC and DOPS of 95:5, 90:10, and 85:15, respectively.
Observation Chambers.
The chambers (20 × 10 × 1 mm) were made of two coverslips, which were first cleaned with a mixture of H2SO4/H2O2 (ratio 70:30), rinsed with distilled water, and conserved in methanol before use. The chamber was built by intercalating 1 mm of melted parafilm between the coverslips.
SLBs.
The SLBs were formed by the spreading of SUVs on the coverslip (47). For preparation of the SUVs, DOPC and DOTAP were mixed in chloroform in adequate lipid fractions and supplemented by 1% fluorescent 2-(12-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino)dodecanoyl-1-hexadecanoyl-_sn_-glycero-3-phosphocholine (C12-NBD-HPC). The chloroform was evaporated, and the lipids were resuspended in distilled water at a concentration of 1 mg/ml by a few minutes of moderate vortexing. The lipid dispersion was then probe-sonicated for a few minutes until the solution became transparent. One volume of this SUV solution was diluted five times with a solution of 5 mM Tris/50 mM NaCl (pH 8) and incubated for 10 min in the observation chamber, in order for the fluorescent supported lipid membrane to be formed on the surfaces. The chamber was rinsed with 10 ml of distilled water and 10 ml of a glucose solution with the same osmolarity as the sucrose solution used for GUV formation.
Imaging the GUVs and Supported Bilayers.
RICM and epifluorescence have been performed on an Axiovert 200 microscope (Zeiss, Oberkochen, Germany) equipped with interference and fluorescence filters and a 63× Antiflex objective.
A Zeiss LSM 510 Meta inverted confocal microscope was used for imaging GUVs on bilayers containing 1% 2-(12-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino)dodecanoyl-1-hexadecanoyl-_sn_-glycero-3-phosphocholine (C12-NBD-PC) lipids. The microscope was equipped with an argon ion laser working at 488 nm.
Atomic force microscopy measurements were performed on a Nanoscope VI-MultiMode (Veeco, Dourdan, France), equipped with a J-scanner (120 μm) and silicon nitride cantilevers with a nominal spring constant of 0.06 N/m (Digital Instruments, Santa Barbara, CA) and coated with poly(l-lysine)-_g_-poly(ethylene glycol) (PLL-_g_-PEG). Details of the imaging procedure can be found elsewhere (48).
Diffusion Coefficient of the Lipids in SLBs.
We used the confocal inverted microscope (Zeiss LSM 510 Meta) to perform FRAP experiments on the SLBs. The full power of the argon laser was 20 mW, corresponding to 10 mW for the 488-nm line. For observation, the laser intensity was reduced to 0.1–1.0%, whereas during bleaching 100% laser intensity was used. We analyzed the FRAP of a 464-μm diameter disk bleached for 380 ms. The diffusion constant of the fluorescent lipids in the membrane of a GUV was obtained from the fit of the fluorescence recovery curve as described in Supporting Text.
Supplementary Material
Supporting Information
Acknowledgments
We thank P. Sens, J.-F. Joanny, S. Cribier, K. Sengupta, and H. Aranda-Espinoza for illuminating discussions; M. Goldmann and M. C. Fauré for the Langmuir experiments, which initiated this work; A. Brisson for the collaboration on the atomic force microscopy experiments; and the microscopy platform team of the Curie Institute for valuable technical support.
Glossary
Abbreviations
DOPC
1,2-dioleoyl-_sn_-glycero-3-phosphocholine
DOPS
1,2-dioleoyl-_sn_-glycero-3-phosphatidylserine
DOTAP
di-oleoyl-3-trimethylammonium-propane
FRAP
fluorescence recovery after photobleaching
GUV
giant unilamellar vesicle
RICM
reflection interference contrast microscopy
SLB
supported lipid bilayer
SUV
small unilamellar vesicle.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Zaidel-Bar R., Cohen M., Addadi L., Geiger B. Biochem. Soc. Trans. 2004;32:416–420. doi: 10.1042/BST0320416. [DOI] [PubMed] [Google Scholar]
- 2.Mooney D. J., Langer R., Ingber D. E. J. Cell Sci. 1995;108:2311–2320. doi: 10.1242/jcs.108.6.2311. [DOI] [PubMed] [Google Scholar]
- 3.Engler A., Bacakova L., Newman C., Hategan A., Griffin M., Discher D. Biophys. J. 2004;86:617–628. doi: 10.1016/S0006-3495(04)74140-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang N., Ostuni E., Whitesides G. M., Ingber D. E. Cell Motil. Cytoskeleton. 2002;52:97–106. doi: 10.1002/cm.10037. [DOI] [PubMed] [Google Scholar]
- 5.Arnold M., Cavalcanti-Adam E. A., Glass R., Blummel J., Eck W., Kantlehner M., Kessler H., Spatz J. P. ChemPhysChem. 2004;5:383–388. doi: 10.1002/cphc.200301014. [DOI] [PubMed] [Google Scholar]
- 6.Tan J. L., Tien J., Pirone D. M., Gray D. S., Bhadriraju K., Chen C. S. Proc. Natl. Acad. Sci. USA. 2003;100:1484–1489. doi: 10.1073/pnas.0235407100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Delanoe-Ayari H., Al Kurdi R., Vallade M., Gulino-Debrac D., Riveline D. Proc. Natl. Acad. Sci. USA. 2004;101:2229–2234. doi: 10.1073/pnas.0304297101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bershadsky A. D., Balaban N. Q., Geiger B. Annu. Rev. Cell Dev. Biol. 2003;19:677–695. doi: 10.1146/annurev.cellbio.19.111301.153011. [DOI] [PubMed] [Google Scholar]
- 9.Boulbitch A., Guttenberg Z., Sackmann E. Biophys. J. 2001;81:2743–2751. doi: 10.1016/S0006-3495(01)75917-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cuvelier D., Nassoy P. Phys. Rev. Lett. 2004;93:228101–228104. doi: 10.1103/PhysRevLett.93.228101. [DOI] [PubMed] [Google Scholar]
- 11.Bernard A.-L., Guedeau-Boudeville M.-A., Jullien L., Di Meglio J.-M. Langmuir. 2000;16:6809–6820. [Google Scholar]
- 12.Nardi J., Bruinsma R., Sackmann E. Phys. Rev. E Stat. Phys. Plasmas Fluids Relat. Interdiscip. Top. 1998;58:6340–6354. doi: 10.1103/physreve.61.4253. [DOI] [PubMed] [Google Scholar]
- 13.Filippov A., Oradd G., Lindblom G. Biophys. J. 2003;84:3079–3086. doi: 10.1016/S0006-3495(03)70033-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hategan A., Sengupta K., Kahn S., Sackmann E., Discher D. E. Biophys. J. 2004;87:3547–3560. doi: 10.1529/biophysj.104.041475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carter S. B. Nature. 1967;213:256–260. doi: 10.1038/213256a0. [DOI] [PubMed] [Google Scholar]
- 16.Cantat I., Misbah C., Saito Y. Eur. Phys. J. E. 2000;3:403–412. [Google Scholar]
- 17.Tordeux C., Fournier J. B., Galatola P. Phys. Rev. E Stat. Phys. Plasmas Fluids Relat. Interdiscip. Top. 2002;65:041912. doi: 10.1103/PhysRevE.65.041912. [DOI] [PubMed] [Google Scholar]
- 18.Brochard-Wyart F., de Gennes P. G. C. R. Acad. Sci. Paris IIb. 1995;321:285–288. [Google Scholar]
- 19.Dos Santos F. D., Ondarcuhu T. Phys. Rev. Lett. 1995;75:2972–2975. doi: 10.1103/PhysRevLett.75.2972. [DOI] [PubMed] [Google Scholar]
- 20.Sumino Y., Magome N., Hamada T., Yoshikawa K. Phys. Rev. Lett. 2005;94:068301. doi: 10.1103/PhysRevLett.94.068301. [DOI] [PubMed] [Google Scholar]
- 21.Berna J., Leigh D. A., Lubomska M., Mendoza S. M., Perez E. M., Rudolf P., Teobaldi G., Zerbetto F. Nat. Mater. 2005;4:704–710. doi: 10.1038/nmat1455. [DOI] [PubMed] [Google Scholar]
- 22.Richter R., Brisson A. Langmuir. 2003;19:1632–1640. [Google Scholar]
- 23.Richter R., Mukhopadhyay A., Brisson A. Biophys. J. 2003;85:3035–3047. doi: 10.1016/S0006-3495(03)74722-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ladha S., Mackie A. R., Harvey L. J., Clark D. C., Lea E. J., Brullemans M., Duclohier H. Biophys. J. 1996;71:1364–1373. doi: 10.1016/S0006-3495(96)79339-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmitt J., Danner B., Bayerl T. M. Langmuir. 2001;17:244–246. [Google Scholar]
- 26.Rädler J., Feder T. J., Strey H. H., Sackmann E. Phys. Rev. E Stat. Phys. Plasmas Fluids Relat. Interdiscip. Top. 1995;51:4526–4536. doi: 10.1103/physreve.51.4526. [DOI] [PubMed] [Google Scholar]
- 27.Kasbauer M., Junglas M., Bayerl T. M. Biophys. J. 1999;76:2600–2605. doi: 10.1016/S0006-3495(99)77412-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kozlovsky Y., Chernomordik L. V., Kozlov M. M. Biophys. J. 2002;83:2634–2651. doi: 10.1016/S0006-3495(02)75274-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lipowsky R., Sackmann E. Structure and Dynamics of Membranes. Amsterdam: Elsevier North–Holland; 1995. [Google Scholar]
- 30.Marchi-Artzner V., Lehn J. M., Kunitake T. Langmuir. 1998;14:6470–6478. [Google Scholar]
- 31.Sapuri A. R., Baksh M. M., Groves J. T. Langmuir. 2003;19:1606–1610. [Google Scholar]
- 32.de Gennes P. G., Brochard-Wyart F., Quéré D. Drops, Bubbles, Pearls, and Waves. New York: Springer; 2004. [Google Scholar]
- 33.Parsegian A., Gingell D. Biophys. J. 1972;12:1192–1204. doi: 10.1016/S0006-3495(72)86155-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brochard-Wyart F. Langmuir. 1989;5:432–438. [Google Scholar]
- 35.Abkarian M., Lartigue C., Viallat A. Phys. Rev. E Stat. Phys. Plasmas Fluids Relat. Interdiscip. Top. 2001;63:041906. doi: 10.1103/PhysRevE.63.041906. [DOI] [PubMed] [Google Scholar]
- 36.Giardini P. A., Fletcher D. A., Theriot J. A. Proc. Natl. Acad. Sci. USA. 2003;100:6493–6498. doi: 10.1073/pnas.1031670100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seifert U. Adv. Phys. 1997;46:13–137. [Google Scholar]
- 38.Thery M., Racine V., Pepin A., Piel M., Chen Y., Sibarita J. B., Bornens M. Nat. Cell Biol. 2005;7:947–953. doi: 10.1038/ncb1307. [DOI] [PubMed] [Google Scholar]
- 39.Fuhr G., Richter E., Zimmermann H., Hitzler H., Niehus H., Hagedorn R. Biol. Chem. 1998;379:1161–1173. doi: 10.1515/bchm.1998.379.8-9.1161. [DOI] [PubMed] [Google Scholar]
- 40.Brinkmann V., Reichard U., Goosmann C., Fauler B., Uhlemann Y., Weiss D. S., Weinrauch Y., Zychlinsky A. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 41.Dai J., Sheetz M. P. Biophys. J. 1995;68:988–996. doi: 10.1016/S0006-3495(95)80274-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cuvelier D., Chiaruttini N., Bassereau P., Nassoy P. Europhys. Lett. 2005;71:1015–1021. [Google Scholar]
- 43.Derényi I., Jülicher F., Prost J. Phys. Rev. Lett. 2002;88:238101. doi: 10.1103/PhysRevLett.88.238101. [DOI] [PubMed] [Google Scholar]
- 44.Rossier O., Cuvelier D., Borghi N., Puech P. H., Derényi I., Buguin A., Nassoy P., Brochard-Wyart F. Langmuir. 2003;19:575–584. [Google Scholar]
- 45.Koster G., Cacciuto A., Derenyi I., Frenkel D., Dogterom M. Phys. Rev. Lett. 2005;94:068101. doi: 10.1103/PhysRevLett.94.068101. [DOI] [PubMed] [Google Scholar]
- 46.Mathivet L., Cribier S., Devaux P. F. Biophys. J. 1996;70:1112–1121. doi: 10.1016/S0006-3495(96)79693-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tamm L. K., McConnell H. M. Biophys. J. 1985;47:105–113. doi: 10.1016/S0006-3495(85)83882-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Richter R. P., Brisson A. R. Biophys. J. 2005;88:3422–3433. doi: 10.1529/biophysj.104.053728. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information