Astrocytes and NG2-glia: what's in a name? (original) (raw)
Abstract
Classic studies recognize two functionally segregated macroglial cell types in the central nervous system (CNS), namely astrocytes and oligodendrocytes. A third macroglial cell type has now been identified by its specific expression of the NG2 chondroitin sulphate proteoglycan (NG2-glia). These NG2-glia exist abundantly in both grey and white matter of the mature CNS and are almost as numerous as astrocytes. It is well established that NG2-glia give rise to oligodendrocytes. However, the majority of NG2-glia in the adult CNS proliferate very slowly and are non-motile. Both astrocytes and NG2-glia display a stellate morphology and express ion channels and receptors to neurotransmitters used by neurons. Both types of glia make intimate contacts with neurons in grey and white matter, and their functional differences and similarities are only beginning to be unravelled. Recent observations emphasize the need to examine the relationship between astrocytes and NG2-glia, and address the question of whether they represent overlapping or two distinct glial cell populations. To be of any relevance, this classification must relate to specific functions in the neural network. At present, the balance of evidence is that NG2-glia and astrocytes are functionally segregated populations.
Keywords: astrocytes, glutamate receptor, glutamate transporter, NG2, oligodendrocytes
‘What's in a name? That which we call a rose By any other name would smell as sweet.
’ William Shakespeare, Romeo and Juliet
Introduction
The mammalian central nervous system (CNS) consists of neurons, which make up 10% of the cellular elements, and glia, which make up the remaining 90%. The name glia is derived from Nervenkitt or nerve glue/putty, as originally proposed by Rudolf Virchow (1856; reviewed in Kettenmann & Ransom, 2005). Unfortunately, this name has perpetuated the misconception that glia are inert cells, which merely provide support or serve as the putty to hold neurons together. A window with 90% putty and only 10% glass would not be very illuminating, but this restricted vision of the neural network is gradually changing. The first indication that glia are not merely passive cells came from studies of the amphibian optic nerve, which showed depolarization of glial membranes in response to neuronal activity (Orkand et al. 1966). Glia are now recognized to perform wide-ranging functions which are essential for neuronal function and viability, including myelination, structural support, extracellular potassium ([K+]o) regulation, neurotransmitter removal, glial scar formation and induction of blood–brain barrier function in endothelial cells. In addition, there is now considerable evidence that glia express many of the same ion channels and neurotransmitter receptors as neurons, signal to each other via propagative intercellular calcium waves and can themselves release neurotransmitters, thus contributing dynamically to the neural network (Haydon, 2001; Lin & Bergles, 2002).
Since their discovery and description by Ramon y Cajal (1913) and later by del Rio-Hortega (1928) and Penfield (1932), astrocytes and oligodendrocytes have been recognized as the two major subtypes of glia in the CNS, and as having entirely segregated functions. Oligodendrocytes exclusively form the myelin, which ensheaths CNS axons and facilitates rapid saltatory conduction, whereas astrocytes are linked to the diverse ‘glial’ functions listed above. However, the functional distinctions between oligodendrocyte and astrocyte lineage cells have become blurred since the discovery of NG2-expressing glia (NG2-glia). There is a large body of evidence that NG2-glia generate myelinating oligodendrocytes (Horner et al. 2000; Nishiyama et al. 2002; Reynolds et al. 2002), but NG2-glia persist in large numbers long after oligodendrocytes are generated. This raises the possibility that NG2-glia carry out some of the functions previously attributed generally to ‘glia’ or ‘astrocytes’. Both astrocytes and NG2-glia have a stellate morphology, are widely distributed throughout the grey and white matter of the mature CNS, and extend their processes to nodes of Ranvier and synapses where they interact with neurons (Butt et al. 2002; Lin & Bergles, 2004). The existence of two glial cell populations with similar morphology and distribution has prompted a need to re-evaluate their relationship in light of our current knowledge of the different glial populations. In the present review, we focus on the question of whether astrocytes and NG2-glia represent two distinct or overlapping glial cell populations.
Astrocytes and NG2-glia are antigenically discrete populations of glia
Astrocytes are currently defined as stellate cells that contain glial fibrillary acidic protein (GFAP), the molecular constituent of glial filaments (Eng et al. 2000; see also recent review on astrocyte identity by Kimelberg, 2004). However, the abundance of GFAP and glial filaments varies among astrocytes and can be altered by changes in the environment. For example, glial filaments and GFAP are much more abundant and readily detectable in fibrous astrocytes of white matter than in protoplasmic astrocytes of grey matter (Peters et al. 1991). GFAP is greatly increased when protoplasmic astrocytes in grey matter undergo reactive changes in response to neuronal damage, unmasking a nascent capacity for GFAP expression in cells that normally express low or undetectable levels of GFAP. The abundance of glial filaments also varies in different processes of single astrocytes. Astrocytic end-feet, which subserve blood vessels or the pial surface, have abundant glial filaments and are strongly immunopositive for GFAP, whereas the fine processes that surround synapses or nodes of Ranvier generally have fewer glial filaments and are immunonegative for GFAP. A single astrocyte can extend all of these processes (Butt et al. 1994). Thus, the abundance of glial filaments or GFAP is not uniform or constant but is dynamically regulated within astrocytes, and can be dramatically altered by changes in the environment. The identification of GFAP-poor cells as astrocytes relies on markers such as glutamine synthetase, an essential enzyme in the conversion of glutamate to glutamine, or the calcium-binding protein S-100β. Although the presence of glial filaments provides a good operational definition of an astrocyte, a limitation lies in the sensitivity of detection of these filaments at the light microscopic level by GFAP immunohistochemistry. A more desirable definition of astrocytes would be one that links them to a set of functional properties.
There is another abundant glial population in white and grey matter that can be identified by their specific expression of the integral membrane proteoglycan NG2. They comprise 8–9% of the total cell population in white matter, and 2–3% of total cells in grey matter (Dawson et al. 2003). Extensive studies from several investigators have shown that NG2 is never expressed on GFAP+ cells in vivo, even in the glial scar, where GFAP is dramatically up-regulated in astrocytes. Ultrastructurally, glial filaments have not been found in NG2-glia. Neither do NG2-glia express glutamine synthetase or S-100β, the two other antigens present in many astrocytes, including GFAP− protoplasmic astrocytes, although the distribution of the latter varies among reports. Thus, GFAP-expressing astrocytes and NG2-glia appear to be antigenically distinct cell populations.
Astrocytes and NG2-glia are both stellate but display differences in process arborization
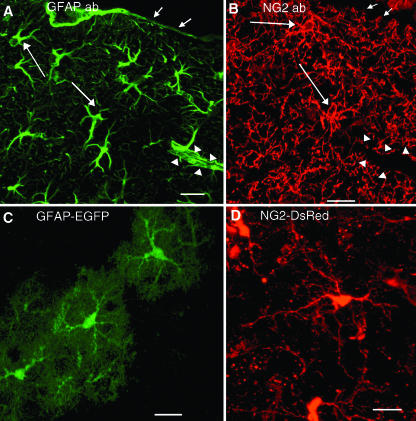
GFAP-immunolabelled astrocytes and NG2-glia in white and grey matter are both stellate, multiprocess-bearing cells and often appear very similar in shape (Fig. 1A,B). However, this merely reflects similarity in the pattern of antigen distribution, and, in the case of GFAP, this does not reflect the true morphology of the cells. Whereas NG2 is distributed uniformly along the cell surface and outlines the entire cell, the area labelled by GFAP antibodies represents only a small fraction of the astrocyte process arborization. The use of transgenic mice expressing diffusible cytosolic fluorescent proteins under the control of astrocyte or NG2 cell-active regulatory sequences has enabled visualization of the entire cell (Fig. 1C,D), similar to the picture depicted by dye-filling or Golgi staining. Astrocytes have asymmetrically radiating processes that consist of several primary thick processes, from which emanate multiple smaller collateral branching secondary processes, giving them a bushy appearance (Fig. 1C; Bushong et al. 2002; Grass et al. 2004). By contrast, NG2-glia have a centrally located soma, from which extend several long, slender primary processes, which bifurcate two or more times, but less extensively than astrocytes, to form a symmetrical process field (Fig. 1D). Astrocyte processes often end in bulbous swellings, or terminal end-feet, which form the vascular and pial glia limitans (Fig. 1A), whereas NG2-glial processes taper to an end and do not appear to contribute to the glia limitans (Fig. 1B; Butt et al. 1994, 2002). Although both NG2-glia and astrocytes extend processes to nodes of Ranvier in white matter (Butt et al. 1994, 1999) and synapses in grey matter, their geometric relationship to these neuronal elements is different. Thus, although astrocytes and NG2-glia bear a superficial resemblance, they are distinguished by their different process arborizations. This will reflect fundamental differences in the way these two glial cell populations interact with other elements in the neural network.
Fig. 1.
Detection of astrocytes and NG2-glia by different methods. (A,B) Confocal images of astrocytes (A) and NG2-glia (B) in the adult mouse cerebral cortex detected with antibodies to GFAP (A) and NG2 (B) showing multiprocessed morphology. Short arrows indicate the position of glia limitans and the pial surface. Arrowheads indicate a blood vessel. Long arrows indicate examples of positively stained cells. GFAP+ astrocytes extend end-feet onto the pial surface and blood vessels but NG2 cells do not. (C,D) Confocal images of astrocytes and NG2 cells in neocortex of adult transgenic mice showing differences in process arborization between astrocytes and NG2-glia. (C) EGFP fluorescence in a transgenic mouse in which EGFP is expressed in weakly GFAP+ protoplasmic astrocytes. (D) DsRed fluorescence in a transgenic mouse generated by injecting linearized BAC DNA containing the entire NG2 gene with DsRed inserted into the first exon. Scale bars, 20 µm.
Astrocytes and NG2-glia develop along different pathways
A number of observations indicate that NG2-glia and oligodendrocytes have a common lineage, separate from that of astrocytes. There is evidence that NG2-glia differentiate into oligodendrocytes in vitro and in vivo (Horner et al. 2000; Nishiyama et al. 2002; Dawson et al. 2003; Bu et al. 2004), indicating that at least some NG2-glia are indeed oligodendrocyte precursor cells (OPCs). During development, not all NG2+ cells give rise to oligodendrocytes but many remain as NG2-glia that persist into adulthood. When isolated, these NG2-glia behave as ‘O-2A progenitor’ cells, first identified by Raff et al. (1983). Although in vitro studies showed the bipotential ability of O-2A progenitor cells to differentiate into type 2 astrocytes and oligodendrocytes, there has been no demonstration of the ability of NG2-glia to differentiate into GFAP+ astrocytes in vivo, despite numerous transplant and lesioning experiments. Direct determination of whether NG2-glia in different brain regions differentiate exclusively into oligodendrocytes and not into astrocytes must await the outcome of studies using novel approaches such as temporally inducible cre-mediated reporter expression. Nonetheless, the available evidence indicates that astrocytes and NG2-glia belong to separate lineages, and a transition from one type of glia to the other has not been reported.
The majority of astrocytes and NG2-glia arise from different regions in the embryonic CNS. In the spinal cord and cerebral cortex, NG2-glia appear from ventral locations under the influence of sonic hedgehog (reviewed by Kessaris et al. 2001). By contrast, astrocytes develop from more dorsal regions, and their differentiation is influenced by a different set of growth factors, such as bone morphogenic proteins (reviewed in Ross et al. 2003). Although some astrocytes are transformed from radial glia following neurogenesis, there is little evidence for direct transformation of radial glia into oligodendrocyte lineage cells or NG2-glia (Malatesta et al. 2000). Developmentally, therefore, NG2-glia and astrocytes arise from different sources and develop along different pathways. However, the embryonic spinal cord may contain a ‘glia-restricted precursor’ population that can give rise to both astrocytes and oligodendrocyte-lineage cells (reviewed in Lee et al. 2000).
Astrocytes and NG2-glia are functionally segregated with respect to ion channels and glutamate responsiveness
In addition to their associations with other elements of the CNS, the function of astrocytes and NG2-glia will be defined by the receptors, transporters, ion channels and cell-surface molecules which determine how they interact with these elements. Electrophysiological studies on mouse hippocampal slices and acutely isolated rat hippocampal glial cells using whole-cell patch clamp revealed at least two clearly distinct populations of glial cells, with passive and complex current characteristics (Walz, 2000). Passive cells display a relatively hyperpolarized resting membrane potential, low input resistance and a linear I–V curve. By contrast, complex cells have characteristic outward rectifying potassium currents with little inward currents, have a resting membrane potential that is more depolarized than that of passive cells and havre a higher input resistance. Further studies have revealed differential response to glutamate in the two glial populations. Outwardly rectifying complex cells respond to glutamate in an AMPA receptor-dependent manner (R+) and do not exhibit glutamate transporter currents or possess GLT-1 or GLAST protein or mRNA (T−), whereas passive cells express glutamate transporters but not AMPA receptors (R−/T+) (Zhou & Kimelberg, 2001; Matthias et al. 2003). Immunocytochemical analysis showed differential expression of GFAP and NG2 in R−/T+ and R+/T− cells (Matthias et al. 2003; Schools et al. 2003; Grass et al. 2004). These observations suggest that passive cells are GFAP+ astrocytes and complex cells correspond to NG2-glia.
However, a number of observations are not entirely consistent with this notion of strictly segregated populations of NG2-glia and GFAP+ astrocytes. For example, transcription of GFAP mRNA seems to be active in both passive astrocytes and complex NG2-glia. Zhou et al. (2000) demonstrated by single-cell RT-PCR the existence of GFAP mRNA in both types of glial cells. More recently, transgenic mice expressing enhanced green fluorescent protein (EGFP) under the human GFAP promoter (Matthias et al. 2003; Grass et al. 2004) demonstrated weak expression of EGFP in a subpopulation of NG2+ cells with a complex cell pattern of currents and strong EGFP expression in more passive cells that were GFAP+/NG2−. Although NG2-glia have complex current patterns, there is not a good correlation with expression of NG2 and EGFP; only 38% of weakly EGFP+ cells were NG2+ in the hippocampus, and 63% of NG2-glia were weakly EGFP+ in the respiratory centres. As mentioned above, GFAP has never been detected in NG2-glia. It is not known at present whether the discrepancy between GFAP mRNA and protein expression is simply a reflection of higher sensitivity of mRNA detection, or whether it is due to a post-transcriptional mechanism that ultimately restricts GFAP synthesis to astrocytes or enhances GFAP stability in astrocytes. Caution is needed when interpreting reporter expression in transgenic mice created by using a randomly integrated transgene consisting of an isolated promoter/enhancer segment driving a reporter. The pattern of reporter expression in such mice varies widely from line to line depending on integration site and is often mosaic within a given line (see Feng et al. 2000). This becomes particularly problematic when a highly sensitive reporter is used, as a low level of leaky expression could erroneously lead to highly detectable reporter expression in cells with little or no endogenous mRNA expression. In this regard, targeted insertion of the reporter into the endogenous gene locus or the inclusion of the entire gene by techniques such as the BAC (bacterial artificial chromosome) transgenic approach is preferred (Nishiyama et al. 2004). Furthermore, synthesis of many mRNA species may rapidly change upon loss of normal cell–cell contacts, which is inevitable in acute cell dissociation. It is noteworthy that GFAP mRNA was not detected in NG2-expressing cells isolated by laser capture microdissection (Ye et al. 2003).
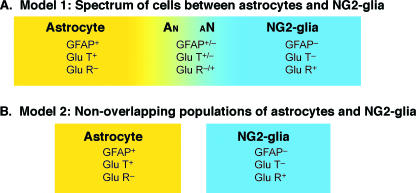
What seems to be a common finding from these studies is that there exist at least two clearly distinct glial cell types in the hippocampus: GFAP+/NG2− astrocytes with R−/T+ currents and GFAP−/NG2+ glia with R+/T− currents. The former corresponds to passive (linear) cells and the latter to complex cells. Whereas the role of the R−/T+ astrocytes may be to clear glutamate in the synaptic cleft released by neuronal activity, NG2+ R+/T− cells may be involved in an entirely different function at the synapse. It is not yet clear whether they represent two non-overlapping cell populations or two extreme ends of a spectrum of glial cells with continuous intermediate phenotypes (see Fig. 2 and next section). In contrast to the cells in hippocampus, R+/T+ cells are found in the brainstem respiratory centre, and the identity of these cells and that of some R+/T− cells that were EGFP+ in the GFAP promoter – EGFP transgenic mice need to be further evaluated.
Fig. 2.
The relationship between astrocytes and NG2-glia. Both models recognize two clearly distinct populations of cells: (1) astrocytes that contain GFAP and have glutamate transporters but not AMPA receptor functions (yellow) and (2) NG2-glia that do not contain GFAP or glutamate transporters but have AMPA receptors (blue). Model A includes an additional group of cells with a phenotype intermediate between astrocytes and NG2-glia. Model B represents two segregated glial cell populations with no overlap. Most currently available data support model B rather than A, but the nomenclature ‘astrocytes’ has been used broadly in some studies to refer to cells with the NG2-glial phenotype.
The relationship between astrocytes and NG2-glia
Before one can begin to discuss the relationship between astrocytes and NG2-glia, there must be an operational definition of an astrocyte. Here we will conform to the classical definition of the astrocyte as a cell that contains glial filaments. NG2-glia are defined simply as CNS glia that carry the NG2 proteoglycan at the cell surface. NG2-glia appear homogeneous with respect to marker expression (GFAP−/NG2+) and their response to glutamate (T−/R+). The functional identity of astrocytes is more complicated. There clearly exists an astrocyte population (GFAP+/NG2−; T+/R−) that is distinct from NG2-glia which are (GFAP−/NG2+ T−/R+). It is less clear whether there also exist cells with a phenotype intermediate between these two extremes. From the observations reviewed above, there are two possibilities to describe the relationship between astrocytes and NG2-glia (Fig. 2). In the first model, astrocytes and NG2-glia represent two ends of a spectrum of cells with a range of intermediate phenotypes (Fig. 2A). In the second model, astrocytes and NG2-glia represent two distinct, non-overlapping glial populations, which together with other specialized glia such as Bergmann glia constitute the non-oligodendrocyte macroglial population (Fig. 2B).
The first model includes the existence of a cell population that shares some properties of astrocytes and NG2-glia (designated as An or aN cells in Fig. 2A, depending on the relative contribution of astrocytic or NG2-glial properties). In this case, these cells would presumably arise from a common progenitor, and the pure NG2-glial population would be the cell type that can potentially differentiate into oligodendrocytes. This model can be used to explain the presence of a low level of GFAP mRNA among some GFAP−/NG2+ cells and the observation that some GFAP+/NG2− cells in the brainstem express both AMPA receptors and glutamate transporters (Grass et al. 2004).
In the second model, which we currently favour, astrocytes and NG2-glia are completely segregated populations, with separate lineages and functions (Fig. 2B). In this model, astrocytes contain glial filaments with high or low levels of GFAP, but do not express NG2, and exhibit glutamate transporter activity. NG2-glia do not contain glial filaments and never express GFAP, although some may express low levels of GFAP mRNA under certain circumstances, and have functional AMPA receptors. NG2-glia express outwardly rectifying ion currents, whereas astrocytes have inwardly rectifying currents and a negative resting membrane potential with low input resistance. Most of the available evidence supports this model. In hippocampal slices and acutely isolated hippocampal cells, there is a clear segregation of cells with AMPA receptors and astrocytes with glutamate transporters (Zhou & Kimelberg, 2001; Matthias et al. 2003; School et al. 2003; Lin & Bergles, 2004). Immunohistochemical studies have shown that NG2+ cells and GFAP+ astrocytes represent antigenically non-overlapping populations (reviewed in Nishiyama et al. 2002). GFAP has never been detected in NG2-glia, nor has a transition from an NG2 glial cell into a GFAP+ cell been observed in vivo during development or in injury response. These observations are all consistent with the second model of segregated astrocyte and NG2-glial populations. Although the NG2+ population of glia appears to be homogeneous with respect to morphology, antigenic expression and AMPA receptor expression, there appears to be some degree of heterogeneity among astrocytes with respect to the level of GFAP expression and glutamate receptor function. For example, there exists a GFAP+ population that has both glutamate transporter and receptor function, such as the Bergmann glia in the cerebellum and possibly some cells in the brainstem respiratory centre (Grass et al. 2004). It is not yet clear whether there are astrocytes that are T−/R+ or whether such cells that were called ‘astrocytes’ are functionally equivalent to T−/R+ NG2 cells.
Do astrocytes and NG2-glia have overlapping functions in the adult CNS? NG2-glia do not express GFAP and do not help form the glia limitans, and are therefore not adapted for the classical roles of astrocytes in structural support, formation of the pial surface of the brain and interactions at the blood–brain barrier. Astrocytes also have established functions in K+ regulation and the uptake of glutamate released at synapses. Both astrocytes and NG2-glia extend processes to nodes of Ranvier and synapses, the sites of K+ and glutamate release during neuronal electrical activity, but they may play entirely different roles at these sites (reviewed in Butt et al. 2002; Lin & Bergles, 2004). Potassium regulation will depend in part on a strongly negative resting membrane potential and inwardly rectifying potassium channels, which are characteristic of GFAP+ passive astrocytes. By contrast, NG2-glia have less negative membrane potentials and outwardly rectifying potassium channels, which are not suited for K+ uptake. Astrocytes express glutamate transporters and are therefore responsible for terminating its action at synapses and protecting against excitotoxic accumulation. Because NG2-glia do not have glutamate transporters, their role at the synapse would be different from that of transmitter uptake functions of astrocytes. Synaptic activation of AMPA receptors results in depolarization and calcium influx in NG2-glia (reviewed in Lin & Bergles, 2004). Importantly, astrocytes have a mechanism of communicating among themselves through calcium waves (reviewed by Haydon, 2001), whereas this property is not apparent in NG2-glia, as reflected in the differences in input resistance between these cell types. These represent fundamental differences in the functions of astrocytes and NG2-glia in the neuronal circuitry.
In conclusion, it seems likely that astrocytes and NG2-glia represent two distinct glial cell populations, each playing a distinct role in the neural network. Newly generated transgenic mice that contain fluorescent markers and inducible gene expression mechanisms will provide us with the tools needed to obtain a clearer view of the functional differences between these populations of glia. As stated by Hubert H. Humphrey: ‘In real life, unlike in Shakespeare, the sweetness of the rose depends upon the name it bears. Things are not only what they are. They are, in very important respects, what they seem to be.’
Acknowledgments
We thank Dr Maria Rubio (Department of Physiology and Neurobiology, University of Connecticut) for her critical review of the manuscript and many helpful discussions.
References
- Bu J, Banki A, Wu Q, Nishiyama A. Increased NG2+ glial cell proliferation and oligodendrocyte generation in the hypomyelinating mutant shiverer. Glia. 2004;48:51–63. doi: 10.1002/glia.20055. [DOI] [PubMed] [Google Scholar]
- Bushong EA, Martone ME, Jones YZ, Ellisman MH. Protoplasmic astrocytes in CA1 stratum radiatum occupy separate anatomical domains. J Neurosci. 2002;22:183–192. doi: 10.1523/JNEUROSCI.22-01-00183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt AM, Colquhoun K, Tutton M, Berry M. Three-dimensional morphology of astrocytes and oligodendrocytes in the intact mouse optic nerve. J Neurocytol. 1994;23:469–485. doi: 10.1007/BF01184071. [DOI] [PubMed] [Google Scholar]
- Butt AM, Duncan A, Hornby MF, et al. Cells expressing the NG2 antigen contact nodes of Ranvier in adult CNS white matter. Glia. 1999;26:84–91. [PubMed] [Google Scholar]
- Butt AM, Kiff J, Hubbard P, Berry M. Synantocytes: new functions for novel NG2 expressing glia. J Neurocytol. 2002;31:551–565. doi: 10.1023/a:1025751900356. [DOI] [PubMed] [Google Scholar]
- Cajal Ramony. Contribucion al conocimmiento de la neuroglia del cerebro humano. Trab Lab Invest Biol. 1913;11:255–315. [Google Scholar]
- Dawson MR, Polito A, Levine JM, Reynolds R. NG2-expressing glial progenitor cells: an abundant and widespread population of cycling cells in the adult rat CNS. Mol Cell Neurosci. 2003;24:476–488. doi: 10.1016/s1044-7431(03)00210-0. [DOI] [PubMed] [Google Scholar]
- Eng LF, Ghirnikar RS, Lee YL. Glial fibrillary acidic protein: GFAP-thirty-one years (1969–2000) Neurochem Res. 2000;25:1439–1451. doi: 10.1023/a:1007677003387. [DOI] [PubMed] [Google Scholar]
- Feng G, Mellor RH, Bernstein M, et al. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron. 2000;28:41–51. doi: 10.1016/s0896-6273(00)00084-2. [DOI] [PubMed] [Google Scholar]
- Grass D, Pawlowski PG, Hirrlinger J, et al. Diversity of functional astroglial properties in the respiratory network. J Neurosci. 2004;24:1358–1365. doi: 10.1523/JNEUROSCI.4022-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydon PG. GLIA: listening and talking to the synapse. Nat Rev Neurosci. 2001;2:185–193. doi: 10.1038/35058528. [DOI] [PubMed] [Google Scholar]
- Horner PJ, Power AE, Kempermann G, et al. Proliferation and differentiation of progenitor cells throughout the intact adult rat spinal cord. J Neurosci. 2000;20:2218–2228. doi: 10.1523/JNEUROSCI.20-06-02218.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessaris N, Pringle N, Richardson WD. Ventral neurogenesis and the neuron–glial switch. Neuron. 2001;31:677–680. doi: 10.1016/s0896-6273(01)00430-5. [DOI] [PubMed] [Google Scholar]
- Kettenmann H, Ransom BR. The concept of neuroglia: a historical perspective. In: Kettenmann H, Ransom BR, editors. Neuroglia. 2nd. Oxford: Oxford University Press; 2005. pp. 1–8. [Google Scholar]
- Kimelberg HK. The problem of astrocyte identity. Neurochem Res. 2004;29:267–274. doi: 10.1016/j.neuint.2003.08.015. [DOI] [PubMed] [Google Scholar]
- Lee JC, Mayer-Proschel M, Rao MS. Gliogenesis in the central nervous system. Glia. 2000;30:105–121. doi: 10.1002/(sici)1098-1136(200004)30:2<105::aid-glia1>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Lin SC, Bergles DE. Synaptic signaling between GABAergic interneurons and oligodendrocyte precursor cells in the hippocampus. Nat Neurosci. 2004;7:24–32. doi: 10.1038/nn1162. [DOI] [PubMed] [Google Scholar]
- Malatesta P, Hartfuss E, Gotz M. Isolation of radial glial cells by fluorescent-activated cell sorting reveals a neuronal lineage. Development. 2000;127:5253–5263. doi: 10.1242/dev.127.24.5253. [DOI] [PubMed] [Google Scholar]
- Matthias K, Kirchhoff F, Seifert G, et al. Segregated expression of AMPA-type glutamate receptors and glutamate transporters defines distinct astrocyte populations in the mouse hippocampus. J Neurosci. 2003;23:1750–1758. doi: 10.1523/JNEUROSCI.23-05-01750.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama A, Watanabe M, Yang Z, Bu J. Identity, distribution, and development of polydendrocytes: NG2-expressing glial cells. J Neurocytol. 2002;31:437–455. doi: 10.1023/a:1025783412651. [DOI] [PubMed] [Google Scholar]
- Nishiyama A, Zhu X, Bergles DE, Wang H, Rubio ME. Extensive contact between NG2 cell processes and axons in NG2-DsRed transgenic mice. Abstract Soc Neurosci. 2004:414.13. [Google Scholar]
- Orkand RK, Nicholls JG, Kuffler SW. Effect of nerve impulses on the membrane potential of glial cells in the central nervous system of amphibia. J Neurophysiol. 1966;29:788–806. doi: 10.1152/jn.1966.29.4.788. [DOI] [PubMed] [Google Scholar]
- Penfield W. Neuroglia, normal and pathological. In: Penfield W, editor. Cytology and Cellular Pathology of the Nervous System. New York: Hafner; 1932. pp. 421–479. [Google Scholar]
- Peters A, de Palay SLF, Webster H. The Fine Structure of the Nervous System. New York: Oxford University Press; 1991. [Google Scholar]
- Raff MC, Miller RH, Noble M. A glial progenitor cell that develops in vitro into an astrocyte or an oligodendrocyte depending on culture medium. Nature. 1983;303:390–396. doi: 10.1038/303390a0. [DOI] [PubMed] [Google Scholar]
- Reynolds R, Dawson M, Papadopoulos D, et al. The response of NG2-expressing oligodendrocyte progenitors to demyelination in MOG-EAE and MS. J Neurocytol. 2002;31:523–536. doi: 10.1023/a:1025747832215. [DOI] [PubMed] [Google Scholar]
- Rio-Hortega P. Tercera aportacion al conocimiento morfologico e interpretacion functional de la oligodendroglia. Mem Real Soc Esp Hist Nat. 1928;14:5–122. [Google Scholar]
- Ross SE, Greenberg ME, Stiles CD. Basic helix–loop–helix factors in cortical development. Neuron. 2003;39:13–25. doi: 10.1016/s0896-6273(03)00365-9. [DOI] [PubMed] [Google Scholar]
- Schools GP, Zhou M, Kimelberg HK. Electrophysiologically ‘complex’ glial cells freshly isolated from the hippocampus are immunopositive for the chondroitin sulfate proteoglycan NG2. J Neurosci Res. 2003;73:765–777. doi: 10.1002/jnr.10680. [DOI] [PubMed] [Google Scholar]
- Virchow R. Gesammelte Abhandlungen zur Wissenschaftlichen Medizin. Frankfurt: Verlag von Medizinger Sohn & Comp; 1856. [Google Scholar]
- Walz W. Controversy surrounding the existence of discrete functional classes of astrocytes in adult gray matter. Glia. 2000;31:95–103. doi: 10.1002/1098-1136(200008)31:2<95::aid-glia10>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Ye P, Bagnell R, D'Ercole AJ. Mouse NG2+ oligodendrocyte precursors express mRNA for proteolipid protein but not its DM-20 variant: a study of laser microdissection-captured NG2+ cells. J Neurosci. 2003;23:4401–4405. doi: 10.1523/JNEUROSCI.23-11-04401.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M, Schools GP, Kimelberg HK. GFAP mRNA positive glia acutely isolated from rat hippocampus predominantly show complex current patterns. Brain Res Mol Brain Res. 2000;76:121–131. doi: 10.1016/s0169-328x(99)00341-1. [DOI] [PubMed] [Google Scholar]
- Zhou M, Kimelberg HK. Freshly isolated hippocampal CA1 astrocytes comprise two populations differing in glutamate transporter and AMPA receptor expression. J Neurosci. 2001;21:7901–7908. doi: 10.1523/JNEUROSCI.21-20-07901.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]